Abstract
In humans dietary circulating nitrate accumulates rapidly in saliva through active transport in the salivary glands. By this mechanism resulting salivary nitrate concentrations are 10–20 times higher than in plasma. In the oral cavity nitrate is reduced by commensal bacteria to nitrite, which is subsequently swallowed and further metabolized to nitric oxide (NO) and other bioactive nitrogen oxides in blood and tissues. This entero-salivary circulation of nitrate is central in the various NO-like effects observed after ingestion of inorganic nitrate. The very same system has also been the focus of toxicologists studying potential carcinogenic effects of nitrite-dependent nitrosamine formation. Whether active transport of nitrate and accumulation in saliva occurs also in rodents is not entirely clear. Here we measured salivary and plasma levels of nitrate and nitrite in humans, rats and mice after administration of a standardized dose of nitrate.
After oral (humans) or intraperitoneal (rodents) sodium nitrate administration (0.1 mmol/kg), plasma nitrate levels increased markedly reaching ~300 µM in all three species. In humans ingestion of nitrate was followed by a rapid increase in salivary nitrate to >6000 µM, ie 20 times higher than those found in plasma. In contrast, in rats and mice salivary nitrate concentrations never exceeded the levels in plasma. Nitrite levels in saliva and plasma followed a similar pattern, ie marked increases in humans but modest elevations in rodents. In mice there was also no accumulation of nitrate in the salivary glands as measured directly in whole glands obtained after acute administration of nitrate.
This study suggests that in contrast to humans, rats and mice do not actively concentrate circulating nitrate in saliva. These apparent species differences should be taken into consideration when studying the nitrate-nitrite-nitric oxide pathway in rodents, when calculating doses, exploring physiological, therapeutic and toxicological effects and comparing with human data.
Keywords: Nitric oxide, Nitrite, Nitrate, Diet, Microbiome, Salivary gland
Graphical abstract
Highlights
-
•
In humans, dietary nitrate is effectively concentrated in saliva through active transport in the salivary glands.
-
•
In humans salivary nitrate levels are10–20 times higher than in plasma.
-
•
In contrast to humans, rats and mice do not actively concentrate nitrate in saliva.
-
•
These species differences have implcations for translational research.
1. Introduction
The nitrate-nitrite-NO pathway has emerged as an important additional source of NO generation in mammals complementing the classical NO synthase (NOS)-dependent pathway [1], [2]. Inorganic nitrate formed following endogenous oxidation of NOS-derived NO or from exogenous dietary sources can be serially reduced to form nitrite and subsequently NO and a variety of other potentially bioactive nitrogen oxides. Ingestion of inorganic nitrate results in measurable NO-like bioactivity in both humans and animals including reductions in blood pressure [3], [4], inhibition of platelet aggregation [5] as well as improvement of metabolic functions [6], [7]. The fact that nitrate and nitrite can be substrates for generation of NO has challenged the dogma that dietary nitrate has detrimental health effects. Prior to the discovery of the nitrate-nitrite-NO pathway, nitrate was mostly known for being a substrate for the generation of potentially carcinogenic nitrosamines [8]. Interestingly, the route to nitrosamine formation from nitrate is the same as that leading to NO since both require intermediate formation of the more reactive nitrite anion.
A central process in the bioactivation of nitrate in humans is its active uptake from blood by the salivary glands and excretion in saliva. In the oral cavity commensal bacteria reduce salivary nitrate to the more reactive nitrite anion [9]. Nitrite is then swallowed and can form a number of nitrogen oxides locally in the acidic gastric environment and systemically in blood and tissues after absorption [1]. In humans the degree of nitrate accumulation in saliva is remarkable and it is believed that 25% of all circulating nitrate is taken up by the salivary glands with resulting salivary levels that are up to 20 times higher than in plasma [10], [11], [12]. The requirement of salivary accumulation of nitrate and reduction to nitrite for the nitrate-nitrite-NO pathway to operate is clearly illustrated in experiments where the test persons either avoids swallowing saliva during a period following nitrate ingestion [5], [11] or uses an antibacterial mouthwash to prevent bacterial nitrate reduction [12]. In both cases the physiological effects of nitrate are abolished.
While most of the favorable effects of dietary nitrate observed in humans occur also in rodents, the required doses to observe similar effects in rodents are typically considerably higher [13]. There have been suggestions that rodents do not concentrate nitrate in saliva [14], [15], [16] but direct measurements and comparisons with humans are currently missing. In this study we set out to directly compare salivary nitrate concentration in humans, rats and mice by administering a standardized amount of sodium nitrate followed by repeated blood- and saliva samples over time.
2. Material and methods
The human part of the study was approved by the Karolinska Institute regional ethics committee in Stockholm, Sweden. The experimental protocols were approved by Institutional Animal Care and Use Committee (IACUC) at the Karolinska Institute in Stockholm, Sweden, and performed according to the National Institutes of Health guidelines for the conduct of experiments in animals.
2.1. Study participants and experimental animals
Five healthy volunteers aged 27–57 years (3 females) participated in the study. The subjects arrived in the lab in the morning after an overnight fast and after having been instructed to avoid nitrate-rich food the preceding day. A catheter was placed in the antecubital vein for sampling. After collection of basal blood and saliva samples, sodium nitrate (0.1 mmol/kg dissolved in 10 mL of drinking water) was ingested and blood and saliva were collected repeatedly during 60 min.
Rats (Sprague Dawley) weighting 280–500 g were anesthetized using Inactin (60 mg/kg) and ketamine (60 mg/kg) and tracheostomy was carefully performed to avoid any damage to the submandibular glands. A catheter (PE50) was inserted in the femoral vein for blood collection. After this, a low dose of pilocarpine (64 µg/kg) was intraperitoneally administrated to induce a low flow of saliva sufficient to allow for collection using a capillary tube. After baseline collection of saliva and blood, sodium nitrate was administrated intraperitoneally (0.1 mmol/kg) and saliva and blood were collected at 15, 30, 45 and, 60 min. In mouse experiments, animals (C57Bl/J6) were anesthetized using ketamine (100 mg/kg) and then pilocarpine (64 µg/kg) was administrated intraperitoneally. Saliva and blood were collected before (baseline) and 60 min after an intraperitoneal injection of sodium nitrate (0.1 mmol/kg). In another set of experiments, 6 mice received a single gastric gavage of sodium nitrate (0.1 mmol/kg). After 60 min the mice were anesthetized and blood was collected along with excision and rapid removal of the sublingual and parotid salivary glands. No pilocarpine was used in these experiments.
2.2. Sample preparation
Blood samples were immediately centrifuged at +4 °C (4700×g, 5 min) and plasma was stored at −80 °C until analyses. The plasma samples were extracted using HPLC grade methanol (CROMASOLV, Sigma-Aldrich) and the methanol was pre chilled in the freezer using a glass bottle to avoid nitrite/nitrate contamination from different plastic materials. In brief, 100 µL methanol was added to 100 µL plasma in 1.5 mL Eppendorf tubes tested for nitrite/nitrate contamination. The tubes were vortexed and then centrifuged for 10 min 4 °C 10,000g.
The saliva samples were centrifuged for 10 min at 4 °C and 10,000g before dilution with carrier solution containing 10% HPLC grade methanol.
The salivary glands removed from the animals were weighted, cut in smaller pieces and immediately placed in ice-cold methanol 1 mL/g tissue. The tissue was then homogenized using a bullet blender with Zirccox beads. After homogenization, the samples were centrifuged for 10 min at 4 °C 10,000g. The supernatant was then transferred to another tube and stored overnight at −20 °C.
2.3. Nitrate and nitrite assessment
A HPLC system dedicated to assessment of nitrite and nitrate (ENO-20; EiCom, Japan) and attached to an auto-sampler (840, EiCom, Kyoto, Japan) was used. The method is sensitive and specific to nitrite and nitrate, and is based on the separation of nitrate by reverse-phase/ion exchange chromatography, followed by inline reduction of nitrate to nitrite with cadmium and reduced copper. Derivatization of reduced nitrate was performed with Griess reagent, and the level of diazo compounds was measured at 540 nm. The reactor solution was freshly prepared before analysis. A standard curve was prepared from sodium nitrite and sodium nitrate, diluted with carrier solution in the range of 0.1–20 µM. Aliquots of standards were stored at −20 °C. The slope was examined and used to calculate the concentration in the samples.
Immediately before running the samples in the HPLC, 100 µL of the samples were transferred to a 96 well plate with conical wells (Costar, nitrate- and nitrite-free). To avoid evaporation and also contamination from the auto sampler that contains a lot of electrical components releasing NO to the surrounding air, we sealed the plate with a film that was sterile and easy for the auto sampler needle to penetrate. The samples were kept at 4 °C by a cooling device in the auto sampler. The data collected and analyzed using the PowerChrom software (V 2.7.9, eDAQ).
2.4. Drugs and solutions
All drugs and reagents used were purchased from Sigma Chemical Co. (St Louis, MO, USA). All drugs were dissolved in saline solution immediately before use.
2.5. Statistical analysis
The results are expressed as means±S.E.M. The comparisons between groups were assessed by t tests, two-way analysis of variance using Bonferroni correction or one-way analysis of variance followed by Dunnett's multiple comparison tests. A p-value<0.05 was considered significant.
3. Results
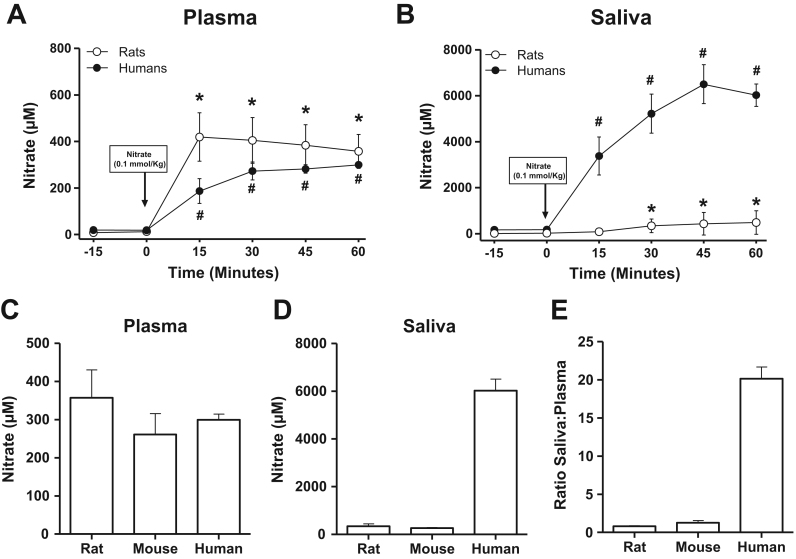
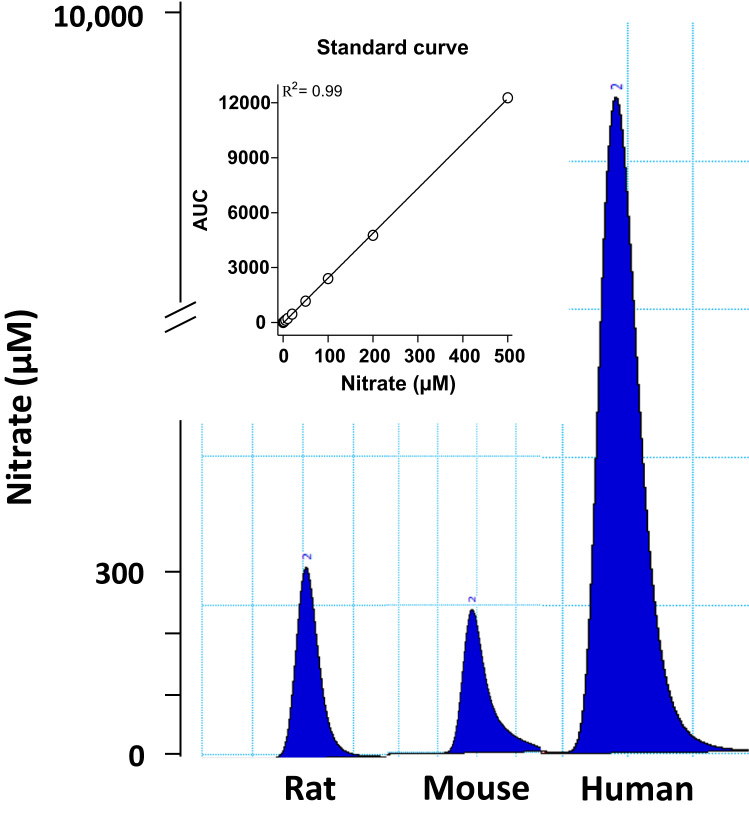
Plasma and salivary levels of nitrate after administration of nitrate are shown in Fig. 1. In humans, plasma and salivary nitrate increased as expected after ingestion of sodium nitrate. At 60 min, plasma nitrate was 299±14 µM whereas the levels in saliva had reached 6022±485 µM, giving a ratio saliva to plasma of around 20:1 (Fig. 1E). In rats and mice injected intraperitoneally with nitrate, plasma nitrate also greatly increased during the observation period. At 60 min, plasma nitrate levels had increased to 357±72 µM in rats and 261±54 µM in mice (Fig. 1C) and were similar to those found in saliva (343±91 and 260±29 for rats and mice, respectively, Fig. 1D). Thus, in rats and mice, the ratio saliva:plasma for nitrate was close to 1:1 (Fig. 1E). Representative HPLC tracings of nitrate levels in saliva samples from the three species studied are shown in Fig. 2.
Fig. 1.
Effects of nitrate administration on levels on nitrate in plasma and saliva of humans, rats and mice. Nitrate was measured repeatedly in plasma (A)and saliva (B)of humans and rats after administration of 0.1 mmol/kg sodium nitrate orally (humans, n=5) or intraperitoneally (rats, n=12). Comparison of nitrate levels in plasma (C) and saliva (D) and ratio saliva:plasma (E) in rats, mice (n=6) and humans measured 60 min after administration of nitrate. # p<0.01 and * p<0.05. vs. baseline (0 min) within the same group by using repeated measures ANOVA and Dunnett's multiple comparison test. Data are shown as mean±S.E.M.
Fig. 2.
Representative HPLC tracings showing levels of nitrate measured in saliva collected after administration of sodium nitrate 0.1 mmol/kg.
The levels of nitrite in plasma and saliva increased markedly in humans after administration of nitrate (Table 1). This increase occurred also in rodents but to a much lesser extent.
Table 1.
Nitrite levels (μM) measured in saliva and plasma before and 60 min after administration of sodium nitrate (0.1 mmol/kg).
| Humans (n=5) | Rats (n=12) | Mice (n=6) | ||
|---|---|---|---|---|
| Saliva | Basal | 63 (38) | 0.654 (0.12) | 1.52 (0.51) |
| 60 min | 2023 (495)* | 14.28 (4.18)* | 5.04 (2.33) | |
| Plasma | Basal | 0.220 (0.04) | 0.245 (0.02) | 0.265 (0.05) |
| 60 min | 0.716 (0.15)* | 0.461 (0.10)* | 0.337 (0.04) | |
Data are shown as mean±(S.E.M.)
p<0.05. vs. baseline (0 min)
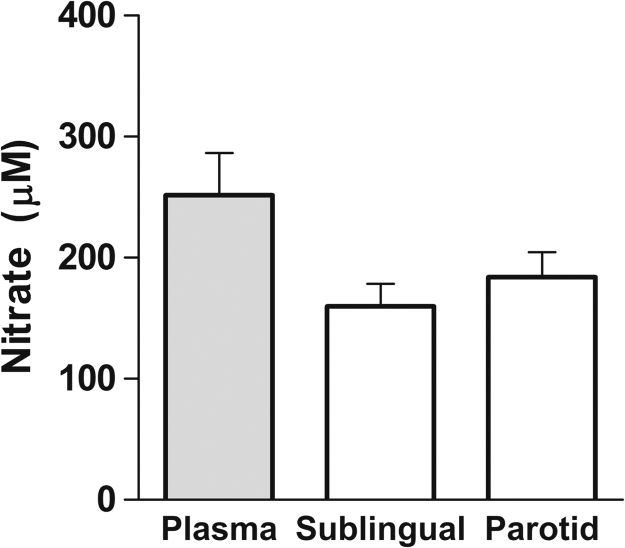
In whole salivary glands obtained from anesthetized mice having received nitrate 60 min earlier, the levels of nitrate were no different from those seen in plasma collected at the same time point (Fig. 3).
Fig. 3.
Levels of nitrate measured in plasma and whole salivary glands of mice 60 min after administration of sodium nitrate (0.1 mmol/kg). Differences between groups (n=6) were not significant.
4. Comment
The results presented here demonstrate that rodents, in contrast to humans, do not effectively concentrate nitrate in saliva. As shown in humans here and earlier, nitrate rapidly accumulates in saliva after ingestion giving concentrations >20 times higher than those in plasma. This is apparently a very powerful system allowing much nitrate to be reduced to nitrite by oral bacteria, eventually giving rise to a number of potentially bioactive nitrogen oxides [17].
If mice and rats do not effectively concentrate nitrate in saliva, why then is dietary nitrate still bioactive also in these species? There are several possible explanations. First, the passive diffusion of nitrate from blood to saliva indicated here might be sufficient to enable enough nitrite formation by oral commensal bacteria. If true this may then explain why higher doses of nitrate are typically needed in rodent studies to achieve a physiological NO-like effect. Second, rodents may have compensated the ineffective nitrate transport and subsequent oral microbial nitrate reduction by instead upregulating mammalian systems to reduce nitrate to nitrite. Indeed, xanthine oxidase is capable of this and is responsible for the increase in systemic nitrite seen in germ-free mice after administration of nitrate [18], [19]. Moreover, many of the described favorable effects of nitrate (in vivo) or nitrite (in vitro) are abolished by inhibition of XOR in rodents [20], [21], [22], [23], [24]. Third, rodents have been reported to secrete nitrate in the upper gastrointestinal tract [25], which apparently does not happen in humans [12]. This nitrate could undergo similar metabolism by bacteria residing at these locations, thereby enhancing the bioactivation of nitrate through its initial reduction to nitrite.
A puzzling fact is that the effects of dietary nitrate in rodents seem to be effectively blocked by local use of an antibacterial mouthwash [13], [26] like they are in humans [12]. This indeed suggests that some enterosalivary circulation of nitrate occurs also in mice and rats and indicates that the lower salivary nitrate and nitrite levels obtained even without active concentration might be sufficient to elicit effects. This is supported by a recent study in which minute amounts of nitrite (resembling levels found in fasting humans saliva) added to drinking water of germ-free mice resulted in a robust physiological response manifested as an increase in gastric mucus formation [27].
The rise in salivary nitrate after administration of nitrate was followed by an impressive increase also in salivary nitrite in humans. This was expected from previous studies and is a consequence of an effective bacterial nitrate reductase activity [10], [11], [12]. Salivary nitrite increased also in rodents but to a much lesser degree. There are likely several reasons for this. First, salivary nitrate accumulation was much lower in rodents. Secondly, nitrate was exposed to oral bacteria during a shorter time in rodents due to the method of collection and pharmacological stimulation of salivary flow. In addition to this, the oral microbiome in rodents may differ from humans in its nitrate reducing capacity although this was not specifically studied here. Plasma levels of nitrite followed the same trends in the three species studied, with greater increases seen in humans. In aggregate, it seems as if the enterosalivary recirculation of nitrate and resulting bioactivation to form the more reactive nitrite anion is considerably more effective in humans compared to rodents.
Besides being of interest to researchers looking at beneficial cardiovascular effects of dietary nitrate, this study may also be relevant to toxicologists looking into the potential harmful effects of nitrate including the formation of nitrosamines [8], [10]. Indeed, the resulting accumulation of nitrite in saliva after a nitrate load is much greater in humans compared to rodents and thereby also the nitrosating capacity.
There are some limitations in this study that deserves to be mentioned. It is difficult to obtain saliva from rodents and hence salivary flow was stimulated with the use of pilocarpine. In humans this is not needed and no pilocarpine was used. One might then argue that the pilocarpine-induced salivary flow is so high that effective concentration of nitrate becomes impossible. While this is most certainly true in the sense that higher flow rates will lead to lower absolute nitrate concentrations, it is still unlikely that the dilution factor would completely mask the concentration ability in rodents. In an effort to minimize this dilution factor we used a low dose of pilocarpine, just enough to enable collection of saliva using capillary tubes. Thus, if active nitrate concentration had occurred in rodents the ratio saliva:plasma would likely not have been as low as 1:1. Indeed, in rabbits that also concentrate nitrate in saliva [28], a high ratio saliva:plasma is existing even though the animals were treated with a high dose of pilocarpine. Another potential confounder is the use of anesthetics in the rodents, which theoretically may affect the function of the salivary glands. However, in earlier studies looking at how iodine is concentrated in rat saliva (the mechanisms for iodine transport in the salivary glands is the same as for nitrate) anesthesia did not influence the capacity to concentrate the anion [15].
In conclusion: The results from the present study support the notion that in contrast to humans and some other mammals, rats and mice do not actively concentrate nitrate in saliva.
Sources of funding
This study was supported by grants from the William Harvey Research Institute Academy (project 608765) and the Swedish Heart and Lung Foundation (project 20140673).
Conflict of interest
Drs Lundberg and Weitzberg are co-inventors on patent applications relating to the medical uses of nitrate- and nitrite salts.
Acknowledgements
We thank Anette Ebberyd, Annika Olsson, and Margareta Stensdotter (Dept. of Physiology and Pharmacology, Karolinska Institutet) for their excellent technical contribution.
References
- 1.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 2.van Faassen E.E. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 4.Kapil V., Khambata R.S., Robertson A., Caulfield M.J., Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb A.J. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen F.J. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabolism. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Larsen F.J., Weitzberg E., Lundberg J.O., Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 8.Tannenbaum S.R., Correa P. Nitrate and gastric cancer risks. Nature. 1985;317:675–676. doi: 10.1038/317675b0. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg J.O., Weitzberg E., Cole J.A., Benjamin N. Opinion–nitrate, bacteria and human health. Nature Reviews Microbiology. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 10.Spiegelhalder B., Eisenbrand G., Preussman R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivoformation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg J.O., Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Govoni M., Jansson E.A., Weitzberg E., Lundberg J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Petersson J. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Larauche M. Protective effect of dietary nitrate on experimental gastritis in rats. Br J Nutr. 2003;89:777–786. doi: 10.1079/BJN2003845. [DOI] [PubMed] [Google Scholar]
- 15.Cohen B., Myant N.B. Concentration of salivary iodide: a comparative study. J Physiol. 1959;145:595–610. doi: 10.1113/jphysiol.1959.sp006165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vittozzi L. Toxicology of nitrates and nitrites. Food Addit Contam. 1992;9:579–585. doi: 10.1080/02652039209374111. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg J.O., Gladwin M.T., Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 18.Jansson E.A. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4(7):411. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 19.Huang L.Y., Borniquel S., Lundberg J.O. Enhanced xanthine oxidoreductase expression and tissue nitrate reduction in germ free mice. Nitric Oxide-Biology and Chemistry. 2010;22:191–195. doi: 10.1016/j.niox.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Carlstrom M. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2011;89:574–585. doi: 10.1093/cvr/cvq366. [DOI] [PubMed] [Google Scholar]
- 21.Gao X. NADPH oxidase in the renal microvasculature is a primary target for blood pressure-lowering effects by inorganic nitrate and nitrite. Hypertension. 2015;65:161–170. doi: 10.1161/HYPERTENSIONAHA.114.04222. [DOI] [PubMed] [Google Scholar]
- 22.Liu M. Nitrite-mediated renal vasodilatation is increased during ischemic conditions via cGMP-independent signaling. Free Radical Biology and Medicine. 2015;84:154–160. doi: 10.1016/j.freeradbiomed.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Yang T. Inorganic nitrite attenuates NADPH oxidase-derived superoxide generation in activated macrophages via a nitric oxide-dependent mechanism. Free Radical Biology and Medicine. 2015;83:159–166. doi: 10.1016/j.freeradbiomed.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Zuckerbraun B.S. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 25.Witter J.P., Balish E. Distribution and metabolism of ingested NO3- and NO2- in germfree and conventional-flora rats. Appl. Environ Microbiol. 1979;38:861–869. doi: 10.1128/aem.38.5.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendgen-Cotta U.B. Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation. 2012;126 doi: 10.1161/CIRCULATIONAHA.112.112912. 1983-+ [DOI] [PubMed] [Google Scholar]
- 27.Petersson J. Physiological recycling of endogenous nitrate by oral bacteria regulates gastric mucus thickness. Free Radical Biology and Medicine. 2015;89:241–247. doi: 10.1016/j.freeradbiomed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Djekoun-Bensoltane S., Kammerer M., Larhantec M., Pilet N., Thorin C. Nitrate and nitrite concentrations in rabbit saliva Comparison with rat saliva. Environ Toxicol Pharmacol. 2007;23:132–134. doi: 10.1016/j.etap.2006.07.007. [DOI] [PubMed] [Google Scholar]