Abstract
Synapse loss in Alzheimer's disease (AD) correlates with cognitive decline. Involvement of microglia and complement in AD has been attributed to neuroinflammation, prominent late in disease. Here we show in mouse models that complement and microglia mediate synaptic loss early in AD. C1q, the initiating protein of the classical complement cascade, is increased and associated with synapses before overt plaque deposition. Inhibition of C1q, C3 or the microglial complement receptor CR3, reduces the number of phagocytic microglia as well as the extent of early synapse loss. C1q is necessary for the toxic effects of soluble β-amyloid (Aβ) oligomers on synapses and hippocampal long-term potentiation (LTP). Finally, microglia in adult brains engulf synaptic material in a CR3-dependent process when exposed to soluble Aβ oligomers. Together, these findings suggest that the complement-dependent pathway and microglia that prune excess synapses in development are inappropriately activated and mediate synapse loss in AD.
Genome-wide association studies (GWAS) implicate microglia and complement-related pathways in AD (1). Previous research has demonstrated both beneficial and detrimental roles of complement and microglia in plaque-related neuropathology (2, 3); however, their roles in synapse loss, a major pathological correlate of cognitive decline in AD (4), remain to be identified. Emerging research implicates microglia and immune-related mechanisms in brain wiring in the healthy brain (1). During development, C1q and C3 localize to synapses and mediate synapse elimination by phagocytic microglia (5-7). We hypothesized that this normal developmental synaptic pruning pathway is activated early in the AD brain and mediates synapse loss.
The degree of region-specific synapse loss is a stronger correlate of cognitive decline in AD than counts of plaques, tangles and neuronal loss (8, 9). To determine how early synapse loss occurs, we used super-resolution structured illumination microscopy (SIM) (10) to quantify synapse density in hippocampal CA1 stratum radiatum of familial AD-mutant hAPP (“J20”) transgenic mice (11). Quantification of colocalized pre- and postsynaptic puncta (synaptophysin and PSD95 (Fig. 1A); synaptotagmin and homer (figs. S1A-D)) revealed a significant loss of synapses in J20 hippocampus at 3-4 months old (mo), an age that precedes plaque deposition (11, 12). Synapse loss in pre-plaque J20 CA1 was confirmed using electron microscopy (Fig. S1G). Confocal imaging also showed synapse loss in CA1, CA3, and dentate gyrus of 3 mo J20 hippocampus but not in striatum (Fig. S1E). Synapse levels were not altered in 1 mo J20 brains vs. wild-type (WT) littermates (Fig. S1F), suggesting that the hippocampal synaptic loss at 3 mo is likely not a result of abnormal synaptic development.
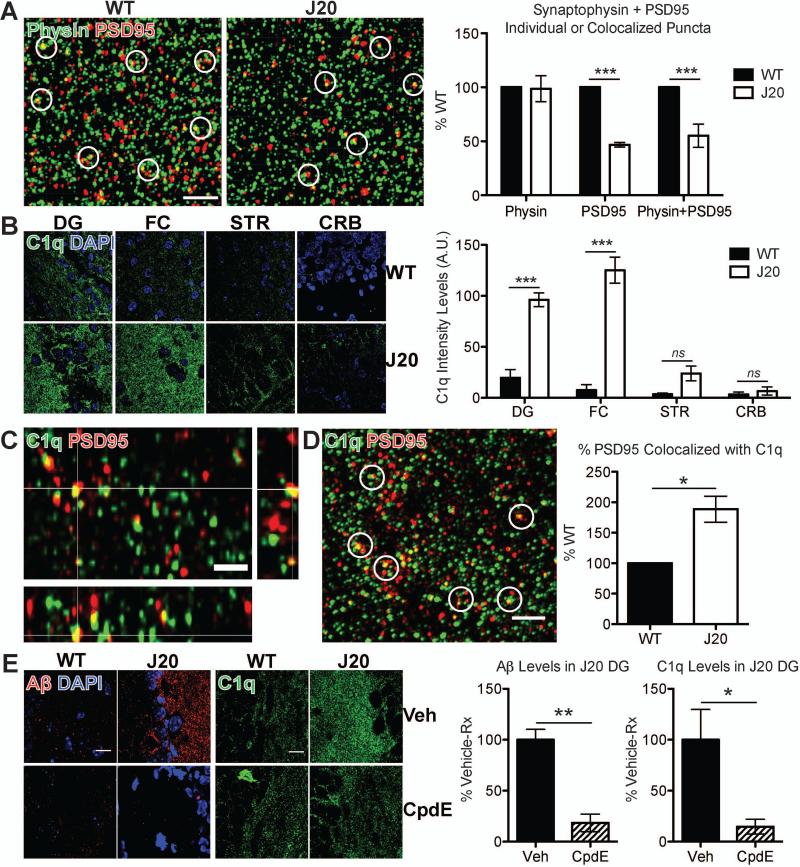
Fig. 1. C1q upregulation and deposition onto synapses precede pre-plaque synapse loss in J20 mice.
(A) Super-resolution SIM images of synaptophysin (green)- and PSD95 (red)-immunoreactive puncta in stratum radiatum of 3 mo J20 or WT hippocampus (CA1). Quantification of synaptic puncta or their apposition using Imaris indicates selective loss of PSD95 in J20 hippocampus as compared to their WT littermate controls. See Fig. S1. (B) Region-specific upregulation of C1q (green) in 1 mo J20; DG=dentate gyrus, FC=frontal cortex, STR=striatum, CRB=cerebellum. See Fig. S2. (C) Orthogonal view of SIM image showing colocalization of C1q (green) and PSD95 (red). (D) Higher % PSD95 colocalized with C1q in 1 mo J20 dentate gyrus vs. WT. (E) Compound E reduces deposited soluble Aβ (red) and C1q (green) in 1 mo J20 dentate gyrus, with minimal effect on C1q levels in WT mice. Scale bar = 2 (A, C and D) or 10 (B and E) μm. Means ± SEM; n = 3-4 mice per genotype or per treatment group per genotype. *P < 0.05 or ***P < 0.001 using two-way ANOVA followed by Bonferroni posttest (A and B), two-tailed one-sample t-test (D) or two-tailed unpaired t-test (E).
We asked whether the classical complement cascade is upregulated in pre-plaque brains when synapses are already vulnerable. C1q immunoreactivity (13; antibody now available at Abcam) was elevated in J20 brains, as early as 1 mo and preceding synapse loss (Fig. 1B and fig. S1). C1q elevation was region-specific, particularly in hippocampus and frontal cortex, two regions vulnerable to synapse loss (14) (Fig. 1B and fig. S2A). C1q immunoreactivity was comparable between J20 and WT at P21 (Fig. S2B), suggesting that elevated levels at 1 mo are likely not a developmental artifact. C1q was also similarly increased in hippocampus of another model of AD, the APP/PS1 mice (15) (Fig. S2C). Importantly, SIM demonstrated colocalization of C1q with PSD95-positive puncta in 1 mo J20 hippocampus (Fig. 1C). A higher percentage of PSD95 colocalized with C1q in hippocampus of J20 mice than that of WT (Fig. 1D and fig. S3), suggesting that the C1q-associated synapses may be marked for elimination.
Punctate Aβ was found deposited in J20 hippocampus at 1 mo (Fig. S4), long before Aβ plaques deposit (11, 12), raising the question of whether C1q increase in these pre-plaque brains is dependent on soluble Aβ levels. To test this, we injected the mice with Compound E, a γ-secretase inhibitor that rapidly decreases Aβ production (12). Compound E markedly reduced soluble Aβ levels in J20 mice; there was a corresponding reduction of C1q deposition (Fig. 1E), suggesting that Aβ upregulates C1q. To further address whether the increase of C1q is dependent on soluble Aβ, and if so, which species, we injected soluble Aβ oligomers or monomers into lateral ventricles of WT mice. Hippocampus contralateral to injection site was examined to avoid any surgery-related effects. Oligomeric Aβ (oAβ), which is prefibrillar in nature and acts as a mediator of synapse loss and dysfunction in AD (4), but not the relatively innocuous monomeric Aβ or vehicle, induced C1q deposition (Fig. 2A and fig. S5). A higher percentage of PSD95 colocalized with C1q in oAβ-injected mice vs. monomer-injected (Fig. 2B), in a manner similar to this colocalization in J20 mice. Together, these findings show an early and aberrant increase and synaptic localization of C1q in multiple AD model systems. Furthermore, fluorescent in situ hybridization demonstrated upregulated C1qa expression in microglia (Fig. S6), implicating microglia as a major source of C1q in these pre-plaque brains.
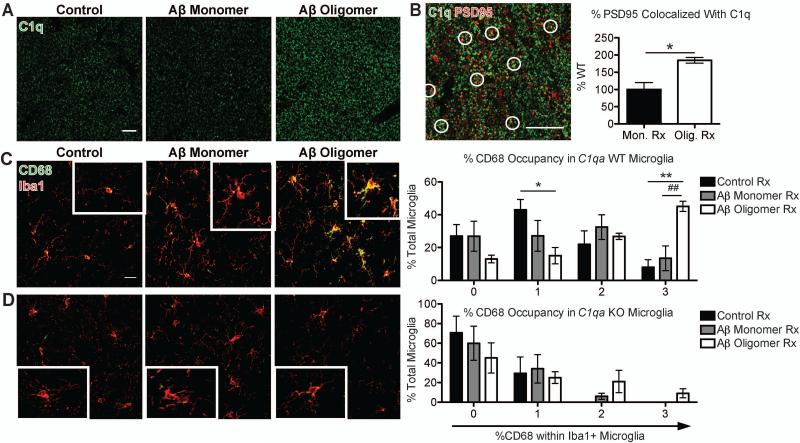
Fig. 2. Oligomeric Aβ increases C1q and microglial phagocytic activity.
(A and B) Soluble Aβ oligomers in WT mice led to elevation of C1q (green) (A) and a higher % PSD95 (red) colocalization with C1q vs. monomers (B). (C and D) oAβ induced high levels of CD68 (green) immunoreactivity in Iba1-positive (red) microglia in WT mice (C), but not in those of C1qa KO mice (D). Both had negligible changes in morphology. See Fig. S10. Scale bar = 10 (A), 5 (B) or 20 (C) μm. Means ± SEM; n = 3-5 mice per treatment group per genotype. *P < 0.05 using two-tailed t-test (B) or *P < 0.05, **P < 0.01 vs. control-treated or ##P < 0.01 vs. Aβ monomer-treated using two-way ANOVA followed by Bonferroni posttest (C).
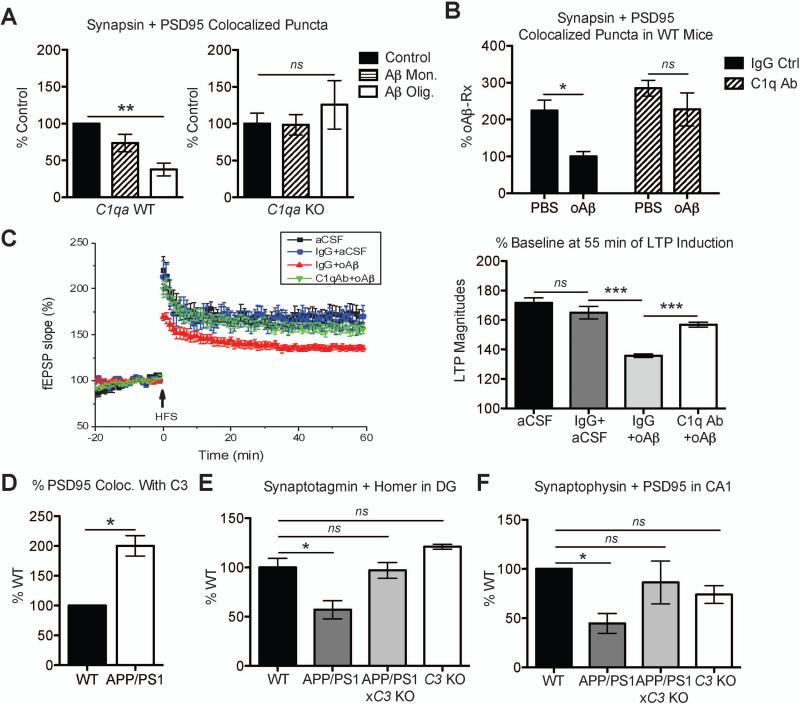
To test whether C1q and oAβ act in a common pathway to eliminate synapses, we injected oAβ into lateral ventricles of C1qa knockout (KO) mice (16). Soluble oAβ induced a significant loss of colocalized synapsin- and PSD95-immunoreactive puncta in WT mice within 72 h (Fig. 3A, left panel) (17). In contrast, oAβ failed to induce synapse loss in C1qa KO mice (Fig. 3A, right panel), suggesting that C1q is required for oAβ-induced synapse loss in vivo. To determine whether local, acute inhibition of C1 activation could similarly blunt the synaptotoxic effects of oAβ, we utilized an anti-C1q antibody, which blocks the classical complement cascade in vitro (ANX-M1 (Annexon Biosciences); see Fig. S7 and Supplemental Methods). Co-administration of the ANX-M1 anti-C1q antibody, but not its IgG isotype control, prevented oAβ from inducing synapse loss in WT mice (Fig. 3B). Thus, blocking C1 activation by either genetic or antibody-mediated means lessened oAβ's synaptotoxic effects.
Fig. 3. Complement is necessary for synapse loss and dysfunction in AD models.
(A) Aβ oligomers induced loss of colocalized synapsin- and PSD95-immunoreactive puncta in contralateral hippocampus of 3 mo WT mice (left panel); however, they failed to do so in C1qa KO mice (right panel). (B) Co-injection of Aβ oligomers with the function-blocking antibody against C1q, ANX-M1, but not with its IgG isotype control, prevented synapse loss in WT mice. (C) Pre-treatment of hippocampal slices with the C1q antibody, ANX-M1, prevented Aβ-mediated LTP inhibition (green) vs. IgG (red). IgG alone had a minimal effect (blue) vs. aCSF vehicle (black). n = 6-11 slices per group. (D) % PSD95 colocalized with C3 is increased in APP/PS1 hippocampus vs. that of WT mice. (E and F) Genetic deletion of C3 prevents synapse loss in 4 mo APP/PS1 mice. Quantification of colocalized immunoreactive puncta for synaptotagmin and homer in dentate gyrus (E) or synaptophysin and PSD95 in CA1 stratum radiatum (F) of WT, APP/PS1, APP/PS1xC3 KO and C3 KO hippocampi. Means ± SEM; n = 3-5 mice per genotype or per treatment group per genotype. *P < 0.05, **P < 0.01 or ***P < 0.001 using two-tailed one-sample t-test (D), one-way (A, C, E, F) or two-way (B) ANOVA followed by Bonferroni posttest.
To determine whether C1q is associated with synaptic dysfunction, we asked whether the established ability of oAβ to potently inhibit LTP (4) was dependent on C1q. We tested the functional effects of the ANX-M1 anti-C1q antibody in acute hippocampal slices treated with oAβ. IgG alone had negligible effects on LTP induction in WT mouse hippocampal slices and on the ability of oAβ to inhibit LTP; however, pre-treatment of hippocampal slices with the C1q antibody significantly prevented the impairment of LTP by oAβ (Fig. 3C). Neither the ANX-M1 nor its IgG control altered basal synaptic neurotransmission (Fig. S8). Collectively, these results in hippocampal slices and in mice support C1q as a key mediator of oAβ-induced synaptic loss and dysfunction.
In the healthy developing brain, C1q promotes activation of C3, which opsonizes subsets of synapses for elimination, a process that is significantly downregulated in the mature brain (5, 6). However, oAβ induced a significant C3 deposition in WT adult mice (Fig. S7A, upper panel). This was significantly reduced in both the C1qa KO (Fig. S7A, lower panel) and the ANX-M1 C1q antibody-treated WT mice (fig. S7B), suggesting that the C3 deposition in this model is downstream of the classical complement cascade. Consistent with these findings, a higher percentage of PSD95 colocalized with C3 in J20 and APP/PS1 brains (Fig. 3D and fig. S9). To determine whether C3 is necessary for early synapse loss in AD genetic models, we crossed APP/PS1 mice, which, similar to the J20 mice, had significant elevation and localization of C1q and C3 onto hippocampal synapses (Figs. S2C and S9), to C3-deficient mice (18). Quantification of colocalized pre- and postsynaptic puncta demonstrated synapse loss in 4 mo APP/PS1 hippocampus as compared to WT; however, APP/PS1xC3 KO mice did not display this synapse loss (Figs. 3E and F). Together, our data indicate that genetic deletion of C3 ameliorates synapse loss in APP/PS1 mice, providing further evidence that the classical complement cascade mediates early synapse loss in AD mouse models.
Microglia express complement receptors and mediate synaptic pruning in the developing brain (1, 6), raising the question of whether this normal developmental pruning pathway could be activated to mediate synapse loss in the pre-plaque AD brain. Consistent with this hypothesis, microglia had elevated levels of the lysosomal protein CD68 in J20 hippocampus compared to WT, and less so in striatum, a less vulnerable region (Fig. S1C and fig. S10). Furthermore, in WT mice challenged with oAβ, microglia had significantly increased levels of CD68 immunoreactivity (Fig. 2C). However, in C1qa KO mice in which synapse loss was rescued, oAβ failed to induce such an increase (Fig. 2D), suggesting that microglia eliminate synapses through the complement pathway.
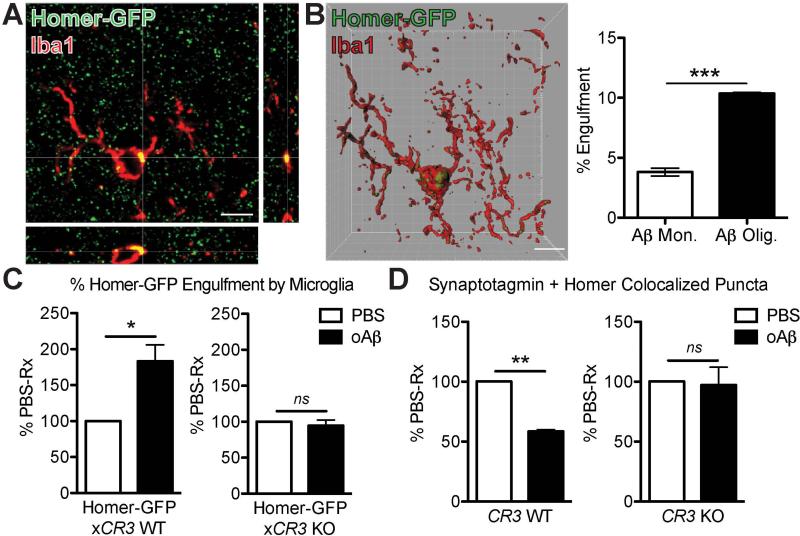
To directly test whether phagocytic microglia engulf synaptic elements, we adapted our in vivo synaptic engulfment assay (19) using intracerebroventricular injections of Aβ in Homer-GFP mice (20) (Fig. 4A). oAβ induced a significantly higher volume of internalized Homer-GFP in microglia than monomeric Aβ controls did at the contralateral hippocampus (Fig. 4B), indicating that microglia engulf synaptic elements when challenged with oAβ. Internalized Homer-GFP often colocalized with CD68 (Fig. S11A), suggesting that the engulfed synapses are internalized into lysosomal compartments in a manner similar to developmental synaptic pruning (6). Importantly, oAβ failed to increase synaptic engulfment in microglia lacking CR3 (21), a high affinity receptor for C3 expressed on macrophages (Homer-GFPxCR3 KO vs. Homer-GFP mice, which received tail vein injections of PBS or oAβ (Fig. 4C)). These data demonstrate that CR3 is necessary for oAβ-dependent engulfment of synapses by microglia.
Fig. 4. Microglia engulf synapses via CR3 upon oligomeric Aβ challenge.
(A) Orthogonal view of high-resolution confocal image shows colocalization of Homer-GFP and Iba1 (red). (B) 3D reconstruction and surface rendering using Imaris demonstrate bigger volumes of Homer-GFP puncta inside microglia of oAβ-injected contralateral hippocampus vs. those of monomer-injected. (C) Microglia of Homer-GFPxCR3 KO mice (right panel) show less engulfment of Homer-GFP when challenged with oAβ vs. those of Homer-GFP mice (left panel). (D) Aβ oligomers failed to induce synapse loss in contralateral hippocampus of CR3 KO mice (right panel) as they did in WT mice (left panel). Scale bar = 5 μm (A and B). Means ± SEM; n = 3 mice per treatment group per genotype (n = 6-17 microglia analyzed per mouse). *P < 0.05 or **P < 0.01 using two-tailed t-test (B) or two-tailed one-sample t-test (C and D).
To test whether inhibition in microglial engulfment leads to protection against oAβ-induced synapse loss, we performed tail vein injections of oAβ into WT and CR3 KO mice. oAβ induced synapse loss in hippocampus of WT mice, but not in CR3 KO mice (Fig. 4D). All CR3-positive microglia were P2RY12-positive (Fig. S11), indicating they are resident cells (22). Altogether, these results suggest that resident microglia engulf synaptic material when challenged by oAβ through a complement-dependent mechanism.
Synaptic deficits occur in early AD and mild cognitively impaired patients before onset of plaques and are some of the first signs of the neuronal degenerative process (4, 23-25). Here we identify critical synaptotoxic roles of complement and microglia prior to plaque formation and neuroinflammation, in regions of hippocampus undergoing synapse loss. Using multiple experimental approaches, we demonstrate a region-specific increase of phagocytic microglia and accumulation of C1q and C3 on synapses in pre-plaque brains. Microglia in the adult brain, when challenged with synaptotoxic, soluble Aβ oligomers, engulf synapses in the absence of plaque aggregates; deletion of CR3 blocks this process. Finally, inhibiting C1q, C3 or CR3 activity rescues synaptic loss and dysfunction.
Our data suggest a local activation of a developmental pruning pathway (5, 6) as a key mechanism underlying oAβ-induced synapse loss in pre-plaque AD brain. C1q is aberrantly increased by diffusible oAβ in a region-specific manner and deposits onto synapses, triggering the activation of downstream classical complement pathway and phagocytic microglia. Blocking Aβ production in J20 mice significantly ameliorated C1q deposition in the hippocampus, and genetic or antibody-mediated inhibition of complement blocks oAβ from inducing microglial synaptic engulfment, synapse loss, and LTP inhibition. These complementary findings have direct therapeutic relevance.
We propose a model in which C1q and oAβ operate in a common pathway to activate the complement cascade and drive synapse elimination by microglia through CR3 (Fig. S12). This could occur in multiple ways: soluble oAβ associates with synaptic membranes and other synaptic markers (4, 26); thus, oAβ bound to synapses may anchor C1q directly. Alternatively, oAβ binding to synapses may weaken the synapse (4) and expose a C1q receptor. Although specific receptors for C1q at synapses are not yet known, C1q binds synapses in vulnerable regions undergoing synapse loss (our data here; (5, 27)). It is also plausible that oAβ and C1q may work indirectly to mediate synapse loss through cytokines such as TGFβ (7), through microglial or astrocytic activation, or through other mechanisms including MHCI/PirB, another immune pathway critical for synapse elimination in development and AD (28-30).
Finally, our studies show that resident microglia in the adult CNS phagocytose synapses when challenged by synaptotoxic oAβ, implicating microglia as potential cellular mediators of synapse loss. Although microglia and complement activation are prominently involved in plaque maintenance and related peri-plaque neuropathology, their roles have heretofore been largely regarded as a secondary event related to neuroinflammation (2). Our studies directly challenge this view and suggest that microglia and immune-related pathways can act as early mediators of synapse loss and dysfunction that occur in AD models before plaques form. Although the complement pathway may not be involved in all pathological routes to AD, including plaque-associated synapse loss, the work reported here provides new insights into how synapses are lost in AD. It will be important in future studies to examine whether this microglia or the complement-dependent pathway also plays a role in plaque-associated synapse loss or in other synaptopathies including tauopathies and Huntington's disease. If so, the work reported here may suggest complement and microglia as potential early therapeutic targets in AD and other neurodegenerative diseases involving synaptic dysfunction and memory decline.
Supplementary Material
One sentence summary.
Complement and microglia mediate synapse loss in pre-plaque AD models, and inhibiting complement rescues Aβ-induced synapse loss.
Acknowledgements
We thank B. Sabatini (HMS), T. Bartels (BWH), and Stevens laboratory members for critical reading of manuscript; L. Dissing-Olesen (BCH) for help with conceptual figure (Fig. S12), M. Ericsson (HMS EM) for EM imaging, K. Kapur (BCH) for advice on statistics, D.M. Walsh (BWH) for Aβ oligomers (S26C), S. Okabe (Univ. Tokyo) for Homer-GFP mice, and Ted Yednock (Annexon Biosciences) for advice on the ANX-M1 anti-C1q antibody; D. Richardson (HCBI), A. Hill (BCH IDDRC), and H. Elliot and T. Xie (HMS IDAC) for assistance with imaging and data analysis; and S. Kim (BWH), K. Colodner (BCH) and S. Matousek (BWH) for assistance with mice. The J20 mice, C1qa KO mice, P2RY12 antibody and the ANX-M1 C1q function blocking antibody are available from L. Mucke, M. Botto, O. Butovsky and A. Rosenthal under material transfer agreements with UCSF Gladstone, Imperial College London, Brigham and Women's Hospital, and Annexon Biosciences, respectively. A.R. is a co-founder, consultant and chairman of the board of directors; B.A.B. is a co-founder and chairman of the Scientific Advisory Board; and B.S. serves on the Scientific Advisory Board of Annexon LLC. A.R., B.A.B. and B.S. are minor shareholders of Annexon LLC. All other authors declare no competing financial interests related to this project. The following patents related to this project have been granted or applied for: PCT/2015/010288 (S.H. and B.S.), US14/988387 and EP14822330 (S.H., A.R. and B.S.), and US8148330, US9149444, US20150368324, US20150368325, US20150368326 and US20120328601 (B.S. and B.A.B.). This work was funded by Edward R. and Anne G. Lefler Fellowship (S.H.), Coins for Alzheimer's Research Trust (B.S.), Fidelity Biosciences Research Initiative (F-Prime) (B.S. and C.A.L.), JPB Foundation (B.A.B.), and the National Institutes of Health AG000222 (S.H.), NINDS-NIH R01NS083845 (D.J.S.) and NIA-NIH 1RF1AG051496A (B.S.). Supplement contains additional data, including Materials and Methods.
Footnotes
Author Contributions. S.H. and B.S. designed the study and wrote the manuscript, with help from all authors. S.H. performed most experiments and data analysis, V.B.G. and B.M.N. performed microglial activation and engulfment experiments along with immunohistochemistry, S.R. and K.M.M. performed C1q immunohistochemistry, A.F. performed FISH, S.L. performed electrophysiology, Q.S. and C.A.L. assisted with design and collection of APP/PS1 tissue, A.R. and B.A.B. designed and characterized the ANX-M1 anti-C1q antibody, and D.J.S. contributed in the discussions and experimental design.
Reference
- 1.Hong S, Dissing-Olesen L, Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol. 2016 doi: 10.1016/j.conb.2015.12.004. doi:10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit ME, et al. C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid-β neurotoxicity. Journal of Biological Chemistry. 2013;288:654–665. doi: 10.1074/jbc.M112.400168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mucke L, Selkoe DJ. Neurotoxicity of Amyloid β-Protein: Synaptic and Network Dysfunction. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Schafer DP, et al. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bialas AR, Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 9.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 10.Hong S, Wilton D, Stevens B, Richardson DS. Structured Illumination Microscopy for the investigation of synaptic structure and function. Methods in Molecular Biology; Synapse Development: Methods and Protocols. doi: 10.1007/978-1-4939-6688-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mucke L, et al. High-Level Neuronal Expression of Abeta 1-42 in Wild-Type Human Amyloid Protein Precursor Transgenic Mice: Synaptotoxicity without Plaque Formation. Journal of Neuroscience. 2000;20:4050. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong S, et al. Dynamic Analysis of Amyloid -Protein in Behaving Mice Reveals Opposing Changes in ISF versus Parenchymal A during Age-Related Plaque Formation. Journal of Neuroscience. 2011;31:15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephan AH, et al. A Dramatic Increase of C1q Protein in the CNS during Normal Aging. Journal of Neuroscience. 2013;33:13460–13474. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris JA, et al. Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer's disease are independent of caspase cleavage of the amyloid precursor protein. J Neurosci. 2010;30:372–381. doi: 10.1523/JNEUROSCI.5341-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jankowsky JL, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 16.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 17.Freir DB, et al. Aβ oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiology of Aging. 2011;32:2211–2218. doi: 10.1016/j.neurobiolaging.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wessels MR, et al. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schafer DP, Lehrman EK, Heller CT, Stevens B. An engulfment assay: a protocol to assess interactions between CNS phagocytes and neurons. J Vis Exp. 2014 doi: 10.3791/51482. doi:10.3791/51482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebihara T, Kawabata I, Usui S, Sobue K, Okabe S. Synchronized formation and remodeling of postsynaptic densities: long-term visualization of hippocampal neurons expressing postsynaptic density proteins tagged with green fluorescent protein. Journal of Neuroscience. 2003;23:2170–2181. doi: 10.1523/JNEUROSCI.23-06-02170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coxon A, et al. A Novel Role for the β2 Integrin CD11b/CD18 in Neutrophil Apoptosis: A Homeostatic Mechanism in Inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 22.Butovsky O, et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat Neurosci. 2013;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 24.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiology of Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, et al. Soluble Aβ Oligomers Are Rapidly Sequestered from Brain ISF In Vivo and Bind GM1 Ganglioside on Cellular Membranes. Neuron. 2014 doi: 10.1016/j.neuron.2014.02.027. doi:10.1016/j.neuron.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 28.Datwani A, et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim T, et al. Human LilrB2 Is a -Amyloid Receptor and Its Murine Homolog PirB Regulates Synaptic Plasticity in an Alzheimer's Model. Science. 2013;341:1399–1404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H, et al. Synapse elimination and learning r. Nature. 2014:1–18. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.