Abstract
With few exceptions, all animals acquire the ability to produce eggs or sperm at some point in their lifecycle. Despite this near universal requirement for sexual reproduction, there exists an incredible diversity in germ-line development. For example, animals exhibit a vast range of differences in the timing at which the germ line, which retains reproductive potential, separates from the soma, or terminally differentiated, non-reproductive cells. This separation may occur during embryonic development, after gastrulation, or even in adults, depending on the organism. The molecular mechanisms of germ line segregation are also highly diverse, and intimately intertwined with the overall transition from a fertilized egg to an embryo. The earliest embryonic stages of many species are largely controlled by maternally supplied factors. Later in development, patterning control shifts to the embryonic genome and, concomitantly with this transition, the maternally supplied factors are broadly degraded. This chapter attempts to integrate these processes – germ line segregation, and how the divergence of germ line and soma may utilize the egg to embryo transitions differently. In some embryos, this difference is subtle or maybe lacking altogether, whereas in other embryos, this difference in utilization may be a key step in the divergence of the two lineages. Here we will focus our discussion on the echinoderms, and in particular the sea urchins, in which recent studies have provided mechanistic understanding in germ line determination. We propose that the germ line in sea urchins requires an acquisition of maternal factors from the egg and, when compared to other members of the taxon, this appears to be a derived mechanism. The acquisition is early – at the 32 cell stage – and involves active protection of maternal mRNAs, which are instead degraded in somatic cells with the maternal to embryonic transition. We collectively refer to this model as the Time Capsule method for germ line determination.
Keywords: Oocyte, egg, embryo, echinoderm, RNA turnover, Primordial germ cells, germ line, soma
The Big Picture
Multicellularity in an organism allows for a division of labor. Different cellular functions enhance the organism’s overall competitiveness in the ecosystem, and expand its range and niche occupancy. Formation of these different functions often results from a progressive loss of developmental potency, as uncommitted cells terminally differentiate into their diverse fates. In most animals, these developmental decisions are controlled by both maternal information and by regulatory decisions engendered during embryogenesis. Differentiation involves not just the acquisition of gene function, but also the repression of recent gene activity and the degradation of previously important mRNAs. As development progresses, patterning controls shift increasingly to those newly made in the embryo. This passing of the controls involves several conserved features which occur coordinately, including: 1) a change in the transcriptional activity of embryonic blastomeres compared to the egg, 2) an extensive degradation of maternally-supplied mRNA, and 3) remodeling of the cell cycle from rapid, often synchronous cleavages to longer, more asynchronous divisions. Here, we will collectively refer to these changes as Maternal to Embryonic Transitions (METs). METs represent a conserved requirement for clearing of the maternal slate, which may facilitate cellular specialization by allowing embryonic gene regulation to rule. Eggs from different organisms exhibit vast differences in the extent that determinants are maternally supplied, and how rapidly these investments are removed in the shift to embryonic developmental control.
The extent of maternal loading of developmentally important information is intimately related to the manner in which embryos accomplish their division of labor. From the yellow crescent material that dictates muscle formation in ascidians, to the anucleate polar lobes that direct mesodermal fates in mollusk embryos, to even the differential yolk accumulation in birds, reptiles, and amphibians that influences cleavage patterns , to the germ plasm important for specifying the germ line in many organisms, differential loading of maternal components greatly influences when and how development proceeds (Conklin, 1905; Crampton & Wilson, 1896). Here we will specifically explore differences in the segregation of germ line from somatic cells, and how maternal control and the MET may be differentially utilized for this process.
In contrast to the terminal differentiation of the soma, the germ line retains the capacity for totipotency, since it will pass all heritable information to the next generation. From an evolutionary perspective, the establishment of the germ line is therefore of utmost importance. The result of germ line segregation is that committed primordial germ cells are developmentally sequestered from the soma and into a reproductive niche that will be solely tasked with creating the germ line stem cells, which once in the gonad will produce gametes. Despite the near universal requirement of the animal germ line, there exists a surprising diversity in the mechanisms by which it is segregated. In some animals, the germ line is formed very early, before the MET, whereas in others, germ line determination occurs after the MET has passed, and may even occur into adulthood. The timing of germ line segregation with respect to the MET necessitates differences in mechanism; that is, does it occur by post-transcriptional control, through induction and gene regulatory networks, or by some combination of these mechanisms? The goal of the work presented here is to unravel the relative contributions of maternal information and embryonic gene regulation in the separation of the germ line from the soma. We will explore the interrelationships between METs and germ line development across species, with particular emphasis on the echinoderms. This phylum comprises familiar examples such as the sea lily, the sea star, the brittle star, the sea cucumber, the sea urchin, and the sand dollar (Figures 1 and 2). Echinoderms offer readily accessible eggs and embryos for studying the mechanisms of developmental transition, and representatives within the phylum exhibit distinct mechanisms of germ line determination, valuable for comparative analysis in developmental function.
Figure 1. Metazoan Phylogenetic Tree (Reproduced from Juliano, Swartz, and Wessel, 2010).

Phylogenetic relationships of major animal groups, with representative organisms highlighted within parenthesis and illustrations.
Figure 2. Species relationships of echinoderm classes (Reich, Dunn, Akasaka, & Wessel, 2015).
The relationships between major echinoderm groups are indicated, with illustrations provided for species discussed in the text. The inferred stem at which the micromere lineage was acquired is indicated in purple. Extant species with micromeres are indicated in red.
Are METs universal?
Analyses of different organisms have taught us that the individual characteristics of METs are indeed widespread. An archetypal example is provided by the midblastula transition (MBT) in the frog Xenopus laevis. In this animal, the rapidly-dividing early embryo first undergoes a morphologically apparent change: blastomeres no longer cleave synchronously. Secondly, based on radioactive nucleotide precursor incorporation experiments as well as more recent genomics, a major activation of embryonic transcription occurs (embryonic genome activation; EGA). Thirdly, a major portion of the maternally supplied transcriptome is actively degraded (Tadros & Lipshitz, 2009). In animals such as Drosophila, Xenopus, and the zebrafish Danio rerio, whose phylogenetic positions are delineated in Figure 1, loss of cell cycle synchrony, EGA, and maternal transcript degradation happen simultaneously. Dramatic examples like these have broadly influenced the terminology (e.g. midblastula transition or maternal-to-zygotic transition) in a way that perhaps does not accurately reflect the diversity in development. There exist remarkable differences in when, where, and how, embryonic genome activation and maternal RNA clearance occur.
General activation of the embryonic genome
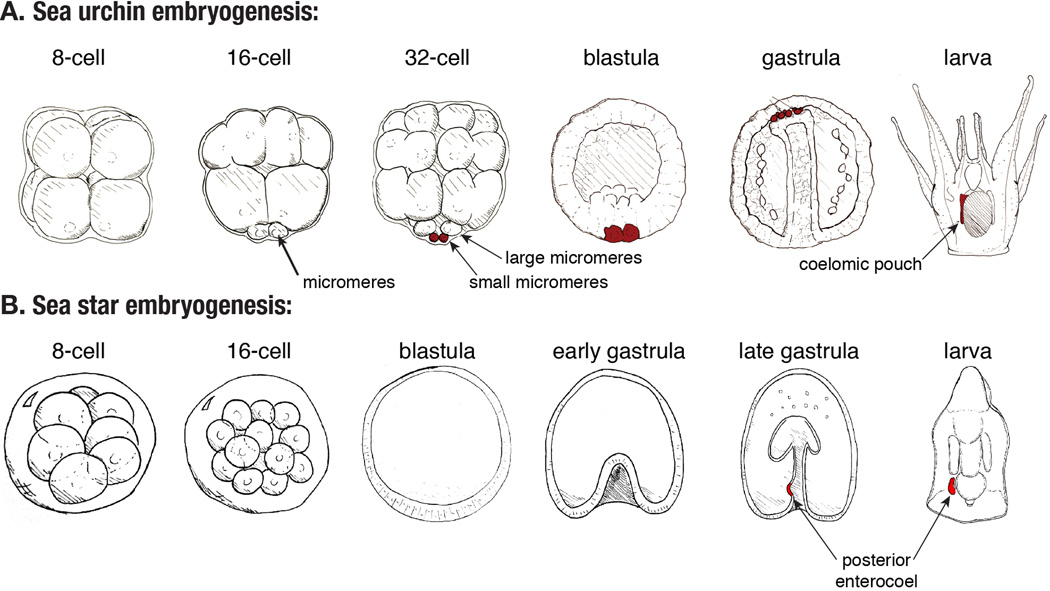
Several species of sea urchins, including California’s purple Strongylocentrotus purpuratus, have been particularly fruitful for investigating transcriptional regulation of embryogenesis. Sea urchin eggs are fertilized externally and adults can produce large cultures of synchronously developing embryos. Early cleavage stages in S. purpuratus are rapid (about 1 hour per cleavage), and complete. The first 3 cleavages are equal, but the fourth is asymmetric, and produces a 16-cell embryo of three tiers: the animal (top, by convention) most and mid-sized mesomeres, the large macromeres, and the small vegetal (bottom) micromeres. At this stage, germ layer fates have already begun to be specified. Subsequently, a ciliated blastula forms, which hatches from the fertilization envelope and becomes free swimming. Gastrulation initiates at the vegetal pole, and embryogenesis culminates in a feeding pluteus larva (Figure 3A).
Figure 3. Representative embryonic stages of echinoderms.
(A) Embryogenesis in the sea urchin is distinguished by highly regular cleavages, the third of which is asymmetric, yielding a 16-cell embryo of differently sized blastomeres. At the vegetal pole, a quartet of 4 small blastomeres, the micromeres, is produced. At the next cleavage, the micromeres divide asymmetrically to produce the large and small micromeres (sMics). The large micromeres ingress into the blastocoel and form the skeleton, while the sMics (red) are the PGCs in the sea urchin. The sMics translocate along the tip of the gut during gastrulation, and subsequently assort into the two coelomic pouches. The sMics contained in the left pouch will contribute to the adult germ line. (B) Embryogenesis of the sea star, which may represent the ancestral mode of germ line segregation in echinoderms. The early cleavages are equal, yielding embryos of equally sized blastomeres with no morphologically overt polarity. A large, hollow blastula forms, and gastrulation initiates before any mesenchyme enters the blastocoel. In the later gastrula, two coelomic pouches form at the anterior tip of the gut. Subsequently, a smaller posterior pouch called the posterior enterocoel (indicated in red) forms on the left of the gut, which is visibly distinct from the gut in the larva. The posterior enterocoel contains the likely PGCs.
Several important discoveries in the sea urchin embryo were transformative to the field of development: 1) the early embryo can develop independently of transcription and even independently of a nucleus, 2) the fertilized egg (zygote1) begins transcriptional activity as quickly as can be measured, and 3) protein synthesis begins at fertilization and begins independently of new transcriptional activity (Davidson, 1986). Development independent of transcription was shown in a variety of ways. One early indication was by E.B. Harvey, when she stratified the egg into nucleated and non-nucleated fragments and then tested developmental potentials in the resultant pieces. Using a highly pigmented species for this work, the local Arbacia punculata at the Marine Biological Laboratory in Woods Hole, she was able to visualize stratification of major organelles with isopycnic sucrose gradients, and even separate intact halves and quarters of eggs. From this approach, she learned that each egg fragment was capable of fertilization and development regardless of whether it contained the egg pronucleus, either as a diploid organism (male and female pronuclear contributions), as a merogone (an enucleated egg fragment that was fertilized), or even as a parthenogenetically activated merogone (with no nucleus). These experiments documented that early cleavage and development can occur in this animal even in the absence of a nucleus and that maternal information was important in early development (Harvey, 1940). Earlier, Theodor Boveri, while working at Stazione Zoologica Anton Dohrn di Napoli, even made use of sea urchin merogones fertilized by the sperm of other species to distinguish between contributions from the maternal stores, relative to the paternal nucleus (Boveri, 1893; Laubichler & Davidson, 2008). Overall, these experiments introduced the nuclear theory of determination but, more importantly for our discussion here, showed that all the RNAs needed for early development in this animal, including mRNAs, rRNAs, tRNAs, and small RNAs, must already be present in the egg prior to fertilization.
Paul Gross made use of the newly-identified toxin actinomycin D as an inhibitor of DNA-dependent RNA synthesis (Gross & Cousineau, 1963; Gross, Malkin, & Moyer, 1964). First, he and his colleagues tested how soon newly synthesized RNA was being made by incorporation of a radiolabeled uridine. Although technically limited to global RNA analysis and only by quantitation of radioactive counts, his group was able to detect significant incorporation within the first time point possible in these experiments, by 20 minutes after fertilization. Further, actinomycin D treated embryos exhibited no detectable transcriptional signal, yet the embryos developed relatively normally. They therefore concluded that new transcription was not necessary for early development, and that protein synthesis was templated by RNAs stored in the egg. It was subsequently demonstrated that the egg contained such information by measuring protein synthesis in the presence of radiolabeled amino acids. David Epel learned that amino acid incorporation occurred following fertilization, also as quickly as could be measured, within 15 minutes (Epel, 1967). Thus, the egg has substantial stored information that can support early development, and the transition from egg to embryo includes a rapid activation of both transcription and translation. While transcription is not essential for early development, the normal embryo does indeed initiate transcription with fertilization. So while some embryos (frog, fly) may not transcribe significant RNAs immediately after fertilization, others (sea urchins) clearly do, supporting the concept that the orchestrated METs of different species are highly variable.
These early functional investigations into sea urchin embryonic transcription and translation guided much of the thinking in the field for decades. Recently, high-throughput RNA sequencing and other technologies documented these processes in transcriptome-level detail. Sampling multiple time points from 10 to 72 hours of sea urchin development onward indicated diverse genome activity (Tu, Cameron, & Davidson, 2014). However, this study did not include time points between fertilization and 10 hours of development (approximately the first 9 cleavages); thus, the earliest upregulation of transcription was not captured. A further limitation of these approaches is that they do not directly distinguish between maternally supplied and embryonically transcribed RNA; embryonic activation could only be inferred by an increase in relative transcript abundance. In the future, it will be important to test additional and earlier time points with new approaches for capturing nascent RNA, such as groSEQ, or blocking of splicing to detect newly-transcribed intronic sequence (Core, Waterfall, & Lis, 2008; Lee et al., 2013). For now, it can at least be deduced that there is an increasing reliance upon embryonic transcriptional activity as development progresses that is perhaps accentuated by important milestones in embryogenesis (summarized in Figure 4).
Figure 4. Summary of METs in sea urchin embryogenesis.
Important embryonic milestones are denoted with embryo illustrations. General transcription based on incorporation of radioactive nucleotides initiates at fertilization and steadily increases through early development (green line). The regionally specific gene regulatory network and fate specification first occurs at the 16-cell stage, when beta catenin is nuclearized in the micromeres (purple line). We hypothesize that the differential GRN further diversifies in activity when the cell cycle is remodeled at approximately 10 h.p.f. and cleavage synchrony is lost. Based on mRNA expression dynamics, we suggest that there is a major degradation of maternal RNA that occurs as the embryo prepares to gastrulate. Maternal mRNA is represented in blue in embryo descriptions, and is lost in somatic cells in the gastrula, but retained in the sMics (green arrows).
Activation of regionally-specific Gene Regulatory Networks (GRNs)
Through gene regulatory analysis, it is clear that lineage-specific gene expression initiates in the sea urchin by the 4th cleavage, when the micromeres express vegetal inductive signals, such as Wnt 8 (Cui, Siriwon, Li, Davidson, & Peter, 2014; Wikramanayake et al., 2004). The earliest activation of this localized gene regulatory network (GRN) is downstream of the canonical Wnt/β-catenin pathway (Logan, Miller, Ferkowicz, & McClay, 1999; Wikramanayake, Huang, & Klein, 1998) (Figure 4). Blocking nuclear β-catenin prevents Wnt8 expression, vegetal fate specification, and animalizes the embryo with complete failure in gastrulation. Typically, the canonical Wnt pathway is activated by a secreted Wnt ligand binding to a Frizzled type receptor. Subsequently, membrane-associated Disheveled protein binds and disassembles a destruction complex, allowing for β-catenin to enter the nucleus and activate transcription. However, the earliest activation of the pathway in the sea urchin embryo may occur independently of a Wnt ligand. Maternally-supplied Disheveled protein is enriched in the vegetal cortex of the sea urchin egg and, based on gel mobility, is modified in a form that perhaps indicates constitutive activation (Peng & Wikramanayake, 2013). In effect, the pathway may be hard-wired, implying that the early embryo activates differential axial specification autonomously, and not until later does inductive signaling influence cell fate decisions. Indeed, when cultured in the presence of an inhibitor that blocks all Wnt secretion, embryos still establish animal-vegetal polarity and form a blastopore (Cui et al., 2014). Nuclearization of maternal β-catenin thus may be a primary maternal activator of the embryonic genome. Downstream of this initial anisotropy, regionally specific GRNs pattern the various ectodermal, endodermal, and mesodermal territories of the pluteus larva (Oliveri, Tu, & Davidson, 2008; Peter & Davidson, 2015).
Loss of cleavage synchrony: a cause or effect of embryonic control?
In Drosophila and Xenopus, major activations of the embryonic genome coincide with a loss of cleavage synchrony amongst blastomeres (Farrell & O'Farrell, 2014; Tadros & Lipshitz, 2009). While cleavage patterns exhibit species differences amongst echinoderms, careful investigation of several sea urchin species reveals some commonalities. Only the first 4 cleavages are completely synchronous—that is, until the birth of the micromere lineage. This timing of synchrony loss would seem to coincide with a major activation of the differential GRN. Subsequently, embryos display “regional synchrony” – that is, particular tiers of related blastomeres divide together, but at different rates than their neighbors. Intriguingly, these regional synchronies follow an animal-vegetal polarity gradient, with vegetal cells (e.g. micromeres) dividing more slowly than the more animal cells. This animal-vegetal wave of cleavage is reminiscent of the metasynchronous cleavage cycles in the early syncytial Drosophila embryo (Edgar & O'Farrell, 1989). All cleavage synchrony in the sea urchin is lost by the 8th or 9th cycle, at the onset of ciliogenesis and hatching (Figure 4). Furthermore, the cell cycle is substantially lengthened at this stage (Dan, Tanaka, Yamazaki, & Kato, 1980; Masuda & Sato, 1984). The complete loss of synchrony detected in blastula stages may coincide with the arrests observed when embryos are cultured in transcriptional inhibitors. That is, even though embryos can develop without new transcriptional activity, essential gene activity is present at the time of cell cycle loss of synchrony. More investigation is required, but it is tempting to speculate that there may be an intimate and even causal relationship between the cell cycle and EGA in the sea urchin. For example, the lagging of vegetal blastomere cleavage (e.g. the micromeres) relative to animal blastomeres may permit the early vegetal activation of GRN expression. Downstream GRNs become activated once other blastomeres in the embryo have divided more, producing enough raw cellular material to create the other territories. The lengthening of the cell cycle at the 9th division may be important to facilitate robust embryonic transcription, as occurs in Drosophila (Shermoen & O'Farrell, 1991). The ability of embryos to reach blastula stages with transcriptional inhibitors may simply reflect their maternally-endowed ability to divide into many cells, whereas the differentiation of those cells is under embryonic control.
The loss of synchrony, lengthening of the cell cycle, and possible relationship between cell cycle and transcription are consistent MET characters in other organisms. Cell cycle modifications in embryogenesis have been extensively characterized in Drosophila, which displays a dramatic extension of cleavage cycle 14—the Drosophila MBT. With cycle 14, a typical G2 phase is introduced – in the first ten cycles, there are no gap phases, and a short G2 only begins to be introduced at cycle 10. One mechanism for this extension is the degradation of the maternally supplied ortholog of the Cdc25 phosphatase, Twine (Farrell & O'Farrell, 2014). The lengthening of the cell cycle may be required for robust transcription, particularly from large genes. Given the extremely rapid early cleavages of the Drosophila embryo, transcription is limited by the ability of RNAPII to elongate before being displaced by the DNA replication machinery or by condensation in the beginning of mitosis. Taking advantage of detailed knowledge of cell cycle timing and in situ hybridization, it was found that Ubx gene transcription is interrupted by mitosis prior to cycle 14, resulting in abortive transcripts (Shermoen & O'Farrell, 1991). Thus, it is possible that the remodeling of the cell cycle observed in the sea urchin and other animals also has direct consequences for transcriptional regulation.
Degradation of the maternally supplied transcriptome
In diverse organisms, such as the zebrafish, frog, and fruit fly, degradation of the maternally supplied transcriptome is essential for developmental progression (Tadros & Lipshitz, 2009). Genomic studies strongly support a major transcriptome remodeling around the blastula to gastrula transition in the sea urchin. Temporal analysis by microarrays suggested that a major fraction of maternally supplied transcripts is degraded by the onset of gastrulation (Wei, Angerer, & Angerer, 2006). Subsequent deep sequencing and cluster analysis indicated that the transcripts of 34% of all genes are maternally deposited into the egg, and sharply degraded by the onset of gastrulation (Figure 4) (Tu et al., 2014).
How these RNAs are turned over in the sea urchin remains unknown. In other animals, maternal transcriptome degradation is effected by RNA binding proteins, as well as by small RNA machinery. Drosophila has two phases of degradation: first, a maternal pathway that can occur in an activated, unfertilized eggs (i.e. without a fertilizing sperm) and a second pathway that requires embryonic transcription (Bashirullah et al., 1999; Tadros et al., 2007). The first pathway uses the RNA binding protein Smaug, which binds stem loop elements in the 3’UTRs of its targets, and can both repress translation and target the mRNA for degradation, typically performing both functions (L. Chen et al., 2014; Smibert, Wilson, Kerr, & Macdonald, 1996). Translational repression is achieved by recruiting the Drosophila-specific factor Cup, which interacts with eIF4E to prevent interaction with eIF4G and recruitment of the 40S ribosomal subunit (Nelson, Leidal, & Smibert, 2004). Transcript degradation is achieved by recruiting the CCR4/POP2/NOT complex, called the CNOT complex in vertebrates (Semotok et al., 2005). This multisubunit complex is a major cytoplasmic deadenylase, which functions by shortening poly-A tails and making the mRNAs substrates for various RNA decay pathways, including the exosome (Collart & Panasenko, 2012). Smaug binding to mRNA in Drosophila can recruit the CNOT complex and degrades over 1000 transcripts, at least 339 of which were identified as direct targets (L. Chen et al., 2014). While most transcripts were both translationally repressed (based on lack of polysome association) and degraded by Smaug, many transcripts were repressed but not destabilized. This result suggests separable functions for Smaug, and future work may illuminate the combinatorial control that can lead to these distinct outcomes of Smaug binding. The sea urchin contains two putative Smaug paralogs, which should be tested in the future for conservation of function in maternal transcript turnover.
A second pathway mechanism for mRNA decay is via the micro RNA (miRNA) pathway. Together with their associated Argonaute proteins, miRNAs recognize seed sequences in 3’UTRs and promote both translational repression and transcript decay, also by recruiting the CNOT complex (Tritschler, Huntzinger, & Izaurralde, 2010). In Drosophila, a second pathway requiring embryonic transcription further reinforces the transcript degradation initiated by Smaug, and requires the miR-309 miRNA cluster. This cluster of miRNAs degrades several hundred maternally supplied mRNAs, and its deletion results in larval lethality (Bushati, Stark, Brennecke, & Cohen, 2008). The miRNA machinery is used in several organisms for maternal transcript clearance. In the zebrafish, miR-430 is expressed at the MBT and causes the degradation of several hundred maternal transcripts by deadenylation (Giraldez et al., 2006). The Xenopus ortholog of miR-430, called miR-427, is required to degrade maternally supplied cyclin A1 and B2 mRNAs; however, whether miR-427 targets a broad subset of transcripts like miR-430 is unknown (Lund, Liu, Hartley, Sheets, & Dahlberg, 2009). The miRNA pathway has also been interrogated in the sea urchin, whose genome contains at least 49 miRNAs present in the egg and embryo. Complete blockage of miRNA biogenesis prevents normal gastrulation and differentiation of the embryo but, strikingly, this defect can be rescued by adding back four abundant miRNAs: miR-1, 31, 2012, and 71 (Song et al., 2012). This result is reminiscent of the rescue obtained when miR-430 is injected into Dicer-mutant zebrafish (Giraldez, 2005). The link has not yet been directly tested in sea urchins; however, it seems likely that these miRNAs would play a large role in the maternal transcript degradation detected by temporal deep sequencing (Tu et al., 2014). Together, these results indicate that maternal transcriptome clearance is an essential transition in embryogenesis. However, since it occurs by different mechanisms in different species, we can infer that convergent evolution has favored multiple acquisitions of this form of MET. There must then exist a strong selective pressure for the process.
We propose that maternal transcriptome degradation is essential for clearing of the slate of the totipotent egg, allowing for specific gene regulatory processes to pattern the different cell fates of the embryo. Differences in developmental strategy that favor rapid development have influenced the evolution of the timing of the event. For example, it occurs late in Drosophila, in an embryo of several thousand syncytial nuclei. In contrast, the mouse initiates degradation of maternal transcripts in response to fertilization and is largely complete by the 2-cell stage (Hamatani, Carter, Sharov, & Ko, 2004). The segregation of the germ line is uniquely intertwined with maternal RNA acquisition, and also has evolved multiple developmental mechanisms, which we document below.
Separating the soma from the germ line: a critical fork in the road
The survival of a species depends on a means for the individual to transmit its heritable information to its offspring. In the lifecycle of most animals, important reproductive cells called the germ line are typically segregated away from the terminally differentiating soma, which lacks reproductive potential and will die with that individual. Species differences in the timing of germ line/soma separation have led to ambiguity in how terminology should be applied. For example, the P blastomeres of the early Caenorhabditis elegans embryo are often referred to as a germ line cells because they contain characteristic germ granules (or P-granules) and will give rise to de facto germ line stem cells later (Seydoux & Braun, 2006). However, this blastomere will also create a number of somatic cell fates for several cleavage cycles, until finally giving rise to the Z blastomeres, which are solely germ line competent. In this work, we will designate any cell as being within the germ line if it is capable of giving rise to a gamete, whether it is the direct progenitor of that gamete, or a general founding blastomere whose later progeny becomes gametogenic. It may be more straightforward to define the germ line by what it is not: a blastomere may be called somatic when its fate no longer includes germ line potential. Similarly, we will refer to a blastomere that is solely germ line competent and without somatic potential as a primordial germ cell (PGC). A germ line stem cell (GSC) is the mature product of the PGC, which is found in the gonad and undergoes self-renewing divisions to produce meiotically capable cells that will give rise to gametes (eggs or sperm). This discussion also excludes the consideration of cells that can make germ line by a variety of experimental or abnormal conditions but do not do so in normal development.
Different roads lead to a conserved germ line program
Comparative analyses of both expression pattern and function have illuminated a set of highly conserved germ line regulators, which have been extensively reviewed elsewhere (Ewen-Campen, Schwager, & Extavour, 2010). The most conserved of these factors are post-transcriptional regulators of mRNA stability and translation. Here, we will focus our attention primarily on a translational repressor, Nanos, but also an Argonaute family member, Piwi, and an RNA helicase, Vasa. In all species examined previously, overlapping expression of these three factors in embryonic blastomeres is predictive of germ-line fate, though occasional non-germ-line stem-cell functions have been identified for them individually (Juliano, Swartz, & Wessel, 2010a). Nanos orthologs are zinc finger-containing RNA-binding proteins that act upon 3’UTRs of target mRNAs in the germ line. Nanos itself possesses only nonselective RNA binding activity, and instead derives its specificity from associating with a partner called Pumilio. Pumilio is the founding member of the PUF-family RNA binding factors, and recognizes highly conserved Pumilio Response Elements (PREs) of a “UGUAAAU” consensus (D. Chen et al., 2012). The primary consequence of Nanos/Pumilio binding a target mRNA is to effect its translational repression and/or destabilization. A conserved mechanism of Nanos action is by recruitment of the CNOT complex, similar to that described for Smaug earlier (Bhandari, Raisch, Weichenrieder, Jonas, & Izaurralde, 2014). Upon recruitment, the nuclease subunits CNOT6 and CNOT7 then degrade the mRNA’s poly-A tail resulting in its destabilization. Piwi family members are nucleases guided by small RNAs (piRNAs), which are primarily thought to protect the germ line by opposing transposable element mobilization in a process called the ping-pong cycle (Mani & Juliano, 2013). The precise role of Vasa has been mysterious since its discovery, but has now been identified as an integral component of piRNA biogenesis and transposon suppression in the germ line (Xiol et al., 2014) as well as a general translation factor (Liu et al., 2009; Yajima and Wessel, 2015).
Despite similarities in the molecular toolkit (e.g. Vasa, Nanos, and Piwi), there exists a surprising amount of diversity in how PGCs are segregated and how germ line specifying genes come to be expressed. The mechanisms of germ line specification across species can be considered within a continuum of what we will call here preloaded versus inductive mechanisms (sometimes referred to as preformation vs. epigenesis, respectively). In brief, induction involves a conversation of cell-cell signaling between embryonic tissues that instructs select cells to adopt germ line fate (Figure 5A,B). Conversely, preloaded specification involves maternally supplied determinants that are often spatially enriched in one region of the egg, which when acquired early by nascent blastomeres, directs them towards a germ line fate. These dense amalgamations of protein and mRNA determinants are collectively referred to as germ plasm (Figure 5C).
Figure 5. Strategies for germ line segregation by induction or preloading.
(A) Germ line specification by induction. Shown is a simplified mouse embryo 6.5 days post fertilization. Bmp4 signaling from the extraembryonic ectoderm (ExE, blue) and Wnt3 expressed in the proximal epiblast (purple) are synergistically required to activate germ line transcriptional regulators in the PGCs (green). (B) Regulatory diagram depicting Wnt3 activating the mesodermal transcription factor T, which in turn activates Blimp1 and Prdm14, which along with AP2γ, form a tripartite network that activates germ line gene expression and represses somatic fate. The activating role of Bmp4 has not been directly delineated, but is required to license the PGCs to respond to the Wnt3 signal. (C) Germ line segregation by preloading. Shown is a syncytial blastoderm staged Drosophila embryo, with maternally-localized germ plasm in green. The PGCs are the first to cellularize in this embryo, and acquisition of the germ plasm directs them towards germ line fate.
PGC specification by induction
Comprehensive syntheses of available data across taxa strongly imply that induction represents the ancestral mode of germ line segregation, though the timing of the event can vary (Extavour, 2007; Extavour & Akam, 2003). Perhaps the most intensely studied example of induction is the mouse, which specifies its germ line late, at about 6.5 days post fertilization. The proximal portion of the epiblast, or embryo proper (in closest proximity to the placental attachment), contains a field of mesodermal progenitors. Synergistic signaling from the extraembryonic ectoderm to this field, notably via the secreted molecules Wnt3 and Bmp4, enacts a germ line transcriptional program in approximately 8 founding PGCs (Figure 5A) (Lawson et al., 1999; Ohinata et al., 2009; Tam & Zhou, 1996). The exact connections between PGC transcriptional regulators are still being elucidated; however, the mesodermal factor Brachyury/T appears to be one of the earliest activators, and a direct target of canonical signaling from Wnt3. Brachyury is then required for the activation of core mouse PGC regulators Blimp1 and Prdm14 (Aramaki et al., 2013; Magnúsdóttir et al., 2013). Together with AP2γ, these three factors comprise a transcriptional program termed the “tripartite network,” which is required to broadly repress somatic cell transcription but enable germ line transcription. Nanos3 is a direct target of AP2γ as determined by ChIP analysis (Magnúsdóttir et al., 2013). It is expressed in early PGCs following their initial segregation and during their migration into the somatic gonad, and its knockout leads to loss of PGCs by apoptosis and sterility of both males and females (Suzuki, Tsuda, Kiso, & Saga, 2008; Tsuda et al., 2003).
While induction is mechanistically best understood in the mouse, ample morphological, gene expression, and some functional data indicate its broad usage across species. Some effort has been made into human PGC investigation, but several considerations make their isolation impractical. Recently, in vitro schemes have been devised that appear to accurately mimic the developmental course of human PGCs in vivo. Already such investigation has revealed some striking differences from the established mouse paradigm – for example, Sox17, which was previously considered an endodermal regulator, is required to activate Blimp1; however, these PGC-like cells also upregulate Brachyury, normally associated with somatic mesoderm formation, and it will be important to test in the future whether Brachyury is required for Blimp1 and Prdm14 activation in human cells, or whether these are competing elements for PGC formation (Irie et al., 2015). These human PGC-like cells also upregulate Nanos3 in a Blimp1 and Sox17 dependent fashion, though it is not yet known whether they are a direct target of these regulators. A third and powerful vertebrate comparison is the axolotl or Mexican salamander, a urodele amphibian. In contrast to mice and humans, axolotl PGC specification seems to require both Fgf and Bmp4 signaling for robust germ line gene activation. Brachyury also enhances germ line induction in the axolotl (Chatfield et al., 2014). Intriguingly, PGC specification appears to be Blimp1 independent in the axolotl, raising the possibility that the upstream Blimp1 expression in mouse PGCs is a derived trait. Further comparisons will be required to more confidently resolve the evolutionary history of vertebrate PGCs. In any case, a conserved theme of vertebrate PGC segregation is intercellular signaling via Bmp signaling and activation of the translational repressor Nanos, whose molecular functions we will return to later.
Accumulating data in non-vertebrates suggest that induction via Bmp signaling is indeed a deeply conserved mechanism for PGC segregation. The cricket Gryllus gryllus has been developed into a functional model organism, and has been suggested to be more representative of ancestral insect development than Drosophila melanogaster. In the cricket, PGC clusters arise in posterior segments, and are recognized both by morphology as well as by expression of germ line factors. Knockdown of Bmp pathway components leads to a loss of PGCs, whereas overactivation of the pathway leads to supernumerary germ cells (Donoughe et al., 2014). Whether the Wnt pathway is also required, as observed in the mouse, remains to be tested. Functional data for PGC specification in other taxa are limited; however, the expression patterns of these genes merged onto the embryological considerations broadly imply conservation of induction. For example, morphological and molecular examination indicates a lack of germ plasm or early-forming PGCs in several other arthropods, Lophotrochozoans such as the mollusk Ilyanassa, and the cnidarian Nematostella, among others (Ewen-Campen, Donoughe, Clarke, & Extavour, 2013a; Ewen-Campen, Jones, & Extavour, 2013b; Extavour, Pang, Matus, & Martindale, 2005; Rabinowitz, Chan, Kingsley, Duan, & Lambert, 2008; Swartz, Chan, & Lambert, 2008). Highly regenerative animals, such as hydrazoan cnidarians like Hydra, demonstrate a capacity to segregate germ cells throughout their life cycles, rather than just in embryogenesis, precluding reliance upon inherited embryonically germ plasm (Juliano et al., 2014). In these animals, a self-renewing multi- or totipotent stem cell called the i-cell continually replenishes somatic lineages, and also contains the germ line, as it will give rise to sperm and eggs upon sexual reproduction (Müller, Teo, & Frank, 2004). The planarian Schmidtea mediterranea can regenerate its germ line after being dissected into very small pieces, demonstrating an impressive capacity for induction in the adult (Y. Wang, Zayas, Guo, & Newmark, 2007). Broad comparisons such as these and many others reviewed elsewhere all point to induction being the likely ancestral route for PGC segregation in Metazoa (Extavour, 2007).
PGC specification by preloading
Investigation in diverse animals has yielded multiple examples of early PGC segregation by preloading. In contrast to the aforementioned strategies, PGC segregation here is thought to occur both autonomously and independently of PGC transcriptional activity. Perhaps the most famous example of germ line specification by preloading is by Drosophila, whose PGCs are indeed the very first cells to form, approximately 1.5 hours after fertilization (Figure 5B). In this archetypal example, a cytologically obvious germ plasm is spatially localized to the posterior of the oocyte. Germ plasm localization involves an active process of transport along microtubules, cytoplasmic dumping from the nurse cells followed by streaming in the oocyte, and their anchoring to the posterior cortex (Bergsten & Gavis, 1999; Seydoux & Braun, 2006). Germ plasm assembly follows a distinct assembly hierarchy, with the insect-specific protein, Oskar, serving as an anchor upon which other mRNAs and proteins aggregate. In an intriguing example of evolutionary co-option, the ancestral role of Oskar was likely in the nervous system, while its utility as a scaffold was later employed in the germ plasms of Dipteran species (Ewen-Campen, Srouji, Schwager, & Extavour, 2012). Classic experiments have revealed that germ plasm is both necessary and sufficient to specify PGCs in Drosophila: transplant of germ plasm to an irradiated (and infertile) recipient can restore fertility, or even create ectopic PGCs in the anterior region of the embryo (Illmensee & Mahowald, 1974). Revisiting these experiments with genetic approaches has shown that Oskar overexpression results in ectopic, anterior PGCs (Ephrussi & Lehmann, 1992; Smith, Wilson, & Macdonald, 1992).
The nematode worm Caenorabiditis elegans also provides a genetically tractable example of preloaded germ line specification. In this organism, the germ plasm comprises a collection of ribonucleoprotein granules called P-granules. The P-granules contain conserved germ line factors, such as the C. elegans Vasa orthologs, called GLH-1 and 2 (Gruidl et al., 1996). Before fertilization, P-granules are uniformly distributed in the cytoplasm, but after fertilization and during cleavage, these granules are segregated towards the P blastomere lineage. As development progresses, P-granules become increasingly associated with the nuclei, and are eventually restricted to the Z-blastomeres, which are the de facto PGCs (Updike & Strome, 2010). While some analyses have suggested that P-granules are neither strictly required nor sufficient for the embryonic segregation of PGCs in C. elegans, their intimate association with the germ line is clear, and they likely perform diverse tasks pertaining to RNA metabolism, post-transcriptional regulation, and germ-line protection (Gallo, Wang, Motegi, & Seydoux, 2010; Voronina, 2013).
Numerous examples of preloaded specification exist within vertebrates as well. Xenopus displays a prominent germ plasm, called the Balbiani body, which translocates to the vegetal cortex during oogenesis. The Xenopus germ plasm contains numerous germ line-associated RNAs and proteins, in addition to a collection of mitochondria, which are commonly associated with germ granule structures in different species. Nanos1 RNA is transcribed during oogenesis and incorporates into the germ plasm and, following fertilization, is inherited by germ line fated vegetal blastomeres (Forristall, Pondel, Chen, & King, 1995; MacArthur, Bubunenko, Houston, & King, 1999). The zebrafish also segregates its PGCs by preloading, displaying remarkable similarities with the frog including a vegetally localized Balbiani body during oogenesis. Nanos and Vasa RNAs, as well as that of Dazl, another translational regulator, assemble into the zebrafish Balbiani body. Following fertilization, the germ plasm material translocates to the animal pole of the zygote, and becomes enriched at the distal ends of cleavage furrows. The four blastomeres that inherit this material are directed to germ-line fate (Kosaka, Kawakami, Sakamoto, & Inoue, 2007). A gene called Bucky ball is required for germ plasm assembly and transport and, while it does not bear evolutionary homology to Oskar, its protein product performs an analogous function (Bontems et al., 2009).
Evolutionary transition of PGC segregation in the echinoderms
Echinoderms provide an intriguing test case for the evolution of preloaded germ-line specification, supported by a rich fossil record (Figure 2). Expression pattern and embryological analyses indicate that the ancestral mode of germ line segregation in the echinoderms was by induction (Wessel et al., 2014). In the sea star, embryological investigations suggest that a posterior coelomic pouch (posterior enterocoel, PE) in the larvae is required for fertility in the adult (Figure 3B); when this structure is removed by microsurgery, juveniles contain fewer putative PGCs by cytological criteria (C. Inoue, Kiyomoto, & Shirai, 1992). Patterns of germ line gene expression also support an inductive mode for the sea star. Transcripts for Vasa and Piwi are ubiquitously distributed in early embryos, with no indications of a localized germ plasm. However, these transcripts become progressively restricted to vegetal cells that form the PE as gastrulation progresses. In contrast, Nanos expression is activated embryonically within the PE cells, reminiscent of Nanos activation in the mouse PGCs after stimulation by Bmp and Wnt signaling (Fresques, Zazueta-Novoa, Reich, & Wessel, 2014). Both Wnt3 and Bmp2/4 are expressed in territories close to the PE progenitors, suggesting that these pathways could have a conserved role for germ line induction, though this premise remains to be tested functionally.
In contrast, the echinoid echinoderms display some features of preloaded PGC segregation. Echinoids comprise a relatively recently diverging clade that includes the euechinoids, or sea urchins and sand dollars, and the cidaroids, or pencil urchins (Figure 3B). A derived trait of this clade is the micromere lineage, which forms by an asymmetric division at the 4th cleavage. This division produces a quartet of small cells at the vegetal pole called the micromeres. The micromeres then divide asymmetrically again to produce the more animal-oriented large micromeres, and the vegetal small micromeres (hereafter abbreviated sMics) (Figure 3B). This separation of fates between the large and small micromeres is particularly dramatic, because the large micromeres are unipotent and will only construct the larval skeleton, while the sMics are the likely PGCs (Wessel et al., 2014).
Numerous lines of molecular evidence support the concept that the sMics are the bona fide PGCs, formed by preloaded specification. Upon formation, the sMics are highly enriched for Vasa protein, which occurs via post-translational mechanisms including ubiquitination and, perhaps, spindle association (Gustafson, Yajima, Juliano, & Wessel, 2011; Yajima & Wessel, 2011a). Shortly after their formation, the sMics express Nanos2, which along with the forkhead transcription factor FoxY, is one of only two known genes embryonically and selectively expressed in the sMics prior to gastrulation (Juliano, Yajima, & Wessel, 2010b; Materna, Swartz, & Smith, 2013) (Figure 6). Nanos is uniquely detectable in the sMics by in situ hybridization as early as the 32/64-cell stage. As in the sea star, Piwi mRNA is maternally supplied and ubiquitous in early embryos, but becomes restricted post-transcriptionally to the sMics by gastrula stages (Swartz et al., 2014; Yajima, Gustafson, Song, & Wessel, 2014). Intriguingly, the vegetal egg cortex and subsequently the sMics of the sea urchin Hemicentrotus pulcherrimus are enriched for mitochondrial rRNAs outside of the mitochondria themselves (Ogawa et al., 1999). Similar observations have been made for the germ plasms of Drosophila and Xenopus, but the significance of extramitochondrial rRNA is unknown (Kobayashi, Amikura, & Mukai, 1998; Kobayashi, Amikura, & Okada, 1993). The sMics show signs of autonomy associated with preloaded PGC segregation: when the micromeres, representing the parent lineage, are surgically removed and cultured in isolation, they divide again asymmetrically to form the sMics, and upregulate both Vasa protein and Nanos RNA on a time scale consistent with the intact embryo (Yajima & Wessel, 2012). After their creation, the sMics divide only once to yield 8 descendants for all of embryogenesis and early larval stages. Thus, the sMics display all characteristics associated with preloaded PGCs, specified much more precociously than in the sea star. Yet, no morphologically-apparent germ plasm has been identified, neither in eggs nor embryos. Lack of evidence is not proof, but these embryos appear to specify germ cells by preloading mechanisms, with a distinct from those seen in other model organisms.
Figure 6. Temporal dynamics of sMic regulatory state (Adapted from Wessel et al., 2014).
Location of the sMic lineage is indicated in green, from 32-cell stage into the larva. Colored bars indicate the presence or absence of gene expression, or enrichment or depletion for specific markers.
Given the unique preloaded characteristics of the sMics, the embryonic expression of Nanos2 is unique. Three Nanos paralogs are present in the sea urchin (termed Nanos1-3), which are expressed at different times in the life cycle. Nanos1 is ovary-specific, while Nanos2 is detectable from shortly after the sMics are created and into the early coelomic pouches. Nanos3 is transiently expressed at the tip of the developing gut late in gastrulation (Juliano et al., 2010b). The regulation of Nanos1 and 3 is unexplored, though some traction has been gained with the early sMic paralog, Nanos2. A late requirement of the Nanos2 gene appears to be FoxY, whose knockdown results in a two-fold reduction in Nanos2 levels at the onset of gastrulation (Song & Wessel, 2012). It is not clear, however, whether Nanos2 is a direct FoxY target. FoxY is directly activated by the Notch/Delta signaling pathway, which is an upstream inducer of mesoderm in the sea urchin (Materna et al., 2013; Materna & Davidson, 2012). Neither FoxY nor Delta/Notch perturbations affect Nanos2 expression in the sMics before gastrulation; therefore, the earliest inputs into this gene remain a critical open question. An important caveat is that there exists significant maternally-supplied FoxY protein in the early embryo, which may be refractory to knockdown. FoxY may serve a supporting role for the sMics though its possible role as a direct regulator of Nanos has not been ruled out. After its initial sMic expression, FoxY expression shifts into adjacent mesodermal precursors and its effect on nanos expression in the sMics then would need to be indirect. Knockdown of FoxY or D/N perturbation both completely prevent coelomic pouch formation, which contain the final niche for the sMics. Delta/Notch signaling through FoxY may therefore be important for establishing the somatic gonad required for GSC maintenance.
Given the early activation of Nanos2 in the sMics, it seems likely that a maternally supplied factor should be responsible. A strong candidate is the Wnt/β-catenin pathway (discussed above). Nuclear beta catenin, the transcriptional effector of the Wnt pathway, is indeed highly enriched in vegetal blastomeres of the sea urchin embryo (Logan et al., 1999). Additionally, the micromere lineage expresses 3 Wnt ligands prior to gastrulation that may reinforce the maternally activated Wnt pathway (Cui et al., 2014). Lithium chloride treatment, which upregulates nuclear beta catenin throughout the embryo, results in an increase in overall Vasa protein levels throughout the embryo (Voronina et al., 2008). Future investigation should further test whether the Wnt pathway regulates Nanos expression directly, particularly in light of conserved roles for Wnt and BMP signaling in germ lines of other species.
Consistent with germ line segregation in other preloaded systems, sea urchin Nanos2 is heavily regulated post-transcriptionally. The 3’UTR contains a stability and translational control element, termed the GNARLE (global nanos associated RNA lability element). The GNARLE element is sufficient to confer sMic localization of injected mRNA reporters, independently of the Nanos open reading frame sequence (Oulhen et al., 2013). Intriguingly, 3’UTR-mediated control of Nanos localization has also been found in zebrafish Nanos1 and mouse Nanos3 (Köprunner, Thisse, Thisse, & Raz, 2001; Suzuki, Saba, Sada, & Saga, 2010). This observation implies that transcriptional regulation alone is insufficient for selective Nanos2 expression in the sMics, and that other post-transcriptional systems are required for separation between PGC and mesodermal associated gene expression at the sea urchin vegetal plate. Instead, Nanos2 may be downstream of a more general mesodermal transcription factor (such as β-catenin), but degradation processes in the cytoplasm further refine its localization. While these processes have not yet been elucidated, they may involve small RNAs or RNA-binding proteins as observed in zebrafish and Drosophila.
The cytological features of the sMics, their gene expression profile, and the fact that they do not contribute to the embryo or larva, but instead are set aside for the adult rudiment, makes them candidates for PGCs in the sea urchin. However, their precise function has been a point of controversy for some time, perhaps because the definitive resolution of their fate requires a challenging lineage trace. While transgenesis is possible in the sea urchin, the long generation times of most species (up to two years for the popular Strongylocentrotus purpuratus) makes the maintenance of stable lines impractical (Arnone et al., 1997). Classic embryological approaches, however, have been used to test whether the micromere lineage is required for fertility of the adult sea urchin. Surprisingly, development can proceed following removal of the micromeres before the 5th cleavage. Such embryos gastrulate, and the resultant larvae even form skeletons, by way of compensatory mesodermal cells (Ettensohn, Kitazawa, Cheers, Leonard, & Sharma, 2007). When raised to adulthood, these manipulated embryos do indeed yield fertile animals (Ransick, Cameron, & Davidson, 1996). It was concluded from this experiment that the micromere lineage contains no obligate germ-cell determinants. However, a subsequent study found that when, instead, the sMics are removed at the subsequent cleavage, the resultant adults are infertile (Yajima & Wessel, 2011b). These seemingly disparate results are actually quite compatible when one considers the organizing capability of the micromeres. When transplanted ectopically to the animal pole of a recipient embryo, a second axis is induced with a complete gut (Ransick & Davidson, 1993). Conversely, experiments in which sMics were transplanted to the animal cap indicated only very weak organizing activity (Kurihara & Amemiya, 2005). Taken together, these results suggest that the micromeres repress the germ line program in what will normally become somatic blastomeres, but upon their removal, another cell lineage can compensate for their loss. Since the sMics lack organizing capability, their removal does not induce compensation. In support of this premise, ectopic Vasa protein strongly accumulates throughout the entire embryo when micromeres are removed but not when sMics are removed (Voronina et al., 2008; Yajima & Wessel, 2011b). Furthermore, following micromere removal, Nanos2 mRNA accumulates in a mesodermal territory that normally neighbors the sMics (Fujii et al., 2009).
Putting your germ cells in the freezer: possible advantages for preloaded specification
While there are apparent similarities in the germ plasms of different systems, mapping these characters to the phylogenetic tree strongly implies that germ-line preloading evolved independently multiple times. Furthermore, examination of the genes involved reveals a lack of homology (e.g. the insect-specific Oskar, the vertebrate-specific Bucky ball), pointing instead to convergent evolution. Multiple realizations of preloading imply a strong selective pressure for the strategy. A consequence of early segregation, particularly before the MET, is that the PGCs must sit and wait for the rest of the embryo to catch up. That is, the PGCs require a somatic support structure – the gonad – to migrate towards and colonize. Until colonization occurs, the PGCs remain locked down and insulated from differentiation cues. Why not specify the germ cells after the MET, in the same location where the mature GSCs will ultimately reside?
Some hypotheses have been proposed. The work of Weismann and others leading to the Modern Synthesis suggested that early segregation of PGCs followed by cell cycle quiescence could confer a protective advantage against the accumulation of DNA replication errors (Buss, 1987). In rapidly developing organisms such as Drosophila, this hypotheses makes intuitive sense; complete genome replication and cleavage in the syncytial blastoderm can occur in as little as 4 minutes (Farrell & O'Farrell, 2014). Drosophila melanogaster can develop normally without a spindle assembly checkpoint machinery, implying that its evolution has perhaps favored rapid development over absolute fidelity (Buffin, Emre, & Karess, 2007). Over many cell-cycle generations, one can envision the accumulation of deleterious mutations and, by setting aside the germ line early in embryogenesis, somatic mutations are unable to gain access to the germ line and thus will not be transmitted to subsequent generations. This premise seems like a reasonable selection mechanism but must be balanced by the fact that in many animals, mammals included, the stem cells that give rise to eggs and sperm will divide enormous numbers of times to give millions of oocytes and sperm. Protecting the germ line early on really only delays onset of the potentially damaging rapid cell divisions, unless the early cell divisions lack a quality control mechanism that instead is present later.
Johnson and colleagues have proposed a complementary hypothesis that early germ line segregation enhances the evolvability of the species. They suggest that by establishing the germ line in isolation instead of as part of the mesoderm, constraints upon the embryo are relaxed. Consequently, somatic tissues can diversify with less risk of compromising the species’ reproductive capability (Johnson, Richardson, Bachvarova, & Crother, 2011). These authors suggest that clades with preloaded germ cell specification should speciate (and/or acquire greater morphological diversity) more rapidly than their sister clades. However, we note here that such a speciation would not occur without the imposition of appropriate selective pressures. Nature has performed this experiment for us several times, and indeed, there may be a correlation between germ line preloading and enhanced speciation. Several examples include the teleost fishes, such as zebrafish, versus inductive, more basally branching ray-finned fishes, such as the sturgeon; and anuran amphibians such as Xenopus, which uses preloading, versus the inductive urodeles like the axolotl. In these examples, the preloaded clade does indeed appear to have greater species diversity than the inductive sister (Johnson et al., 2011). A phylogenetic analysis also suggests that genes in preloading animals tend to evolve more rapidly than their inductive sisters. Evans et al. found that gene trees between anuran and urodele amphibians and mammals often do not recapitulate the proper species relationships, and when they are incongruent, urodele (inductive) sequences tend to cluster with vertebrate sequences, while the anuran (preloaded) sequences are more divergent (Evans, Wade, Chapman, Johnson, & Loose, 2014). This creative analysis may support the premise that germ line preloading correlates with speciation; however, incongruent trees were heavily affected by the choice of outgroup to which they were rooted. Future investigation might also seek to directly measure rates of gene evolution within the preloading-based echinoids, versus other echinoderms, for which transcriptomes in many species are becoming available. Because the sea urchin gene regulatory network is well-characterized, one could test whether particular subnetworks, such as that specifying mesoderm, display different evolutionary rates than others.
We cannot from these correlations conclude that germ line preloading is a universally optimal strategy. Instead, it is more productive to ask what unique attributes of these clades’ environments influenced the convergence. One possibility is that preloading facilitates rapid development; consider that all of the examples provided here, including Drosophila, C. elegans, Xenopus, the sea urchin, and zebrafish each develop through a larval form. By getting germ line specification ‘out of the way’ early, the embryo can then direct its investments towards reaching a motile, feeding form as quickly as possible. Precocial development may be particularly beneficial when the animal is subjected to high predation, or rapid changes in the environment. Put another way, while acquisition of a preloaded germ line may correlate with speciation, it may not be the causal agent. Instead, we suggest the alternative possibility that a preloaded germ line, enhanced speciation, and larval development are parallel consequences of some other environmental pressure towards rapidity.
Are METs different between the germ line and soma? Transcriptional repression in PGCs
Careful study of GRNs in the sea urchin has shown us that transcriptional asymmetries initiate very early in embryogenesis. The embryo is poised for these asymmetries largely thanks to the maternal β-catenin pathway, which is activated in early vegetal blastomeres (as reviewed above). The GRNs put in place by these early asymmetries prime different parts of the embryonic genome for deployment in different embryonic territories. In comparison to the rest of the embryo, however, the sMics are poorly understood in terms of their transcriptional regulation. With a strong collective effort of the community to identify all spatially restricted developmental transcription factors, it is surprising that FoxY is the only one known to be expressed in the sMics before gastrulation (Juliano et al., 2006; Materna et al., 2013; Song & Wessel, 2012; Swartz et al., 2014). Furthermore, its knockdown leads to no obvious defects in the specification of the sMics. It is therefore plausible that the dearth of known transcription factors in the sMics reflects something of biological significance, such as transcriptional repression.
This premise is consistent with the PGCs of other species that undergo periods of broad transcriptional repression, including Drosophila, C. elegans, Xenopus laevis, and the ascidian Ciona intestinalis (Nakamura & Seydoux, 2008; Shirae-Kurabayashi, Matsuda, & Nakamura, 2011). These studies have all benefitted from the generation of antibodies that specifically recognize phosphorylations of the heptapeptide repeat of the C-terminal domain (Seydoux & Dunn, 1997). Antibodies to phosphorylated Serine 2 (pSer2), which is associated with transcriptional elongation, have revealed that early PGCs transcribe at very low levels compared to their somatic neighbors. Surprisingly, each of these species achieves transcriptional repression by distinct mechanisms. Drosophila and C. elegans have both found solutions by interfering with the P-TEFb complex. This complex contains CyclinT and Cdk9, and acts by phosphorylating the C-terminal heptapeptide repeats of RNA polymerase II (RNAPII) at Serine 2. In Drosophila, Pole granule component (pgc) is localized within the germ plasm, and prevents P-TEFb recruitment by directly binding CyclinT (Hanyu-Nakamura, Sonobe-Nojima, Tanigawa, Lasko, & Nakamura, 2008). In C. elegans, a protein called PIE-1 interferes with Cdk9 activity and is thought to function by mimicking the RNAPII C-terminal tail. Both Pgc and PIE-1 are species-specific genes, an example of evolutionary convergence that is intimately associated with having a preloaded germ line. In Xenopus, Nanos is required for pSer2 depletion, though this is almost certainly an indirect effect, given that Nanos is a cytoplasmic regulator of mRNA (Lai, Singh, & King, 2012).
Each of these species uses a preloaded mode of PGC segregation, and has also acquired a mechanism for general transcriptional repression. This correlation may imply that transcriptional repression is a prerequisite for having a preloaded germ line. A possible reason for this requirement is insulation from differentiation. To be kept developmentally naïve, PGCs must not respond to the differentiation cues intended for somatic cells. When transcriptional repression is perturbed in Drosophila, Ciona, and Xenopus, PGCs misexpress somatic genes and can adopt a somatic fate (Y. Hayashi, Hayashi, & Kobayashi, 2004; Lai et al., 2012; Shirae-Kurabayashi et al., 2011). In addition to these preloaded PGC animals, the mouse (which uses induction) also shows signs of broad transcriptional repression, although with some differences. Its PGCs are transcriptionally active at their initial segregation, but lose pSer2 intensity as they migrate toward the somatic gonad. The mechanism of this is unknown, but must involve different regulation than observed in the preloading-based systems. Mouse PGCs are arrested in G2 of the cell cycle during migration, and their transcriptional repression thus may be cell-cycle dependent (Seki et al., 2007).
The sea urchin sMics also display signs of broad transcriptional repression. Immediately after their creation, the sMic nuclei are enriched for the heterochromatin mark H3K9 trimethylation, and this enrichment persists into blastula stages. Subsequently, the sMic nuclei become depleted for elongating RNA polymerase II, based on immunofluorescence with phosphospecific antibodies (Swartz et al., 2014) (Figure 6). The mechanism of this depletion is unknown, but one possibility is that a methyltransferase is either localized to, or more active within, the sMics than in the somatic blastomeres. The subsequent phase of RNA polymerase repression could be dependent upon the earlier phase of H3K9 trimethylation. There also could be cell-cycle dependency; BrdU incorporation studies have found that sMics display a prolonged S-phase from their birth until migration (Tanaka & Dan, 1990). They begin to divide at the early gastrula stage during translocation, and this mitotic activity could be a cause for their reduced RNAPII pSer2.
The sMics are also enriched for a maternally supplied form of linker histone H1 based on immunofluorescence data (Figure 6). While this work was carried out before the sequencing of the genome, and the precise identity of this H1 ortholog has not yet been identified, the sMics retain this variant form from their creation through migration and into the coelomic pouches (Pehrson & Cohen, 1986). The retention of this linker histone may simply reflect mitotic quiescence of the sMics—slow cell cycling would prevent dilution of cleavage stage histones, while somatic cells dilute out their maternally supplied histones amongst the many progeny cells. However, this H1 variant could also actively confer transcriptional repression. Such a role would be consistent with the function of linker histones, which compact chromatin into higher order structure. Furthermore, a histone H1 variant called dBigH1 was recently reported to regulate embryonic genome activation in Drosophila. dBigH1 is exchanged for a shorter variant upon cellularization of the blastoderm and activation of embryonic transcription, but is retained in the PGCs. dBigH1 loss-of-function mutants precociously activate transcription in both the somatic and germ-line cells (Pérez-Montero, Carbonell, Morán, Vaquero, & Azorín, 2013). Thus, histone variants may play a conserved role in regulating the timing of embryonic genome activation in PGCs and somatic cells.
In addition to these clues in the nuclei, some cell surface changes suggest that the sMics may not respond to differentiation signals. Scanning electron microscopy suggested that the sMic plasma membranes lack the characteristic microvilli of blastomeres, and are “smoother” than somatic neighbors (Dale, Yazaki, & Tosti, 1997). This observation could reflect a broad membrane rearrangement in the micromere lineage. Perhaps in support of this premise, the micromeres, and subsequently the sMics, have reduced ABC/multidrug transporter activity based on efflux of fluorescent reporter molecules (Campanale & Hamdoun, 2012). A reduction of multidrug activity in the sMics is surprising, since protection of the germ line from toxicants would seemingly confer an advantage. However, reduction of efflux activity may sensitize the sMics to migration cues, as inhibition of MDRs results in improper coelomic pouch homing. Loss of efflux activity may be a consequence of a greater plasma membrane modification, hinted at by the earlier electron microscopy observations; perhaps, the sMics broadly internalize transmembrane proteins. Furthermore, the sMics are enriched for the mRNA of a Sprouty family member. Sprouty family proteins are negative regulators of receptor tyrosine kinase (RTK) signaling and of proliferation (Kim & Bar-Sagi, 2004). Taken together, these observations depict the sMics as a cell type that “covers its ears” and ignores differentiation signals. What turns them “back on” after gastrulation may be the loss of nanos, transiently expressed in this cell, but the mechanism of this premise is unclear also.
Differential stability of mRNA in the germ line and soma
The sMics have a unique propensity for retaining inherited RNA. Transcriptomic analyses, and specific examination of Vasa and Seawi RNAs, suggest that the sMics generally inherit maternally supplied RNA, rather than transcribing them de novo. These RNAs are typically ubiquitously distributed throughout the early embryo, and retained in the sMics, but turned over in somatic cells later. This pattern of acquisition is consistent with the observation that the sMics are transcriptionally repressed (Swartz et al., 2014; Voronina et al., 2008; Yajima et al., 2014). Furthermore, exogenous mRNA reporters injected into zygotes, while eventually degraded in somatic cells, are retained several days longer in the sMics (Gustafson & Wessel, 2010; Oulhen & Wessel, 2013). This RNA retention phenomenon occurs independently of sequence information, because several reporters, containing mCherry, GFP, different UTRs, and other cross-species derived sequences, all are similarly retained in the sMics. Instead, RNA retention is probably reflective of generally increased stability for mRNA in the sMics relative to somatic cells. The sea urchin embryo achieves this differential stability at least in part by repressing accumulation of the major deadenylase CNOT6 in the sMics, while expressing it in all other blastomeres (Figure 7). Without its full nuclease activity, the CNOT complex would be less capable of shortening poly-A tails, reducing their likelihood as substrates for various degradation processes. CNOT6 is directly depleted in the sMics by Nanos2 repression of its mRNA. The CNOT6 3’UTR contains two consensus PRE motifs, which are each required for CNOT6 mRNA to be degraded in the sMics. Importantly, Nanos knockdown prevents CNOT6 degradation while forcing expression of CNOT6 in the sMics disrupts their ability to retain the germ line determinants Vasa and Piwi. Conversely, broad knockdown of CNOT6 expands the domain of retention of both Piwi RNA and exogenous RNA. These results highlight an important difference in RNA stability between germ line and soma in the sea urchin embryo that is important for inheriting germ line molecules.
Figure 7. Time Capsule model for (Reproduced from Wessel et al., 2014).
(A) CNOT6 mRNA (green) is present in all somatic cells but depleted in the sMics (red, Vasa protein) in the 18 h.p.f. blastula. (B,C) Model for differential RNA stability between the somatic cells and sMics. (B) CNOT6 deadenylase is broadly present, and destabilizes maternal transcripts such as Vasa, Seawi, and Baf250 by shortening their poly-A tails. (B) These RNAs are protected in the sMics, because Nanos (Nos) and its partner Pumilio (Pum) repress the accumulation of CNOT6. Scale bar = 20µ.
Differential RNA degradation and protection has been noted in several different species, particularly in those that use preloaded strategies. Hsp83 and nanos RNAs provide examples in the Drosophila embryo. Both of these RNAs are broadly distributed throughout the oocyte, egg, and syncytial embryo; in the case of nanos, only about 5% of its total transcripts are enriched at the early posterior pole (Bergsten & Gavis, 1999). Thus, some other process must fine-tune their localization to the PGCs. Analysis of the 3’UTRs of nanos and Hsp83 has turned up cis elements that confer lability in somatic cells (Bashirullah et al., 1999; Dahanukar & Wharton, 1996). The RNA-binding protein Smaug has been identified as the maternally supplied factor required to degrade Hsp83 RNA in Drosophila. Smaug targets Hsp83 for degradation by the CNOT complex, and represses translation of unlocalized nanos (Jeske, Moritz, Anders, & Wahle, 2011; Semotok et al., 2005). The primary recognition element for Smaug association was mapped to a region within the Hsp83 open reading frame termed the Hsp83 mRNA instability element (Semotok et al., 2008). The piRNA pathway has been also been implicated in the turnover of maternal nanos mRNA in Drosophila somatic cells by interacting with the CNOT complex (Rouget et al., 2010). In the zebrafish, Nanos1 RNA is also differentially regulated between PGCs and soma by a microRNA: miR-430 (Mishima et al., 2006).
Furthermore, specific 3’UTR elements confer protection to certain RNAs in the PGCs. Such elements have been identified in Drosophila Hsp83; when swapped with the Hsp70 3’UTR, Hsp83 is degraded in both somatic cells and PGCs simultaneously. Thus, the RNA degradation pathway is active in the PGCs, but certain RNAs are masked from its action. The trans-acting factors that mediate protection in the fly have not yet been definitively identified, but protective factors have been discovered in vertebrates. One such factor is Dnd1, an RNA-binding protein that is essential for germ line development in zebrafish and mice (Weidinger et al., 2003; Youngren et al., 2005). In zebrafish, Dnd1 binds the 3’UTR of Nanos in direct proximity to miR-430 sites, and occludes access by the miRNA machinery (Kedde et al., 2007). Another conserved germ-line factor, Dazl, also opposes miR-430 by enhancing polyadenylation of Nanos mRNA (Takeda, Mishima, Fujiwara, Sakamoto, & Inoue, 2009). In each of these cases, specific mRNAs are protected by the addition of cis- and trans-acting factors. Thus, the sea urchin is the only known example which creates a general bottleneck in RNA turnover by the depletion of CNOT6. As both RNA-binding proteins and miRNAs use the CNOT complex to effect target degradation, in the future it will be important to investigate whether deadenylase depletion is a conserved phenomenon in germ cells. Expression analysis in the sea star, which uses an inductive PGC system, suggests that mesodermal progenitors have reduced CNOT6 (Fresques et al., 2014).
METs are delayed in PGCs
The emerging picture is that animals that specify their germ line by preloading display a delay in both EGA and maternal transcript degradation in their PGCs. Some genomic approaches using FACS-isolated PGCs have directly tested the timing and extent of delays for EGA and maternal transcriptome degradation. Drosophila PGCs do not upregulate embryonically transcribed transcripts until 3 to 5 hours post fertilization, compared to the major somatic EGA, which occurs at 2 hours (Siddiqui et al., 2012). A 1–3 hour delay in Drosophila time is substantial, as this period spans when the PGCs were created, into gastrulation, until just before the PGCs begin migrating to the somatic gonad. Furthermore, the Drosophila PGCs are delayed for degradation of maternal RNA by several hours. At least some mechanistic aspects of EGA and transcript degradation are similar between the germ line and soma, such as the shared requirement for the RNA-binding protein, Smaug (Siddiqui et al., 2012). Similarly, FACS of sea urchin sMics indicated that transcription is inhibited in the sMics from their creation, and they retain inherited mRNA substantially longer than somatic cells (Swartz et al., 2014). Future studies should examine how that repression is removed. One possibility is that, once the sMics have finished their migration into the coelomic pouches, transcriptional activity will resume, perhaps based on degradation of Nanos protein.
A continuum of maternal and embryonic contributions to development
We have highlighted a range of differences in maternal versus embryonic control of development amongst diverse animal species. In rapidly developing organisms such as the fruit fly Drosophila melanogaster, the frog Xenopus laevis, and the zebrafish Danio rerio, the early embryo subsists entirely on maternally provided factors for a protracted period of many cell cycles. It is only later in development that the embryonic genome activates. In contrast, organisms such as the sea urchin and the mouse initiate transcription immediately after fertilization. A more conserved feature of animal development instead appears to be a broad degradation of the maternal transcriptome, which is critical for cell fate specification. In animals with a classically defined midblastula transition, like the fruit fly, frog, and zebrafish, maternal transcriptome degradation and embryonic genome activation are coupled, and occur with remodeling of the cell cycle. However, in the sea urchin, maternal transcriptome degradation does not appear to occur until many hours after the embryonic genome has activated, suggesting that these two processes can be decoupled.
Given the pervasiveness of global RNA turnover events throughout the animal tree, we have proposed that this MET may have been essential for the transition from a unicellular ancestor to a multicellular organism with specialized cell fates (Swartz et al., 2014). We have highlighted the importance of miRNA and RNA-binding protein-mediated turnover of maternal mRNA in the sea urchin and other organisms; blocking these processes broadly perturbs cell fate specification in development. The miRNA pathway also has a well-appreciated requirement for the differentiation of many adult stem cells in myogenesis, skin homeostasis, and hematopoiesis (Gangaraju & Lin, 2009). Global RNA turnover “clears the slate” for somatic fate specification. In contrast to the somatic cells in the sea urchin embryo which clear the slate by use of at least CNOT6, the PGCs (i.e. the sMics) protect and retain inherited mRNA and remain transcriptionally inert—in other words, they are insulated from the MET which occurs in the soma. This mechanism of germ line – soma separation, which we call the Time Capsule mode in the sea urchin, bears striking similarity to differential RNA stability between germ line and soma observed in Drosophila and zebrafish, suggesting that it may be a deeply conserved strategy (Bashirullah et al., 1999; Mishima et al., 2006) (Figure 7). With the transition to multicellular animals, the true novelty was the soma—the specialized parts devoid of reproductive potential—since the unicellular ancestor played the role of both germ line and soma. The diverse METs of many species documented here may be functional remnants of how that primordial separation may have occurred: a broad degradation of RNA to allow division of labor, and insulation of a select subset of cells to remain reproductively potent.
Abbreviations
- MET
Maternal to embryo transition
- PGC
Primordial germ cells
- GSCs
Germ line stem cells
- MBT
Mid-blastula transition
- EGA
Embryonic genome activation
- PRE
Pumilio response element
- sMics
small micromeres
- PE
posterior enterocoel
- D/N
delta –notch
- GNARLE
Global Nanos Associated RNA Lability Element
- ORF
open reading frame
- FACS
fluorescence activated cell sorting
Footnotes
The term zygote (Gr, yoked, or joined together) formally refers to the cell in development following fertilization, when the two gametes have joined into one cell, but before the first cell division. Here we have retained this definition and refer to events following this as in embryogenesis.
References
- Aramaki S, Hayashi K, Kurimoto K, Ohta H, Yabuta Y, Iwanari H, Mochizuki Y, et al. A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Developmental cell. 2013;27(5):516–529. doi: 10.1016/j.devcel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Arnone MI, Bogarad LD, Collazo A, Kirchhamer CV, Cameron RA, Rast JP, Gregorians A, et al. Green Fluorescent Protein in the sea urchin: new experimental approaches to transcriptional regulatory analysis in embryos and larvae. Development (Cambridge, England) 1997;124(22):4649–4659. doi: 10.1242/dev.124.22.4649. [DOI] [PubMed] [Google Scholar]
- Bashirullah A, Halsell SR, Cooperstock RL, Kloc M, Karaiskakis A, Fisher WW, Fu W, et al. Joint action of two RNA degradation pathways controls the timing of maternal transcript elimination at the midblastula transition in Drosophila melanogaster. The EMBO journal. 1999;18(9):2610–2620. doi: 10.1093/emboj/18.9.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten SE, Gavis ER. Development (Cambridge, England) 4. Vol. 126. The Company of Biologists Limited; 1999. Role for mRNA localization in translational activation but not spatial restriction of nanos RNA; pp. 659–669. [DOI] [PubMed] [Google Scholar]
- Bhandari D, Raisch T, Weichenrieder O, Jonas S, Izaurralde E. Structural basis for the Nanos-mediated recruitment of the CCR4-NOT complex and translational repression. Genes & development. 2014;28(8):888–901. doi: 10.1101/gad.237289.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontems F, Stein A, Marlow F, Lyautey J, Gupta T, Mullins MC, Dosch R. Bucky ball organizes germ plasm assembly in zebrafish. Current biology : CB. 2009;19(5):414–422. doi: 10.1016/j.cub.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Boveri T. An organism produced sexually without characteristics of the mother. English translation: Morgan, T.H. Am. Nat. 1893;27:222–232. [Google Scholar]
- Buffin E, Emre D, Karess RE. Flies without a spindle checkpoint. Nature Cell Biology. 2007;9(5):565–572. doi: 10.1038/ncb1570. [DOI] [PubMed] [Google Scholar]
- Bushati N, Stark A, Brennecke J, Cohen SM. Temporal reciprocity of miRNAs and their targets during the maternal-to-zygotic transition in Drosophila. Current Biology. 2008;18(7):501–506. doi: 10.1016/j.cub.2008.02.081. [DOI] [PubMed] [Google Scholar]
- Buss LW. The evolution of individuality. 1987 [Google Scholar]
- Campanale JP, Hamdoun A. Programmed reduction of ABC transporter activity in sea urchin germline progenitors. Development (Cambridge, England) 2012;139(4):783–792. doi: 10.1242/dev.076752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield J, O'Reilly M-A, Bachvarova RF, Ferjentsik Z, Redwood C, Walmsley M, Patient R, et al. Stochastic specification of primordial germ cells from mesoderm precursors in axolotl embryos. Development (Cambridge, England) 2014;141(12):2429–2440. doi: 10.1242/dev.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Zheng W, Lin A, Uyhazi K, Zhao H, Lin H. Pumilio 1 suppresses multiple activators of p53 to safeguard spermatogenesis. Current biology : CB. 2012;22(5):420–425. doi: 10.1016/j.cub.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Dumelie JG, Li X, Cheng MH, Yang Z, Laver JD, Siddiqui NU, et al. Genome biology. 1. Vol. 15. BioMed Central Ltd; 2014. Global regulation of mRNA translation and stability in the early Drosophila embryo by the Smaug RNA-binding protein; p. R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart MA, Panasenko OO. The Ccr4--not complex. Gene. 2012;492(1):42–53. doi: 10.1016/j.gene.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Conklin E. Organ-forming substances in the eggs of ascidians. Biological Bulletin. 1905;8(4) 205–U6. [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science (New York, NY) 2008;322(5909):1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampton HE, Wilson EB. Development genes and evolution. 1. Vol. 3. Springer; 1896. Experimental studies on gasteropod development; pp. 1–19. [Google Scholar]
- Cui M, Siriwon N, Li E, Davidson EH, Peter IS. Specific functions of the Wnt signaling system in gene regulatory networks throughout the early sea urchin embryo. Proceedings of the National Academy of Sciences. 2014;111(47):E5029–E5038. doi: 10.1073/pnas.1419141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Wharton RP. The Nanos gradient in Drosophila embryos is generated by translational regulation. Genes & development. 1996;10(20):2610–2620. doi: 10.1101/gad.10.20.2610. [DOI] [PubMed] [Google Scholar]
- Dale B, Yazaki I, Tosti E. Polarized distribution of L-type calcium channels in early sea urchin embryos. The American journal of physiology. 1997;273(3 Pt 1):C822–C825. doi: 10.1152/ajpcell.1997.273.3.C822. [DOI] [PubMed] [Google Scholar]
- Dan K, Tanaka S, Yamazaki K, Kato Y. Cell cycle study up to the time of hatching in the embryos of the sea urchin, Hemicentrotus pulcherrimus. Development, Growth and Differentiation. 1980;22(3):589–598. doi: 10.1111/j.1440-169X.1980.00589.x. [DOI] [PubMed] [Google Scholar]
- Davidson EH. Gene Activity in Early Development – Eric H. Davidson - Google Books. Orlando, FL: Academic Press; 1986. [Google Scholar]
- Donoughe S, Nakamura T, Ewen-Campen B, Green DA, Henderson L, Extavour CG. BMP signaling is required for the generation of primordial germ cells in an insect. Proceedings of the National Academy of Sciences. 2014;111(11):4133–4138. doi: 10.1073/pnas.1400525111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, O'Farrell PH. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989;57(1):177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel D. Proceedings of the National Academy of Sciences of the United States of America. 4. Vol. 57. National Academy of Sciences; 1967. Protein synthesis in sea urchin eggs: a “late” response to fertilization; p. 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358(6385):387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- Ettensohn CA, Kitazawa C, Cheers MS, Leonard JD, Sharma T. Gene regulatory networks and developmental plasticity in the early sea urchin embryo: alternative deployment of the skeletogenic gene regulatory network. Development (Cambridge, England) 2007;134(17):3077–3087. doi: 10.1242/dev.009092. [DOI] [PubMed] [Google Scholar]
- Evans T, Wade CM, Chapman FA, Johnson AD, Loose M. Acquisition of germ plasm accelerates vertebrate evolution. Science (New York, NY) 2014;344(6180):200–203. doi: 10.1126/science.1249325. [DOI] [PubMed] [Google Scholar]
- Ewen-Campen B, Donoughe S, Clarke DN, Extavour CG. Germ cell specification requires zygotic mechanisms rather than germ plasm in a basally branching insect. Current biology : CB. 2013a;23(10):835–842. doi: 10.1016/j.cub.2013.03.063. [DOI] [PubMed] [Google Scholar]
- Ewen-Campen B, Jones TEM, Extavour CG. Evidence against a germ plasm in the milkweed bug Oncopeltus fasciatus, a hemimetabolous insect. Biology open. 2013b;2(6):556–568. doi: 10.1242/bio.20134390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen-Campen B, Schwager EE, Extavour CGM. The molecular machinery of germ line specification. Molecular reproduction and development. 2010;77(1):3–18. doi: 10.1002/mrd.21091. [DOI] [PubMed] [Google Scholar]
- Ewen-Campen B, Srouji JR, Schwager EE, Extavour CG. Oskar predates the evolution of germ plasm in insects. Current biology : CB. 2012;22(23):2278–2283. doi: 10.1016/j.cub.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Extavour CGM. Evolution of the bilaterian germ line: lineage origin and modulation of specification mechanisms. Integrative and comparative biology. 2007;47(5):770–785. doi: 10.1093/icb/icm027. [DOI] [PubMed] [Google Scholar]
- Extavour CG, Akam M. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development (Cambridge, England) 2003;130(24):5869–5884. doi: 10.1242/dev.00804. [DOI] [PubMed] [Google Scholar]
- Extavour CG, Pang K, Matus DQ, Martindale MQ. vasa and nanos expression patterns in a sea anemone and the evolution of bilaterian germ cell specification mechanisms. Evolution & development. 2005;7(3):201–215. doi: 10.1111/j.1525-142X.2005.05023.x. [DOI] [PubMed] [Google Scholar]
- Farrell JA, O'Farrell PH. From egg to gastrula: how the cell cycle is remodeled during the Drosophila mid-blastula transition. Annual Review of Genetics. 2014;48:269–294. doi: 10.1146/annurev-genet-111212-133531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forristall C, Pondel M, Chen L, King ML. Patterns of localization and cytoskeletal association of two vegetally localized RNAs, Vg1 and Xcat-2. Development (Cambridge, England) 1995;121(1):201–208. doi: 10.1242/dev.121.1.201. [DOI] [PubMed] [Google Scholar]
- Fresques T, Zazueta-Novoa V, Reich A, Wessel GM. Selective accumulation of germ-line associated gene products in early development of the sea star and distinct differences from germ-line development in the sea urchin. Developmental dynamics : an official publication of the American Association of Anatomists. 2014;243(4):568–587. doi: 10.1002/dvdy.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Sakamoto N, Ochiai H, Fujita K, Okamitsu Y, Sumiyoshi N, Minokawa T, et al. Role of the nanos homolog during sea urchin development. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238(10):2511–2521. doi: 10.1002/dvdy.22074. [DOI] [PubMed] [Google Scholar]
- Gallo CM, Wang JT, Motegi F, Seydoux G. Cytoplasmic partitioning of P granule components is not required to specify the germline in C. elegans. Science (New York, NY) 2010;330(6011):1685–1689. doi: 10.1126/science.1193697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nature Reviews Molecular Cell Biology. 2009;10(2):116–125. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ. MicroRNAs Regulate Brain Morphogenesis in Zebrafish. Science (New York, NY) 2005;308(5723):833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science (New York, NY) 2006;312(5770):75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- Gross PR, Cousineau GH. Effects of actinomycin D on macromolecule synthesis and early development in sea urchin eggs. Biochemical and Biophysical Research Communications. 1963;10(4):321–326. doi: 10.1016/0006-291x(63)90532-1. [DOI] [PubMed] [Google Scholar]
- Gross PR, Malkin LI, Moyer WA. Templates for the first proteins of embryonic development. Proceedings of the National Academy of Sciences of the United States of America. 1964;51:407–414. doi: 10.1073/pnas.51.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruidl ME, Smith PA, Kuznicki KA, McCrone JS, Kirchner J, Roussell DL, Strome S, et al. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13837–13842. doi: 10.1073/pnas.93.24.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EA, Wessel GM. Exogenous RNA is selectively retained in the small micromeres during sea urchin embryogenesis. Molecular reproduction and development. 2010;77(10):836. doi: 10.1002/mrd.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EA, Yajima M, Juliano CE, Wessel GM. Post-translational regulation by gustavus contributes to selective Vasa protein accumulation in multipotent cells during embryogenesis. Developmental biology. 2011;349(2):440–450. doi: 10.1016/j.ydbio.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatani T, Carter MG, Sharov AA, Ko MSH. Dynamics of global gene expression changes during mouse preimplantation development. Developmental cell. 2004;6(1):117–131. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- Hanyu-Nakamura K, Sonobe-Nojima H, Tanigawa A, Lasko P, Nakamura A. Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature. 2008;451(7179):730–733. doi: 10.1038/nature06498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EB. A comparison of the development of nucleate and non-nucleate eggs of Arbacia punctulata. Biological Bulletin. 1940;79(1):166–187. [Google Scholar]
- Hayashi Y, Hayashi M, Kobayashi S. Nanos suppresses somatic cell fate in Drosophila germ line. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(28):10338–10342. doi: 10.1073/pnas.0401647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illmensee K, Mahowald AP. Transplantation of posterior polar plasm in Drosophila. Induction of germ cells at the anterior pole of the egg. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(4):1016–1020. doi: 10.1073/pnas.71.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue C, Kiyomoto M, Shirai H. Development, Growth and Differentiation. 4. Vol. 34. Wiley Online Library; 1992. Germ cell differentiation in starfish: The posterior enterocoel as the origin of germ cells in Asterina pectinifera; pp. 413–418. [DOI] [PubMed] [Google Scholar]
- Irie N, Weinberger L, Tang WWC, Kobayashi T, Viukov S, Manor YS, Dietmann S, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160(1–2):253–268. doi: 10.1016/j.cell.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske M, Moritz B, Anders A, Wahle E. Smaug assembles an ATP-dependent stable complex repressing nanos mRNA translation at multiple levels. The EMBO journal. 2011;30(1):90–103. doi: 10.1038/emboj.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Richardson E, Bachvarova RF, Crother BI. Evolution of the germ line-soma relationship in vertebrate embryos. Reproduction (Cambridge, England) 2011;141(3):291–300. doi: 10.1530/REP-10-0474. [DOI] [PubMed] [Google Scholar]
- Juliano CE, Reich A, Liu N, Götzfried J, Zhong M, Uman S, Reenan RA, et al. PIWI proteins and PIWI-interacting RNAs function in Hydra somatic stem cells. 2014;111(1):337–342. doi: 10.1073/pnas.1320965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano CE, Swartz SZ, Wessel GM. A conserved germline multipotency program. Development (Cambridge, England) 2010a;137(24):4113–4126. doi: 10.1242/dev.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano CE, Voronina E, Stack C, Aldrich M, Cameron AR, Wessel GM. Germ line determinants are not localized early in sea urchin development, but do accumulate in the small micromere lineage. Developmental biology. 2006;300(1):406–415. doi: 10.1016/j.ydbio.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Juliano CE, Yajima M, Wessel GM. Nanos functions to maintain the fate of the small micromere lineage in the sea urchin embryo. Developmental biology. 2010b;337(2):220–232. doi: 10.1016/j.ydbio.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedde M, Strasser MJ, Boldajipour B, Oude Vrielink JAF, Slanchev K, le Sage C, Nagel R, et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131(7):1273–1286. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nature Reviews Molecular Cell Biology. 2004;5(6):441–450. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Amikura R, Mukai M. Localization of mitochondrial large ribosomal RNA in germ plasm of Xenopus embryos. Current biology : CB. 1998;8(20):1117–1120. doi: 10.1016/s0960-9822(98)70466-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Amikura R, Okada M. Presence of mitochondrial large ribosomal RNA outside mitochondria in germ plasm of Drosophila melanogaster. Science (New York, NY) 1993;260(5113):1521–1524. doi: 10.1126/science.7684857. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Kawakami K, Sakamoto H, Inoue K. Spatiotemporal localization of germ plasm RNAs during zebrafish oogenesis. Mechanisms of development. 2007;124(4):279–289. doi: 10.1016/j.mod.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Köprunner M, Thisse C, Thisse B, Raz E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes & development. 2001;15(21):2877–2885. doi: 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H, Amemiya S. Zoological Science. 8. Vol. 22. Zoological Society of Japan; 2005. Developmental Potential of Small Micromeres in Sea Urchin Embryos; pp. 845–852. [DOI] [PubMed] [Google Scholar]
- Lai F, Singh A, King ML. Xenopus Nanos1 is required to prevent endoderm gene expression and apoptosis in primordial germ cells. Development (Cambridge, England) 2012;139(8):1476–1486. doi: 10.1242/dev.079608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubichler MD, Davidson EH. Boveri's long experiment: sea urchin merogones and the establishment of the role of nuclear chromosomes in development. Developmental biology. 2008;314:1–11. doi: 10.1016/j.ydbio.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes & development. 1999;13(4):424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Fleming ES, Giraldez AJ. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature. 2013;503(7476):360–364. doi: 10.1038/nature12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Miller JR, Ferkowicz MJ, McClay DR. Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development (Cambridge, England) 1999;126(2):345–357. doi: 10.1242/dev.126.2.345. [DOI] [PubMed] [Google Scholar]
- Lund E, Liu M, Hartley RS, Sheets MD, Dahlberg JE. Deadenylation of maternal mRNAs mediated by miR-427 in Xenopus laevis embryos. RNA. 2009;15(12):2351–2363. doi: 10.1261/rna.1882009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur H, Bubunenko M, Houston DW, King ML. Xcat RNA is a translationally sequestered germ plasm component in Xenopus. Mechanisms of development. 1999;84(1–2):75–88. doi: 10.1016/s0925-4773(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Magnúsdóttir E, Dietmann S, Murakami K, Günesdogan U, Tang F, Bao S, Diamanti E, et al. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nature Cell Biology. 2013;15(8):905–915. doi: 10.1038/ncb2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SR, Juliano CE. Untangling the web: the diverse functions of the PIWI/piRNA pathway. Molecular reproduction and development. 2013;80(8):632–664. doi: 10.1002/mrd.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Sato H. Development, Growth and Differentiation. 3. Vol. 26. Wiley Online Library; 1984. Asynchronization of cell division is concurrently related with ciliogenesis in sea urchin blastulae; pp. 281–294. [DOI] [PubMed] [Google Scholar]
- Materna SC, Davidson EH. A comprehensive analysis of Delta signaling in pre-gastrular sea urchin embryos. Developmental biology. 2012;364(1):77–87. doi: 10.1016/j.ydbio.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materna SC, Swartz SZ, Smith J. Notch and Nodal control forkhead factor expression in the specification of multipotent progenitors in sea urchin. Development (Cambridge, England) 2013;140(8):1796–1806. doi: 10.1242/dev.091157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Giraldez AJ, Takeda Y, Fujiwara T, Sakamoto H, Schier AF, Inoue K. Differential regulation of germline mRNAs in soma and germ cells by zebrafish miR-430. Current Biology. 2006;16(21):2135–2142. doi: 10.1016/j.cub.2006.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller WA, Teo R, Frank U. Totipotent migratory stem cells in a hydroid. Developmental biology. 2004;275(1):215–224. doi: 10.1016/j.ydbio.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Seydoux G. Less is more: specification of the germline by transcriptional repression. Development (Cambridge, England) 2008;135(23):3817–3827. doi: 10.1242/dev.022434. [DOI] [PubMed] [Google Scholar]
- Nelson MR, Leidal AM, Smibert CA. Drosophila Cup is an eIF4E-binding protein that functions in Smaug-mediated translational repression. The EMBO journal. 2004;23(1):150–159. doi: 10.1038/sj.emboj.7600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Amikura R, Akasaka K, Kinoshita T, Kobayashi S, Shimada H. Zoological Science. 3. Vol. 16. BioOne; 1999. Asymmetrical distribution of mitochondrial rRNA into small micromeres of sea urchin embryos; pp. 445–451. [Google Scholar]
- Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell. 2009;137(3):571–584. doi: 10.1016/j.cell.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Oliveri P, Tu Q, Davidson EH. Global regulatory logic for specification of an embryonic cell lineage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(16):5955–5962. doi: 10.1073/pnas.0711220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, Wessel GM. Retention of exogenous mRNAs selectively in the germ cells of the sea urchin requires only a 5“-cap and a 3-”UTR. Molecular reproduction and development. 2013;80(7):561–569. doi: 10.1002/mrd.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, Yoshida T, Yajima M, Song JL, Sakuma T, Sakamoto N, Yamamoto T, et al. The 3'UTR of nanos2 directs enrichment in the germ cell lineage of the sea urchin. Developmental biology. 2013;377(1):275–283. doi: 10.1016/j.ydbio.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson JR, Cohen LH. The fate of the small micromeres in sea urchin development. Developmental biology. 1986;113(2):522–526. doi: 10.1016/0012-1606(86)90188-0. [DOI] [PubMed] [Google Scholar]
- Peng CJ, Wikramanayake AH. Differential regulation of disheveled in a novel vegetal cortical domain in sea urchin eggs and embryos: implications for the localized activation of canonical Wnt signaling. PloS one. 2013;8(11):e80693. doi: 10.1371/journal.pone.0080693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter I, Davidson EH. Genomic Control Process: Development and Evolution. Academic Press; 2015. [Google Scholar]
- Pérez-Montero S, Carbonell A, Morán T, Vaquero A, Azorín F. Developmental cell. 6. Vol. 26. Elsevier; 2013. The Embryonic Linker Histone H1 Variant of Drosophila, dBigH1, Regulates Zygotic Genome Activation; pp. 578–590. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JS, Chan XY, Kingsley EP, Duan Y, Lambert JD. Nanos is required in somatic blast cell lineages in the posterior of a mollusk embryo. Current biology : CB. 2008;18(5):331–336. doi: 10.1016/j.cub.2008.01.055. [DOI] [PubMed] [Google Scholar]
- Ransick A, Davidson E. A complete second gut induced by transplanted micromeres in the sea urchin embryo. Science (New York, NY) 1993;259(5098):1134–1138. doi: 10.1126/science.8438164. [DOI] [PubMed] [Google Scholar]
- Ransick A, Cameron RA, Davidson EH. Postembryonic segregation of the germ line in sea urchins in relation to indirect development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(13):6759–6763. doi: 10.1073/pnas.93.13.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich A, Dunn C, Akasaka K, Wessel G. Phylogenomic analyses of echinodermata support the sister groups of asterozoa and echinozoa. PloS one. 2015;10(3):e0119627. doi: 10.1371/journal.pone.0119627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget C, Papin C, Boureux A, Meunier A-C, Franco B, Robine N, Lai EC, et al. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature. 2010;467(7319):1128–1132. doi: 10.1038/nature09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, Saga Y, et al. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development (Cambridge, England) 2007;134(14):2627–2638. doi: 10.1242/dev.005611. [DOI] [PubMed] [Google Scholar]
- Semotok JL, Cooperstock RL, Pinder BD, Vari HK, Lipshitz HD, Smibert CA. Smaug recruits the CCR4/POP2/NOT deadenylase complex to trigger maternal transcript localization in the early Drosophila embryo. Current Biology. 2005;15(4):284–294. doi: 10.1016/j.cub.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Semotok JL, Luo H, Cooperstock RL, Karaiskakis A, Vari HK, Smibert CA, Lipshitz HD. Drosophila Maternal Hsp83 mRNA Destabilization Is Directed by Multiple SMAUG Recognition Elements in the Open Reading Frame. Molecular and cellular biology. 2008;28(22):6757–6772. doi: 10.1128/MCB.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G, Braun RE. Pathway to totipotency: lessons from germ cells. Cell. 2006;127(5):891–904. doi: 10.1016/j.cell.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Dunn MA. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development (Cambridge, England) 1997;124(11):2191–2201. doi: 10.1242/dev.124.11.2191. [DOI] [PubMed] [Google Scholar]
- Shermoen AW, O'Farrell PH. Progression of the cell cycle through mitosis leads to abortion of nascent transcripts. Cell. 1991;67(2):303–310. doi: 10.1016/0092-8674(91)90182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirae-Kurabayashi M, Matsuda K, Nakamura A. Ci-Pem-1 localizes to the nucleus and represses somatic gene transcription in the germline of Ciona intestinalis embryos. Development (Cambridge, England) 2011;138(14):2871–2881. doi: 10.1242/dev.058131. Retrieved from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=21693510&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- Siddiqui NU, Li X, Luo H, Karaiskakis A, Hou H, Kislinger T, Westwood JT, et al. Genome-wide analysis of the maternal-to-zygotic transition in Drosophila primordial germ cells. Genome biology. 2012;13(2) doi: 10.1186/gb-2012-13-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smibert CA, Wilson JE, Kerr K, Macdonald PM. smaug protein represses translation of unlocalized nanos mRNA in the Drosophila embryo. Genes & development. 1996;10(20):2600–2609. doi: 10.1101/gad.10.20.2600. [DOI] [PubMed] [Google Scholar]
- Smith JL, Wilson JE, Macdonald PM. Overexpression of oskar directs ectopic activation of nanos and presumptive pole cell formation in Drosophila embryos. Cell. 1992;70(5):849–859. doi: 10.1016/0092-8674(92)90318-7. [DOI] [PubMed] [Google Scholar]
- Song JL, Wessel GM. The forkhead transcription factor FoxY regulates Nanos. Molecular reproduction and development. 2012;79(10):680–688. doi: 10.1002/mrd.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JL, Stoeckius M, Maaskola J, Friedländer M, Stepicheva N, Juliano C, Lebedeva S, et al. Select microRNAs are essential for early development in the sea urchin. Developmental biology. 2012;362(1):104–113. doi: 10.1016/j.ydbio.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Saba R, Sada A, Saga Y. The Nanos3-3'UTR is required for germ cell specific NANOS3 expression in mouse embryos. PloS one. 2010;5(2):e9300. doi: 10.1371/journal.pone.0009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Tsuda M, Kiso M, Saga Y. Nanos3 maintains the germ cell lineage in the mouse by suppressing both Bax-dependent and -independent apoptotic pathways. Developmental biology. 2008;318(1):133–142. doi: 10.1016/j.ydbio.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Swartz SZ, Chan XY, Lambert JD. Localization of Vasa mRNA during early cleavage of the snail Ilyanassa. Development genes and evolution. 2008;218(2):107–113. doi: 10.1007/s00427-008-0203-6. [DOI] [PubMed] [Google Scholar]
- Swartz SZ, Reich AM, Oulhen N, Raz T, Milos PM, Campanale JP, Hamdoun A, et al. Deadenylase depletion protects inherited mRNAs in primordial germ cells. Development (Cambridge, England) 2014;141(16):3134–3142. doi: 10.1242/dev.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development (Cambridge, England) 2009;136(18):3033–3042. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- Tadros W, Goldman AL, Babak T, Menzies F, Vardy L, Orr-Weaver T, Hughes TR, et al. SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Developmental cell. 2007;12(1):143–155. doi: 10.1016/j.devcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Mishima Y, Fujiwara T, Sakamoto H, Inoue K. DAZL relieves miRNA-mediated repression of germline mRNAs by controlling poly(A) tail length in zebrafish. PloS one. 2009;4(10):e7513. doi: 10.1371/journal.pone.0007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Developmental biology. 1996;178(1):124–132. doi: 10.1006/dbio.1996.0203. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Dan K. Study of the Lineage and Cell Cycle of Small Micromeres in Embryos of the Sea Urchin, Hemicentrotus pulcherrimus. (small micromeres/cell cycle/cell lineage/unequal cleavage/sea urchin) Development, Growth and Differentiation. 1990;32(2):145–156. doi: 10.1111/j.1440-169X.1990.00145.x. [DOI] [PubMed] [Google Scholar]
- Tritschler F, Huntzinger E, Izaurralde E. Role of GW182 proteins and PABPC1 in the miRNA pathway: a sense of déjà vu. Nature Reviews Molecular Cell Biology. 2010;11(5):379–384. doi: 10.1038/nrm2885. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y. Conserved role of nanos proteins in germ cell development. Science (New York, NY) 2003;301(5637):1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- Tu Q, Cameron RA, Davidson EH. Quantitative developmental transcriptomes of the sea urchin Strongylocentrotus purpuratus. Developmental biology. 2014;385(2):160–167. doi: 10.1016/j.ydbio.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D, Strome S. P granule assembly and function in Caenorhabditis elegans germ cells. Journal of andrology. 2010;31(1):53–60. doi: 10.2164/jandrol.109.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E. The diverse functions of germline P-granules in Caenorhabditis elegans. Molecular reproduction and development. 2013;80(8):624–631. doi: 10.1002/mrd.22136. [DOI] [PubMed] [Google Scholar]
- Voronina E, Lopez M, Juliano CE, Gustafson E, Song JL, Extavour C, George S, et al. Vasa protein expression is restricted to the small micromeres of the sea urchin, but is inducible in other lineages early in development. Developmental biology. 2008;314(2):276–286. doi: 10.1016/j.ydbio.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zayas RM, Guo T, Newmark PA. nanos function is essential for development and regeneration of planarian germ cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(14):5901–5906. doi: 10.1073/pnas.0609708104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Angerer RC, Angerer LM. A database of mRNA expression patterns for the sea urchin embryo. Developmental biology. 2006;300(1):476–484. doi: 10.1016/j.ydbio.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, Lovell-Badge R, Thisse C, et al. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Current biology : CB. 2003;13(16):1429–1434. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- Wessel GM, Brayboy L, Fresques T, Gustafson EA, Oulhen N, Ramos I, Reich A, et al. The biology of the germ line in echinoderms. Molecular reproduction and development. 2014;81(8):679–711. doi: 10.1002/mrd.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikramanayake AH, Huang L, Klein WH. beta-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9343–9348. doi: 10.1073/pnas.95.16.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikramanayake AH, Peterson R, Chen J, Huang L, Bince JM, McClay DR, Klein WH. Nuclear beta-catenin-dependent Wnt8 signaling in vegetal cells of the early sea urchin embryo regulates gastrulation and differentiation of endoderm and mesodermal cell lineages. Genesis. 2004;39(3):194–205. doi: 10.1002/gene.20045. [DOI] [PubMed] [Google Scholar]
- Xiol J, Spinelli P, Laussmann MA, Homolka D, Yang Z, Cora E, Couté Y, et al. RNA clamping by Vasa assembles a piRNA amplifier complex on transposon transcripts. Cell. 2014;157(7):1698–1711. doi: 10.1016/j.cell.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Yajima M, Wessel GM. The DEAD-box RNA helicase Vasa functions in embryonic mitotic progression in the sea urchin. Development (Cambridge, England) 2011a;138(11):2217–2222. doi: 10.1242/dev.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Wessel GM. Small micromeres contribute to the germline in the sea urchin. Development (Cambridge, England) 2011b;138(2):237–243. doi: 10.1242/dev.054940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Wessel GM. Autonomy in specification of primordial germ cells and their passive translocation in the sea urchin. Development (Cambridge, England) 2012;139(20):3786–3794. doi: 10.1242/dev.082230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Gustafson EA, Song JL, Wessel GM. Piwi regulates Vasa accumulation during embryogenesis in the sea urchin. Developmental dynamics : an official publication of the American Association of Anatomists. 2014;243(3):451–458. doi: 10.1002/dvdy.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren KK, Coveney D, Peng X, Bhattacharya C, Schmidt LS, Nickerson ML, Lamb BT, et al. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435(7040):360–364. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]