Abstract
INTRODUCTION: Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are a treatment option in the second- or third-line palliative setting in EGFR wild-type (wt) non–small cell lung cancer (NSCLC) patients. However, response rates are low, and only approximately 25% will achieve disease control. Early prediction of treatment resistance could accelerate discontinuation of ineffective treatment and reduce unnecessary toxicity. In this study, we evaluated early changes on 18F-fluoro-D-glucose (F-18-FDG) positron emission tomography/computed tomography (PET/CT) and in total plasma cell-free DNA (cfDNA) as markers of erlotinib response in EGFR-wt patients. METHODS: F-18-FDG-PET/CT scans and blood samples were obtained prior to erlotinib initiation and were repeated after 1 week (PET/CT) and 1 to 4 weeks (blood sample) of treatment. Level of cfDNA was measured by droplet digital polymerase chain reaction. Percentage change (%∆) in SULpeak and total lesion glycolysis (TLG) on FDG-PET/CT and in plasma cfDNA was correlated to radiological response, progression-free survival (PFS), and overall survival (OS). RESULTS: Fifty patients were prospectively enrolled. A significant correlation was found between CT response and %∆TLG (P = .003). All patients with early metabolic progression showed radiological progression. Increased %∆TLG and %∆cfDNA were significantly correlated with shorter PFS (P = .002 and P = .004, respectively) and OS (P = .009 and P = .009, respectively). Multivariate analysis indicated %∆cfDNA to be the strongest predictor of OS. CONCLUSION: Early increase in TLG on F-18-FDG-PET/CT correlates with radiological progression, and shorter PFS and OS. Early increase in cfDNA predicts shorter PFS and OS. Both assessments are promising tools for early detection of nonresponders and reduced OS in TKI-treated EGFR-wt NSCLC patients.
Introduction
Tyrosine kinase inhibitors (TKIs) targeting the epidermal growth factor receptor (EGFR) have emerged as important treatment options in non–small cell lung cancer (NSCLC) patients. Activating mutations in the EGFR gene have proven to be crucial predictors of treatment response [1]. However, mutation status cannot solely predict outcome because a fraction of EGFR wild-type (wt) patients also benefits from the treatment. Hence, EGFR-TKIs are a treatment option in the second- or third-line palliative setting in these patients [2], [3]. However, additional clinical tools are needed to distinguish nonresponders from responders and thereby increase the ability to end an ineffective EGFR-TKI treatment earlier so that a more effective treatment can be offered to the patient.
An early response assessment on a 2′-deoxy-2′-[18F] fluoro-D-glucose (F-18-FDG) positron emission tomography/computed tomography (PET/CT) scan performed during the first 2 weeks of treatment is a promising new tool for treatment response prediction. A change in FDG uptake has been visualized as early as after 2 days of TKI treatment [4], [5], and studies have shown an association between the early metabolic response and treatment outcome [4], [5], [6], [7], [8]. However, these studies have evaluated patients with either unselected or mixed EGFR mutation status. Patients with EGFR activating mutations have a considerably better effect on EGFR-TKIs, and whether early FDG-PET response assessment is predictive in a cohort consisting exclusively of EGFR-wt patients is still unknown. Furthermore, in prior studies, standardized uptake value (SUV) metrics have been used for FDG-PET response assessment; yet, these parameters only represent a change in FDG uptake in a single voxel of the tumor or a small region of interest in the tumor. A more informative parameter could be the volume-based parameter total lesion glycolysis (TLG) because it reflects the entire metabolic tumor burden by combining volumetric data of tumors with the metabolic activity. In chemotherapy-treated NSCLC patients, two studies have reported TLG to be superior to SUVmax and SUVpeak for early response prediction [9], [10] and this could also apply for TKI-treated patients.
Total plasma cell-free deoxyribonucleic acid (cfDNA) is appearing as a new potential biomarker in cancer. cfDNA is believed to be shed by both normal cells and tumor cells. The amount found in the circulation increases when cells are undergoing apoptosis or necrosis. Higher levels have been identified in cancer patients compared with noncancer patients [11], [12], [13], and the level has been suggested to reflect the tumor burden in patients. Therefore, changes in cfDNA concentration could be associated with treatment response; however, the predictive value of an early change in cfDNA value during TKI treatment has not yet been investigated.
Thus, changes in 18-F-FDG-PET signals as well as changes in cfDNA levels are two promising methods for early response assessment. Hence, the aim of this study was to evaluate the predictive value of each of these methods in a cohort of advanced-stage EGFR-wt NSCLC patients treated with erlotinib in second or third line. Moreover, we compared the value of the two FDG-PET–derived parameters, TLG and SULpeak, for the early metabolic response prediction.
Material and Methods
Patients and Study Design
In this prospective, single-center study, 67 patients with stage III or IV NSCLC were enrolled from April 2013 until August 2015 at the Department of Oncology, Aarhus University Hospital, Denmark. Patients were candidates for enrolment if they were eligible to initiate treatment with erlotinib in a palliative setting. Details on inclusion criteria and study treatment have been described previously [14]. The study was approved by the Central Denmark Region Committees on Biomedical Research Ethics (no. 1-10-72-19-12) and reported to ClinicalTrial.gov (NCT02043002). Each patient gave written informed consent before inclusion. For the purpose of this work, we included patients from the enrolled cohort who were EGFR-wt, were treated in second- or third-line, and had undergone paired scans and/or paired blood samples.
18-FDG-PET/CT scans were performed pretreatment and after 7 to 10 days of erlotinib treatment. Blood samples were collected prior to erlotinib initiation and after 1 to 4 weeks of treatment. CT scans of the chest and abdomen were conducted before and after 9 to 11 weeks of treatment or earlier on clinical indication. Further evaluation CT scans were performed every 12 weeks during the treatment period. Neuroimaging was performed on clinical indication. Routine clinical and biochemical evaluation was performed every fourth week in the first 12 weeks and subsequently every sixth week.
Data on clinical characteristics and response were collected from medical files. Testing for EGFR mutations and anaplastic lymphoma kinase (ALK) translocations had been performed as part of the diagnostic workup and is described in detail in Supplementary File 1.
Response Assessment on FDG-PET/CT and CT Imaging
All F-18-FDG-PET/CT scans were performed on a combined PET/CT scanner (Siemens Biograph TruePoint 40) at the Department of Nuclear Medicine and PET-Centre, Aarhus University Hospital, Denmark. The imaging protocol is described in Supplementary File 1. Same scanner model, protocol for acquisition, and reconstruction software were used in all patients. Data on amount of injected 18-F-FDG, uptake time, and plasma glucose concentration are shown in Supplementary Table 1.
An experienced nuclear medicine physician blinded to the patient outcome analyzed all PET/CT scans using Siemens Syngo.via software. All SUV values were normalized to lean body mass (SUL). SULpeak and whole-body TLG were calculated according to the Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) 1.0 guideline [15] (described in Supplementary File 1). TLG could not be evaluated in two patients: in one patient due to carcinomatosis of the lung and in one patient due to multiple small lesions on the follow-up scan making tumor-volume assessment impossible. Percentage change (%∆) in SULpeak and whole-body TLG between pretreatment and follow-up scan was calculated as: (follow-up value − pretreatment value)/pretreatment value × 100. Metabolic response based on %∆SULpeak was classified according to the PERCIST 1.0 guideline, whereas %∆TLG was classified using a cutoff value of 25% based on observations by Kahraman et al. [16] (see Supplementary File 1).
Radiological response was evaluated on the first CT scan performed after initiation of erlotinib and quantified as %∆ in sum of longest diameter (SLD) of target lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria [17].
Quantification of Total Plasma cfDNA
A peripheral blood sample of 10 ml was collected at each time point. The samples were centrifuged (1400g for 15 minutes), and plasma was isolated. Plasma was subsequently frozen at −80°C until further analysis. Total cfDNA was purified from 2 ml of plasma by use of the QIAamp circulating Nucleic Acid kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol and eluted in a volume of 100 μl of TE buffer. To quantify the amount of cfDNA, the beta-2-microglobulin (B2M) gene was measured as previously described [18]. To account for a possible overestimation of the total cfDNA by accidental leukocyte contamination in the preanalytical phase, a unique B-cell immunoglobulin DNA rearrangement (PBC) was quantified [18]. The two genes were quantified in duplicates by droplet digital polymerase chain reaction (ddPCR, Bio-Rad QX200) in a multiplex reaction using 5 μl of sample. Primers (Eurofins Genomics), probes (Integrated DNA technology), and ddPCR conditions are described in Supplementary File 1. Samples with a PBC/B2M ratio larger than 0.1% were excluded from further analysis as previously described [18]. As the level of cfDNA varies between patients, %∆ in cfDNA level from pretreatment to follow-up was calculated.
Statistical Analysis
Correlations between metabolic response, change in total plasma cfDNA, and radiological response were calculated using Fisher’s exact test (categorical variables), Spearman's rank correlation coefficient (continuous variables), and Mann-Whitney U test (median values). In calculation of correlation between metabolic and radiological response, patients classified with stable disease (SD) or partial response (both metabolic and radiologic) were combined because of the low number of patients classified with partial response. Predictive accuracy of PET and cfDNA with respect to nonprogression on the CT scan was evaluated by using receiver operating characteristics (ROC) analysis (area under the curve [AUC]). Overall survival (OS) was measured from start of erlotinib treatment until death of any cause or last follow-up date (November 30, 2015). Progression-free survival (PFS) was defined as the time from initiation of erlotinib to first documentation of either clinical or radiological progression or death. If erlotinib treatment was ended without occurrence of progression or death, patients were censored at the time of discontinuation. Patients still undergoing treatment with erlotinib on the last follow-up date were censored at that day. Estimates of median PFS and OS were calculated by the Kaplan-Meier method and compared by the log-rank test. The Cox proportional-hazards model was used to calculate crude and adjusted hazard ratios (HRs). Clinical variables were dichotomized except for age (continuous). FDG-PET parameters and level of cfDNA were tested as continuous variables to avoid bias created by the cutoff values selected for classification of the variables. All tests were two-sided, and P values less than .05 were considered to be statistically significant. Statistical analyses were performed using SPSS statistics version 20.0 for Windows (IBM SPSS Statistics, Chicago, IL). STATA version 13 (Stata Corporation, College Station, TX) was used for preparation of Kaplan-Meier curves.
Results
Patients
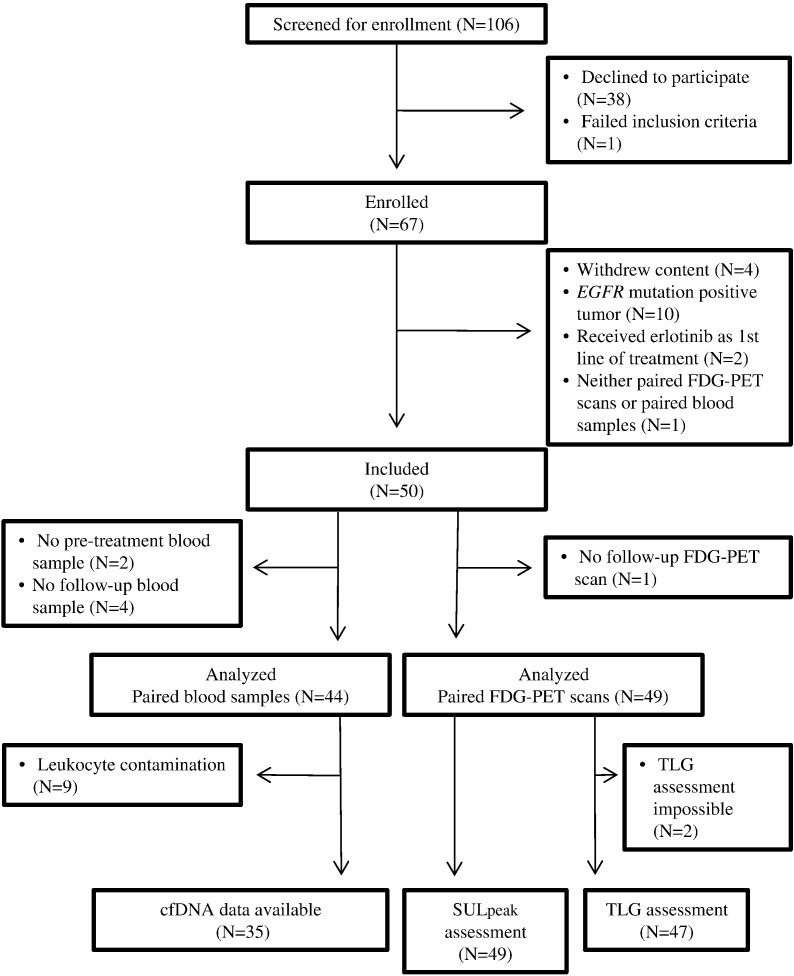
A total of 50 patients were included in the final analysis. A flow diagram of inclusion is shown in Figure 1. Patient characteristics are shown in Table 1. Follow-up data were available for all patients. At the last follow-up date, one patient was still undergoing erlotinib treatment and nine patients were still alive. Erlotinib was discontinued in patients because of either radiological or clinical progression of disease (n = 41), toxicity (n = 7), or death (n = 1).
Figure 1.
Consort diagram of patient inclusion.
Table 1.
Clinical Characteristics (N = 50)
| Characteristics | n (%) |
|---|---|
| Age | |
| Median years (range) | 68 (49-83) |
| Sex | |
| Female | 22 (44) |
| Male | 28 (56) |
| PS, ECOG | |
| 0 | 4 (8) |
| 1 | 36 (72) |
| 2 | 10 (20) |
| Smoking status | |
| Never | 1 (2) |
| Former⁎ | 38 (73) |
| Current | 12 (23) |
| Unknown | 1 (1) |
| Stage | |
| IIIa | 2 (4) |
| IIIb | 2 (4) |
| IV | 46 (92) |
| Brain metastases | |
| Yes | 7 (14) |
| No | 45 (86) |
| Histology | |
| Adenocarcinoma | 42 (84) |
| Squamous cell | 8 (16) |
| EML4-ALK gene fusion† | |
| Positive | 0 |
| Negative | 27 (54) |
| Unknown | 23 (44) |
| Erlotinib treatment | |
| 2nd line | 41 (79) |
| 3rd line | 9 (17) |
| Prior treatment | |
| 1st line | |
| Carboplatin/vinorelbine‡ | 27 (54) |
| Carboplatin/vinorelbine /bevacizumab§ | 23 (46) |
| 2nd line¶ | |
| Pemetrexed | 5 (56) |
| Docetaxel | 4 (44) |
| Timing of PET scans | |
| Days from pretreatment PET to erlotinib start, median (range) | 1 (0-21) |
| Days from erlotinib start to follow-up PET, median (range)# | 8 (2-23) |
| Timing of CT scans | |
| Days from pretreatment CT to erlotinib start, median (range) | 14 (4-120) |
| Days from erlotinib start to evaluation CT, median (range)⁎⁎ | 72 (20-92) |
| Timing of blood samples | |
| Days from pretreatment sample to erlotinib start, median (range) | 3 (0-24) |
| Days from erlotinib start to follow-up sample, median (range)†† | 26 (6-58) |
PS, performance status; ECOG, Eastern Cooperative Oncology Group; EML4-ALK, echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase.
Former smoker was defined as having stopped smoking at time of diagnosis.
Only patients with adenocarcinoma were tested.
Carboplatin day 1 (AUC 5) and vinorelbine day 1 and day 8 (60-80 mg/m2 [PO]) every 3 weeks for a maximum of four cycles.
Bevacizumab (7.5 mg/m2 IV day 1) was given in combination with chemotherapy. Patients with disease control received subsequent maintenance therapy every 3 weeks until progression or toxicity.
Only including patients treated with erlotinib in third line.
Four patients were not scanned between 7 and 10 days after initiation of erlotinib but instead after 2, 5, 14, and 23 days, respectively.
Four patients were scanned later than 9 to 11 weeks of treatment (3 patients 12 weeks after and 1 patient 13 weeks after). Thirteen patients had their CT scan performed earlier because of suspicion of progression.
One patient had the follow-up sample collected before 1 week of erlotinib treatment (after 6 days) and 7 patients later than 4 weeks (30, 31, 32, 35, 40, 48, and 58 days, respectively).
Correlation between FDG-PET/CT and CT Response
A significant correlation was found between radiological response and metabolic response on FDG-PET when TLG was used for response assessment (P = .003) (Table 2). Twelve patients were classified with metabolic progression, and all showed radiological progression. Thereby, 44% (12/27) of patients showing progression on CT could be identified by early FDG-PET. The %∆TLGs found in the 12 patients classified with metabolic progression are shown in Supplementary Table 2. Likewise, assessment of the %∆SULpeak showed a high predictive value of early metabolic progression (Table 2). However, only 21% (6/28) of patients demonstrating radiological progression could be identified by this measure. When continuous %∆ in early PET response was correlated to %∆SLD measured on the CT scan, a correlation was found using both PET parameters (%∆TLG: Spearman's correlation coefficient = 0.356, P = .024; %∆SULpeak: Spearman's correlation coefficient = 0.327, P = .034). The ROC analyses showed that the prediction of nonprogression by %∆TLG was 0.93 (95% CI, 0.84-1.00; P < .001) and by %∆SULpeak was 0.85 (95% CI, 0.74-0.96; P < .001) (Supplementary Figure 1).
Table 2.
PET Response and Change in Level of Total Plasma cfDNA in Correlation to CT Response
| CT Response⁎ |
||||
|---|---|---|---|---|
| PR | SD | PD | Total | |
| PET response using %∆SULpeak assessment† (P = .076‡) | ||||
| PMR | 0 | 3 | 2 | 5 (12%) |
| SMD | 1 | 11 | 20 | 32 (74%) |
| PMD | 0 | 0 | 6 | 6 (14%) |
| Total n (%) | 1 (2%) | 14 (33%) | 28 (65%) | 43 (100%) |
| PET response using %∆TLG assessment§ (P = .003‡) | ||||
| PMR | 1 | 4 | 1 | 6 (15%) |
| SMD | 0 | 9 | 14 | 23 (56%) |
| PMD | 0 | 0 | 12 | 12 (29%) |
| Total n (%) | 1 (2%) | 13 (32%) | 27 (66%) | 41 (100%) |
| Total plasma cfDNA (P = .172¶) | ||||
| Median %∆cfDNA (range) | 5% (−91 to 401) | 58% (−31 to 6249) | 47% (−91 to 6249) | |
| Total n (%) | 12 (40%) | 18 (60%) | 30 (100%) | |
PR, partial response; PD, progressive disease; PMR, partial metabolic response; SMD, stable metabolic disease; PMD, progressive metabolic disease.
An evaluation CT scan was performed in 44 patients. CT response was defined according to RECIST version 1.1 criteria.
Response was defined according to PERCIST 1.0 guideline.
In calculation of the P value, PMR and SMD as well as PR and SD were combined. P value was calculated by the Fisher’s exact test.
PMR was defined as a reduction in TLG of minimum 25%, PMD as an increase in TLG of minimum 25%, and SMD as a change not classified as PMR or PMD.
P value was calculated by the Mann-Whitney U test.
Correlation Between Total Plasma cfDNA Level and CT Response
Leukocyte DNA contamination was found in samples from nine patients, and these patients were excluded from further analysis. The overall median %∆cfDNA was 49% (range, −91 to 6249). A median increase of 58% was seen in patients classified with radiological progression, whereas only a median increase of 5% was found in patients classified with SD (Table 2); however, the difference was not statistically significant. Of the 18 patients showing radiological progression, 13 patients (72%) showed an increase in cfDNA, whereas the same fraction was 50% in patients with SD. No significant correlation was found when the continuous %∆SLD was correlated to continuous %∆cfDNA (Spearman's correlation coefficient = 0.206, P = .284). In line with this, the ROC analysis showed a relative poor AUC of 0.62 (95% CI, 0.45-0.86; P = .162) (Supplementary Figure 1).
Correlation Between FDG-PET/CT Scans, cfDNA Level, and Survival
The overall median PFS of all patients was 2.7 months (95% CI, 2.5-2.9), and the median OS was 6.0 months (95% CI, 3.7-8.3). Kaplan-Meier curves of PFS and OS according to FDG-PET response are shown in Supplementary Figure 2. Patients classified with progression by any of the two assessments had a significantly shorter PFS than patients classified with nonprogression (P = .014 [SULpeak] and P = .024 [TLG]). Univariate Cox regression analyses showed that an increase in %∆TLG and %∆cfDNA was significantly correlated to shorter PFS (Table 3) and shorter OS (Table 4). A trend toward an association with PFS was found for %∆SULpeak, whereas there was no correlation to OS. To evaluate the independent impact of %∆TLG and %∆cfDNA, multivariate Cox regression analyses were performed. Increase in %∆TLG and %∆cfDNA both remained independent predictors of shorter PFS (%∆TLG: adjusted HR = 1.02 [95% CI, 1.00-1.03], P = .045; % ∆cfDNA: adjusted HR = 1.001 [95% CI, 1.00-1.002], P = .017) (Table 3). Furthermore, increase in %∆cfDNA remained an independent predictor of shorter OS (Table 4), whereas %∆TLG did not show an independent correlation.
Table 3.
Univariate and Multivariate Cox Regression Analysis of PFS (N = 50)
| Variables | HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| Age⁎ | 1.01 (0.97-1.05) | .596 | ||
| Sex | ||||
| Female | 0.65 (0.35-1.22) | .180 | ||
| Male | 1.00 | |||
| Histology | ||||
| Adenocarcinoma | 0.95 (0.43-2.07) | .892 | ||
| Squamous cell | 1.00 | |||
| Smoking | ||||
| Never or former† | 0.99 (0.49-1.99) | .978 | ||
| Current | 1.00 | |||
| PS, ECOG | ||||
| 0-1 | 1.08 (0.45-2.57) | .872 | ||
| 2 | 1.00 | |||
| Stage | ||||
| IV | 0.40 (0.12-1.37) | .144 | ||
| III | 1.00 | |||
| Brain metastases | ||||
| Yes | 3.31 (1.34-8.21) | .010 | 34.54 (2.75-433.56) | .006 |
| No | 1.00 | 1.00 | ||
| Erlotinib treatment | ||||
| 1st or 2nd line | 0.85 (0.40-1.80) | .668 | ||
| 3rd line | 1.00 | |||
| %∆TLG⁎ | 1.02 (1.01-1.03) | .002 | 1.02 (1.00-1.03) | .045 |
| %∆SULpeak⁎ | 1.01 (1.00-1.02) | .100 | ||
| %∆cfDNA⁎ | 1.001 (1.00-1.001) | .004 | 1.001 (1.00-1.002) | .017 |
Evaluated as a continuous variable.
Former smoker was defined as having stopped smoking at time of diagnosis.
Table 4.
Univariate and Multivariate Cox Regression Analysis of OS (N = 50)
| Variables | HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| Age⁎ | 1.00 (0.97-1.04) | .845 | ||
| Sex | ||||
| Female | 0.63 (0.33-1.22) | .169 | ||
| Male | 1.00 | |||
| Histology | ||||
| Adenocarcinoma | 0.99 (0.43-2.26) | .977 | ||
| Squamous cell | 1.00 | |||
| Smoking | ||||
| Never or former† | 1.46 (0.69-3.07) | .322 | ||
| Current | 1.00 | |||
| PS, ECOG | ||||
| 0-1 | 0.59 (0.28-1.26) | .177 | ||
| 2 | 1.00 | |||
| Stage | ||||
| IV | 0.55 (0.19-1.58) | .267 | ||
| III | 1.00 | |||
| Brain metastases | ||||
| Yes | 3.47 (1.45-8.11) | .004 | 17.00 (2.94-98.44) | .002 |
| No | 1.00 | 1.00 | ||
| Erlotinib treatment | ||||
| 1st or 2nd line | 1.19 (0.52-2.72) | .676 | ||
| 3rd line | 1.00 | |||
| %∆TLG⁎ | 1.02 (1.00-1.03) | .009 | 1.01 (1.00-1.02) | .178 |
| %∆SULpeak⁎ | 1.00 (0.99-1.02) | .716 | ||
| %∆cfDNA⁎ | 1.001 (1.00-1.001) | .009 | 1.001 (1.00-1.001) | .043 |
Evaluated as a continuous variable.
Former smoker was defined as having stopped smoking at time of diagnosis.
Discussion
In this prospective study, we evaluated two different methods for early response assessment in EGFR-wt NSCLC patients treated with erlotinib because such a method is highly needed in this subgroup of patients. We demonstrated that an early change in TLG, measured on an FDG-PET/CT scan performed after 1 week of erlotinib treatment, was significantly associated with RECIST response after 10 weeks of treatment when a cutoff value of 25% was used. Most importantly, we found a high negative predictive value of early metabolic progression. All patients with progression after 1 week of treatment showed radiological progression. Thereby, 44% of all patients showing CT progression could be identified after only 1 week. Moreover, five out of six patients classified with partial metabolic response on the PET scan showed nonprogression on the CT scan. Lastly, the early metabolic change correlated with both PFS and OS.
Our results are consistent with findings in previous studies assessing the predictive value of an early metabolic response in TKI-treated NSCLC patients [5], [6], [7], [8], [19], [20], [21]. Despite variations in the timing of the early FDG-PET scan (2-14 days), all studies demonstrated an association between early PET response and outcome. However, these studies were performed in patients with either unselected or mixed EGFR mutation status. Our study is the first to show that early metabolic response monitoring is a useful predictor of outcome in a cohort consisting of only EGFR-wt patients. This is clinically important because biomarkers for EGFR-TKI treatment are highly needed in this patient subgroup where disease stabilization is only seen in around 25% and tumor response in approximately 8% [22]. Our findings bear important clinical significance because identification of resistant patients after just 1 week of treatment can lead to early discontinuation of ineffective treatment. This will markedly reduce the risk of unnecessary toxicity and increase the chance of receiving other potentially effective treatments before worsening of performance status.
We compared the effectiveness of two different parameters for PET response assessment. Both parameters were found to correlate with the change in SLD on the CT scan and show a high accuracy for prediction of nonprogression. However, TLG assessment was the only one of the two parameters significantly correlated to PFS and OS. Overall, our data indicate a superiority of early TLG assessment compared with SULpeak for early response monitoring in TKI-treated NSCLC patients. TLG is a promising parameter because it provides information of the complete metabolic tumor burden in the patient and, in addition, includes the metabolic activity of the tumor, which serves as a marker of tumor aggressiveness. One prior study has evaluated TLG assessment for early response monitoring in erlotinib-treated NSCLC patients. In 30 patients, a trend toward a correlation between metabolic response and longer PFS was found when a 20% or a 30% cutoff value was used for defining a response [16]. No comparison to other SUV metrics was performed in their study. However, a comparison between the value of SUVmax, SUVpeak, MTV, and TLG assessments on FDG-PET for detecting early response to chemotherapy was performed in 52 advanced-stage NSCLC patients [9]. In consistency with our data, they found TLG to be the sole parameter significantly correlated to PFS and OS.
In addition, we evaluated the predictive value of an early change in total plasma cfDNA. We demonstrated that an increase in plasma cfDNA after 1 to 4 weeks of TKI treatment was independently associated with a shorter PFS and shorter OS. In the group of patients showing radiological progression, we found a higher median percentage increase in cfDNA and a higher fraction of patients with an increase in cfDNA compared with patients classified with SD. However, this difference did not reach statistical significance. We are the first to evaluate an early change in plasma cfDNA in TKI-treated NSCLC patients. The dynamics of plasma cfDNA have been evaluated in NSCLC patients treated with chemotherapy; however, studies have showed conflicting results [23], [24], [25], [26]. Yet, none of the previous studies have accounted for a potential leukocyte DNA contamination and excluded blood samples with high contribution of leukocyte DNA as done in our study. We found contamination to be a problem in a substantial number of samples (11%), and a possible overestimation of cfDNA concentrations in prior studies could have influenced their results.
Previous studies have proposed total plasma cfDNA level to be a marker of tumor burden. Baseline plasma cfDNA values were found to correlate to both nodal stage and number of metastases in 134 NSCLC patients [27], and a significant decrease in the level of plasma cfDNA was found after tumor resection in 20 low-stage patients [12]. Our findings support these data and suggest plasma cfDNA as a promising predictor of PFS and OS in EGFR-wt NSCLC patients treated with erlotinib.
The strengths of our study are the prospective nature and the standardization of the FDG-PET imaging. All PET scans were performed on the same scanner model and with use of the same protocol for acquisition and reconstruction software, reducing the risk of interindividual variability of the scans. Moreover, handling of blood samples was performed in one laboratory by trained technicians, and we excluded samples with leukocyte contamination. Lastly, complete clinical data including the EGFR mutation status were available in all patients. In contrast, our work had some limitations to consider. Although it is one of the largest studies in the field, the impact of the study could have been increased if a higher number of patients had been included. Additionally, as the follow-up blood samples were collected in a range from 6 to 58 days after initiation of erlotinib, we are unable to define the optimal time point for early assessment of cfDNA.
Conclusion
In conclusion, our study demonstrated TLG assessment on an early FDG-PET/CT scan to be a promising predictor of response and survival in advanced-stage EGFR-wt NSCLC patients treated with an EGFR-TKI and to be a more robust method for response assessment than SULpeak. Moreover, we showed that an early increase in the level of total plasma cfDNA predicted shorter PFS and OS, but no correlation with radiological response was seen. A combination of the two assessments could be promising for response monitoring in this patient population. Because of the low number of patients included in our study, we could not evaluate the combination of the two assessments, and future larger, prospective, randomized studies are needed to accomplish this.
The following are the supplementary data related to this article.
ROC curve analysis performed for predicting the accuracy of PET parameters and cfDNA with respect to nonprogression on the CT scan. (A) ROC curve for %ΔTLG, (B) ROC curve for % ΔSULpeak, (C) ROC curve for %ΔSULpeak.
Kaplan-Meier curves for PFS (A and B) and OS (C and D) according to early PET response assessed with SULpeak and TLG. (A) SULpeak assessment; median PFS of patients classified with progression: 2.5 months (95% CI, 0.7-4.4) and nonprogression: 2.7 months (95% CI: 2.5-3.0). (B) TLG assessment; median PFS of patients classified with progression: 2.4 months (95% CI, 1.6-3.3) and nonprogression: 2.7 months (95% CI, 2.5-2.9). (C) SULpeak assessment; median OS of patients classified with progression: 2.3 months (95% CI, 1.5-3.1) and nonprogression: 8.0 months (95% CI, 5.1-11.0). (D) TLG assessment; median OS of patients classified with progression: 3.2 months (95% CI, 0.0-8.5) and nonprogression: 8.3 months (95% CI, 4.8-11.9). Differences between groups were calculated using the log-rank test.
Data on Plasma Glucose Concentration, F-18-FDG Injection, and Time Interval between FDG Injection and Scanning (Uptake Time) for Pretreatment FDG-PET Scans, Follow-Up FDG-PET Scans, and the Variation between Scans in Each Patient
%∆TLG and %∆cfDNA in the 12 Patients Classified with PMD by the TLG Assessment
Supplementary Figure 2.
Acknowledgements
The authors are thankful to Birgit Westh Mortensen for laboratory help.
Footnotes
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Lee C.K., Brown C., Gralla R.J., Hirsh V., Thongprasert S., Tsai C.-M., Tan E.H., Ho J.C.-M., Chu D.T., Zaatar A. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605. doi: 10.1093/jnci/djt072. 10.1093/jnci/djt072 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 2.Reck M., Popat S., Reinmuth N., De Ruysscher D., Kerr K.M., Peters S. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl. 3):iii27–iii39. doi: 10.1093/annonc/mdu199. 10.1093/annonc/mdu199 [cited 2015 Mar 5] [DOI] [PubMed] [Google Scholar]
- 3.Masters G.A., Temin S., Azzoli C.G., Giaccone G., Baker S., Brahmer J.R., Ellis P.M., Gajra A., Rackear N., Schiller J.H. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33:3488–3515. doi: 10.1200/JCO.2015.62.1342. 10.1200/JCO.2015.62.1342 [cited 2015 Sep 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunaga N., Oriuchi N., Kaira K., Yanagitani N., Tomizawa Y., Hisada T., Ishizuka T., Endo K., Mori M. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer. 2008;59:203–210. doi: 10.1016/j.lungcan.2007.08.012. 10.1016/j.lungcan.2007.08.012 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 5.Tiseo M., Ippolito M., Scarlattei M., Spadaro P., Cosentino S., Latteri F., Ruffini L., Bartolotti M., Bortesi B., Fumarola C. Predictive and prognostic value of early response assessment using 18FDG-PET in advanced non-small cell lung cancer patients treated with erlotinib. Cancer Chemother Pharmacol. 2014;73:299–307. doi: 10.1007/s00280-013-2356-x. 10.1007/s00280-013-2356-x [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 6.Zander T., Scheffler M., Nogova L., Kobe C., Engel-Riedel W., Hellmich M., Papachristou I., Toepelt K., Draube A., Heukamp L. Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [(18)F]fluorodeoxyglucose and [(18)F]fluorothymidine positron emission tomography. J Clin Oncol. 2011;29:1701–1708. doi: 10.1200/JCO.2010.32.4939. 10.1200/JCO.2010.32.4939 [cited 2013 May 23] [DOI] [PubMed] [Google Scholar]
- 7.Hachemi M., Couturier O., Vervueren L., Fosse P., Lacœuille F., Urban T., Hureaux J. [18F]FDG positron emission tomography within two weeks of starting erlotinib therapy can predict response in non-small cell lung cancer patients. PLoS One. 2014;9:e87629. doi: 10.1371/journal.pone.0087629. 10.1371/journal.pone.0087629 [cited 2016 Feb 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi R., Hirata H., Tachibana I., Shimosegawa E., Inoue A., Nagatomo I., Takeda Y., Kida H., Goya S., Kijima T. Early [18F]fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin Cancer Res. 2012;18:220–228. doi: 10.1158/1078-0432.CCR-11-0868. 10.1158/1078-0432.CCR-11-0868 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 9.Moon S.H., Cho S.-H., Park L.C., Ji J.H., Sun J.-M., Ahn J.S., Park K., Choi J.Y., Ahn M.-J. Metabolic response evaluated by 18F-FDG PET/CT as a potential screening tool in identifying a subgroup of patients with advanced non-small cell lung cancer for immediate maintenance therapy after first-line chemotherapy. Eur J Nucl Med Mol Imaging. 2013;40:1005–1013. doi: 10.1007/s00259-013-2400-4. 10.1007/s00259-013-2400-4 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 10.Usmanij E.A., de Geus-Oei L.-F., Troost E.G.C., Peters-Bax L., van der Heijden E.H.F.M., Kaanders J.H.A.M., Oyen W.J.G., Schuurbiers O.C.J., Bussink J. 18F-FDG PET early response evaluation of locally advanced non-small cell lung cancer treated with concomitant chemoradiotherapy. J Nucl Med. 2013;54:1528–1534. doi: 10.2967/jnumed.112.116921. 10.2967/jnumed.112.116921 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 11.Szpechcinski A., Chorostowska-Wynimko J., Struniawski R., Kupis W., Rudzinski P., Langfort R., Puscinska E., Bielen P., Sliwinski P., Orlowski T. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br J Cancer. 2015;113:476–483. doi: 10.1038/bjc.2015.225. 10.1038/bjc.2015.225 [cited 2016 Feb 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szpechcinski A., Chorostowska-Wynimko J., Kupis W., Maszkowska-Kopij K., Dancewicz M., Kowalewski J., Orlowski T. Quantitative analysis of free-circulating DNA in plasma of patients with resectable NSCLC. Expert Opin Biol Ther. 2012;12(Suppl 1):S3–S9. doi: 10.1517/14712598.2012.668519. 10.1517/14712598.2012.668519 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 13.Yoon K.-A., Park S., Lee S.H., Kim J.H., Lee J.S. Comparison of Circulating Plasma DNA Levels between Lung Cancer Patients and Healthy Controls. J Mol Diagn. 2009;11:182–185. doi: 10.2353/jmoldx.2009.080098. 10.2353/jmoldx.2009.080098 [cited 2016 Jan 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winther-Larsen A., Fledelius J., Sorensen B.S., Meldgaard P. Metabolic tumor burden as marker of outcome in advanced EGFR wild-type NSCLC patients treated with erlotinib. Lung Cancer. 2016;94:81–87. doi: 10.1016/j.lungcan.2016.01.024. 10.1016/j.lungcan.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 15.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl. 1):122S–150S. doi: 10.2967/jnumed.108.057307. 10.2967/jnumed.108.057307 [cited 2015 Nov 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahraman D., Holstein A., Scheffler M., Zander T., Nogova L., Lammertsma A.A., Boellaard R., Neumaier B., Dietlein M., Wolf J. Tumor lesion glycolysis and tumor lesion proliferation for response prediction and prognostic differentiation in patients with advanced non-small cell lung cancer treated with erlotinib. Clin Nucl Med. 2012;37:1058–1064. doi: 10.1097/RLU.0b013e3182639747. 10.1097/RLU.0b013e3182639747 [cited 2015 Nov 16] [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. 10.1016/j.ejca.2008.10.026 [cited 2014 Jul 10] [DOI] [PubMed] [Google Scholar]
- 18.Pallisgaard N., Spindler K.-L.G., Andersen R.F., Brandslund I., Jakobsen A. Controls to validate plasma samples for cell free DNA quantification. Clin Chim Acta. 2015;446:141–146. doi: 10.1016/j.cca.2015.04.015. 10.1016/j.cca.2015.04.015 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 19.Mileshkin L., Hicks R.J., Hughes B.G.M., Mitchell P.L.R., Charu V., Gitlitz B.J., Macfarlane D., Solomon B., Amler L.C., Yu W. Changes in 18F-fluorodeoxyglucose and 18F-fluorodeoxythymidine positron emission tomography imaging in patients with non-small cell lung cancer treated with erlotinib. Clin Cancer Res. 2011;17:3304–3315. doi: 10.1158/1078-0432.CCR-10-2763. 10.1158/1078-0432.CCR-10-2763 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 20.Benz M.R., Herrmann K., Walter F., Garon E.B., Reckamp K.L., Figlin R., Phelps M.E., Weber W.A., Czernin J., Allen-Auerbach M.S. (18)F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. J Nucl Med. 2011;52:1684–1689. doi: 10.2967/jnumed.111.095257. 10.2967/jnumed.111.095257 [cited 2016 Feb 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahraman D., Scheffler M., Zander T., Nogova L., Lammertsma A.A., Boellaard R., Neumaier B., Ullrich R.T., Holstein A., Dietlein M. Quantitative analysis of response to treatment with erlotinib in advanced non-small cell lung cancer using 18F-FDG and 3’-deoxy-3'-18F-fluorothymidine PET. J Nucl Med. 2011;52:1871–1877. doi: 10.2967/jnumed.111.094458. 10.2967/jnumed.111.094458 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 22.Arriola E., Taus Á., Casadevall D. Is there a role for epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor wild-type non-small cell lung cancer? World J Clin Oncol. 2015;6:45–56. doi: 10.5306/wjco.v6.i4.45. 10.5306/wjco.v6.i4.45 [cited 2015 Nov 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S., Guleria R., Singh V., Bharti A.C., Mohan A., Das B.C. Plasma DNA level in predicting therapeutic efficacy in advanced nonsmall cell lung cancer. Eur Respir J. 2010;36:885–892. doi: 10.1183/09031936.00187909. 10.1183/09031936.00187909 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 24.Pan S., Xia W., Ding Q., Shu Y., Xu T., Geng Y., Lu Y., Chen D., Xu J., Wang F. Can plasma DNA monitoring be employed in personalized chemotherapy for patients with advanced lung cancer? Biomed Pharmacother. 2012;66:131–137. doi: 10.1016/j.biopha.2011.11.022. 10.1016/j.biopha.2011.11.022 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 25.Gautschi O., Bigosch C., Huegli B., Jermann M., Marx A., Chassé E., Ratschiller D., Weder W., Joerger M., Betticher D.C. Circulating deoxyribonucleic Acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J Clin Oncol. 2004;22:4157–4164. doi: 10.1200/JCO.2004.11.123. 10.1200/JCO.2004.11.123 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
- 26.Li B.T., Drilon A., Johnson M.L., Hsu M., Sima C.S., McGinn C., Sugita H., Kris M.G., Azzoli C.G. A prospective study of total plasma cell-free DNA as a predictive biomarker for response to systemic therapy in patients with advanced non-small-cell lung cancers†. Ann Oncol. 2016;27:154–159. doi: 10.1093/annonc/mdv498. 10.1093/annonc/mdv498 [cited 2016 Feb 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y.J., Yoon K.-A., Han J.-Y., Kim H.T., Yun T., Lee G.K., Kim H.Y., Lee J.S. Circulating cell-free DNA in plasma of never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. Clin Cancer Res. 2011;17:5179–5187. doi: 10.1158/1078-0432.CCR-11-0400. 10.1158/1078-0432.CCR-11-0400 [cited 2016 Feb 2] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curve analysis performed for predicting the accuracy of PET parameters and cfDNA with respect to nonprogression on the CT scan. (A) ROC curve for %ΔTLG, (B) ROC curve for % ΔSULpeak, (C) ROC curve for %ΔSULpeak.
Kaplan-Meier curves for PFS (A and B) and OS (C and D) according to early PET response assessed with SULpeak and TLG. (A) SULpeak assessment; median PFS of patients classified with progression: 2.5 months (95% CI, 0.7-4.4) and nonprogression: 2.7 months (95% CI: 2.5-3.0). (B) TLG assessment; median PFS of patients classified with progression: 2.4 months (95% CI, 1.6-3.3) and nonprogression: 2.7 months (95% CI, 2.5-2.9). (C) SULpeak assessment; median OS of patients classified with progression: 2.3 months (95% CI, 1.5-3.1) and nonprogression: 8.0 months (95% CI, 5.1-11.0). (D) TLG assessment; median OS of patients classified with progression: 3.2 months (95% CI, 0.0-8.5) and nonprogression: 8.3 months (95% CI, 4.8-11.9). Differences between groups were calculated using the log-rank test.
Data on Plasma Glucose Concentration, F-18-FDG Injection, and Time Interval between FDG Injection and Scanning (Uptake Time) for Pretreatment FDG-PET Scans, Follow-Up FDG-PET Scans, and the Variation between Scans in Each Patient
%∆TLG and %∆cfDNA in the 12 Patients Classified with PMD by the TLG Assessment
Supplementary Figure 2.