Abstract
Background
Recent research has clarified the relationship between adipokines, metabolic syndrome (MS) and cardiovascular disease (CVD). The results of animal and clinical studies have revealed a positive relationship between leptin and vascular smooth muscle cell counts and calcification, arterial rigidity, carotid thickness and the incidence of CVD. However, despite leptin fulfilling the definition of a uremic toxin, its exact role in chronic kidney disease (CKD) has yet to be determined.
Methods
One hundred and forty-two CKD patients (stages 2–5D) participated in this study, and were followed for a minimum of 20 months at Amiens University Medical Center.
Results
Leptin was negatively correlated with the glomerular filtration rate (GFR), total adiponectin (TAdip) and high-molecular weight adiponectin and positively correlated with age, waist circumference, body mass index (BMI), aortic calcification score (ACS), C-reactive protein (CRP), triglycerides, insulin and parathormone (PTH). Leptin and insulin were significantly correlated with the MS score. The BMI, insulin, MS score and PTH were independent predictors of leptin levels (P = 0.002, 0.016, 0.028 and 0.017, respectively). Leptin, insulin and TAdip were independent predictors of the presence of MS (P = 0.05, 0.04 and 0.04). However, leptin levels were not significantly predictive of the clinical outcomes.
Conclusions
Our study was the first to demonstrate a significant, independent link between leptin and MS (but not clinical outcomes) and PTH in patients at different CKD stages. Future studies will have to assess the involvement of leptin in MS and clinical outcomes in CKD, and the potential modulation of leptin by PTH.
Keywords: adiponectin, chronic kidney disease, leptin, metabolic syndrome, parathormone
Introduction
Over the last two decades, the introduction of technological and medical innovations in the treatment of chronic kidney disease (CKD) has failed to translate into markedly longer patient survival times. Indeed, mortality due to cardiovascular disease (CVD) remains unacceptably high in CKD patients and so minimizing this parameter is one of the nephrologist's greatest challenges.
This context is especially problematic because CKD per se is a cause of cardiovascular morbidity and mortality [1]. Given that classical risk factors can only partially explain the high rates of CVD mortality observed in CKD populations, researchers have been prompted to study particular factors in the uremic environment (such as uremic toxins) that may interact negatively with biological functions [2]. To this end, the role of leptin in the context of CKD has been studied extensively.
At the cellular level, leptin raises alkaline phosphatase activity and stimulates the proliferation, migration and calcification of vascular smooth muscle cells [3, 4]. For the most part, clinical studies have found a positive correlation between leptin and cardiovascular parameters (such as an increase in arterial rigidity [5] or CVD [6, 7], whereas others have not [8, 9]). However, these studies did not specifically involve CKD patients.
In fact, leptin's role in CKD is complex. Given that leptin fulfills the definition of a uremic toxin [2], one can reasonably speculate about the hormone's influence on the clinical outcomes of CKD patients. Hence, in view of the known relationship between high leptin levels and CVD in other contexts, reducing blood levels (e.g. by haemodiafiltration) in CKD might have a beneficial impact on the clinical outcomes.
In contrast, Scholze et al. [10] found that a low leptin level was an independent predictor of mortality in haemodialysis patients; this may mean that high leptin levels are associated with better outcomes. Similarly, a study by Kalantar-Zadeh et al. [11] suggested that being obese (having more leptin, in other words) is a survival advantage in haemodialysis patients. ‘Reverse epidemiology’ has been invoked as an explanation for these counterintuitive observations, on the basis that risk factors in the general population may be protective factors in particular patient groups, such as the haemodialysis population.
However, a number of studies have failed to support this ‘reverse epidemiology’. In Dutch study of haemodialysis patients, the researchers did not find any survival advantage in obese subjects [12]. It is noteworthy that neither the North American study nor the Dutch cohort study took account of leptin levels or included patients at earlier CKD stages [11, 12].
In addition to the unresolved issue of leptin and clinical outcomes in CKD patients on dialysis, data on leptin's role in early stages of CKD and its correlation with metabolic syndrome (MS) are scarce. Some researchers have suggested that leptin is an independent component of MS [13, 14] because of the hormone's relationship with insulin resistance and waist circumference [15]. However, there is presently a lack of consensus on this question.
Hence, the objective of the present study was to investigate putative links between leptin, MS and clinical outcomes by evaluating biochemical and clinical parameters (including vascular calcification scores, inflammatory and bone markers and overall and cardiovascular mortality) in a cohort of stage 2–5D CKD patients.
Subjects and methods
Patient selection
Over an 18-month period (from January 2006 to June 2007), 150 Caucasian CKD patients were recruited from the Nephrology Department's outpatient clinic at Amiens University Medical Center. All patients gave their informed, written consent to participation. The study was approved by the local Institutional Review Board and performed in accordance with the precepts of the Declaration of Helsinki.
Included patients had to be over the age of 40, with a confirmed diagnosis of CKD (defined as being on haemodialysis or having two previous, estimated creatinine clearance values <90 mL/min/1.73 m2, as calculated according to the Cockcroft–Gault formula and with an interval of 3–6 months). Stage 5D-CKD patients had been on chronic haemodialysis (three times a week, >3 months). The exclusion criteria were the presence of chronic inflammatory disease, atrial fibrillation, complete heart block, abdominal aorta aneurysm, an aortic and/or femoral artery prosthesis, primary hyperparathyroidism, kidney transplantation and an acute cardiovascular event in the 3 months prior to screening for inclusion. The 142 patients who met all the inclusion criteria and had available data for plasma leptin, total adiponectin (TAdip), high-molecular weight adiponectin (HMWAdip) and insulin were included in this analysis. In order to compare the plasma leptin levels values from this cohort with the reference range, we used a control group with apparently normal healthy subjects (n = 10).
Study protocol
All patients were day-hospitalized in order to perform laboratory blood tests, blood pressure measurements, pulse wave velocity determination, a lateral lumbar X-ray and multislice spiral computed tomography (ms-CT). For a given patient, all examinations were performed between 9:00 am and 2:00 pm on the same day. Haemodialysis patients were seen on a dialysis-free day or in the morning before the dialysis session. A patient's interview was conducted focusing on comorbidities and disease history, both in general and previous vascular events. The patients’ medical files were reviewed in order to identify and record any concomitant medications. For descriptive purposes, patients who reported current use of insulin and/or orally administered hypoglycaemic drugs were considered to be diabetics. Previous CVD was defined as a history of any of the following events: myocardial infarction, stroke, heart failure, angina pectoris or surgical procedures for angina or coronary/peripheral artery disease (including percutaneous-transluminal angioplasty).
The MS score was calculated in accordance with the Executive Summary of the Third Report of the National Cholesterol Education Program [16]. One point was attributed to each of the following variables: waist circumference (men >102 cm; women >88 cm), triglycerides (≥1.5 g/L), HDL cholesterol (men < 0.4 g/L; women < 0.5 g/L) and blood pressure (≥130 × 85 mmHg or antihypertensive agents use). For the last scoring point, we used the diagnoses of diabetes mellitus [according to the World Health Organization (WHO) criteria] [17], instead of the fasting glucose level, to get homogeneous criteria since not all patients had their fasting glucose levels available (particularly patients on haemodialysis). Lastly, we considered malnourished-inflamed (MI) patients to be those with plasma albumin levels <35 g/L and plasma C-reactive protein (CRP) levels >2.0 mg/dL.
Laboratory tests
Blood samples were collected in the morning, before any other investigations were undertaken. Selected assays were performed after the samples had been frozen and stored at −80°C. Serum calcium, phosphate, albumin, cholesterol, haemoglobin, creatinine (Scr) and CRP levels were assayed in an on-site biochemistry laboratory using standard auto-analyzer techniques (Modular IIP® system, Roche Diagnostics, Basel, Switzerland). Serum intact 1-84-parathormone (PTH) was determined in a chemiluminometric immunoassay (Liaison N-tact PTH CLIA®, Diasorin, Stillwater). The plasma concentrations of leptin, TAdip, HMWAdip and insulin were determined in the same laboratory by using an ELISA. The leptin and the insulin assay were manufactured by DRG Diagnostics (Marburg, Germany), whereas the adiponectin kit was supplied by ALPCO Diagnostics (Salem). The laboratory's reference values in apparently healthy individuals were 3.84 ± 1.79 ng/mL (males)/7.36 ± 3.73 ng/mL (females) for leptin; 4.30 ± 1.76 µg/mL (males)/6.62 ± 3.04 µg/mL (females) for TAdip; 1.62 ± 1.02 µg/mL (males)/3.24 ± 2.13 µg/mL (females) for HMWAdip and 2–25 µIU/mL for insulin (calibrated against the WHO approved reference material NIBSC-66/304).
Serum cystatin C (CysC) levels were determined by immunonephelometry (N Latex Cystatin C®, Dade Behring, Marburg, Germany). In order to describe the true glomerular filtration rate (GFR), the estimated GFR combining Scr and CysC measurements (‘CKD-epi’) was calculated for all non-dialyzed patients according to the following, recently published equation: 177.6 × Scr−0.65× CysC−0.57× age−0.20× (0.82, if female) [18]. For descriptive purposes, patients were then classified into CKD stages according to the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines [19].
Pulse wave velocity evaluation
The carotid–femoral PWV was determined automatically with a dedicated, validated device (Complior Colson, Createch Industrie, Massy, France), as previously described [20]. The PWV was evaluated with two pressure probes by a trained physician. Simultaneously recorded pulse waveforms were obtained transcutaneously over the common carotid artery and the femoral artery in the groin. The PWV was calculated as the distance between recording sites measured over the body's surface (L), divided by the time interval (t) between the feet of the flow waves (PWV = L/t); this result was averaged over 10 cardiac cycles [21]. This method has been validated previously and has an intraobserver repeatability coefficient of 0.93 and an interobserver reproducibility coefficient of 0.89 [20, 21].
Abdominal aorta imaging with plain radiography
A technique similar to that described by Kauppila et al. [22] was used to obtain images of the lower abdominal aorta and generate an aortic calcification score (ACS). All X-rays were reviewed by two independent investigators and a consensus on the interpretation was reached in all cases.
Multislice spiral computed tomography
In order to quantify the presence and extent of aortic calcifications, each patient underwent an ms-CT scan. All examinations were performed with a 64-detector CT scanner (Lightspeed VCT®, GE Healthcare, Milwaukee, WI). The volume acquisition started at the aortic hiatus of the diaphragm and ended at the third lumbar vertebra. The scanning parameters were as follows: collimation, 64 × 0.625 mm; slice thickness, 0.625 mm; pitch, 1; gantry rotation speed, 0.5 s/rotation; tube voltage, 120 kV; tube current, 300 mA.
The volume acquisition was analysed with commercially available software (Volume Viewer®, GE Healthcare). The abdominal aorta was segmented manually. In order to reduce errors due to noise, a threshold of 160 UH was applied. The total calcification volume was calculated as the sum of all voxels in the remaining volume. The abdominal aorta calcification score was calculated as follows: [(total calcification volume)/(aorta wall surface area) × 100)].
Bone density was measured halfway through the twelfth thoracic vertebra. The CT image analysis was based on the conversion of the bone tissue attenuation (measured in Hounsfield units) into grams of hydroxyapatite per cubic centimeter of bone [23]. In the absence of a mineral reference phantom in the present study, we measured trabecular bone attenuation—the best surrogate marker of density we could provide and the core measurement that all CT programs use to determine bone mineral density. Bone attenuation was assessed by an experienced radiologist from Amiens University Medical Center.
Survival
Death records were established prospectively by considering all patients included at least 20 months before the study end date (i.e. before 1 January 2010). Each medical chart was reviewed and the cause of death was assigned by a physician on the basis of all the available clinical information. For out-of-hospital deaths, the patient's family doctor was interviewed to obtain pertinent information on the cause. Cardiovascular mortality was defined as any death directly related to cardiovascular system dysfunction (stroke, myocardial infarction, congestive heart failure or sudden death). Cardiovascular events were defined as cardiovascular death or any adverse event directly related to cardiovascular system dysfunction (stroke, angina pectoris/myocardial infarction, congestive cardiac failure, peripheral ischaemia or new-onset arrhythmia) or surgical procedures for angina or coronary/peripheral artery disease.
Statistical analysis
Data were expressed as the mean ± SD, median and range or frequency, as appropriate. For descriptive and analytical purposes, patients were stratified according to the median serum leptin level for the entire cohort (serum leptin < 11.42 ng/mL versus serum leptin ≥11.42 ng/mL). Intergroup comparisons were performed using a χ2 test for categorical variables and Student's t-test or a Mann–Whitney test for continuous variables. Pearson's correlation coefficient or Spearman's rank correlation was used to assess the relationships between serum leptin levels (log normalized) and selected clinical or biochemical variables. For variables with a non-Gaussian distribution, log-normalized values were considered in tests that required normally distributed variables. A multivariate stepwise linear regression analysis was performed to identify variables that were independently correlated with serum leptin levels. Variables selected in a univariate regression [age, body mass index (BMI), triglycerides, CRP, insulin, ACS, TAdip, CKD stage, PTH and MS score] were included in a multivariate model. Variables related to MS in a univariate regression but not used to calculate the MS score (haemoglobin, interleukin-6, leptin, insulin, albumin, total adiponectin and CKD stage) were included in the multivariate model to determine independent parameters associated with MS. A Kaplan–Meier actuarial curve was used to estimate overall and cardiovascular mortality as a function of the median leptin level and the MS score (ranging from 0 to 4) for the study population. The log-rank test was used to test intercurve differences. The threshold for statistical significance was set to P < 0.05. All statistical analyses were performed using SPSS software (version 13.0, SPSS Inc., Chicago).
Results
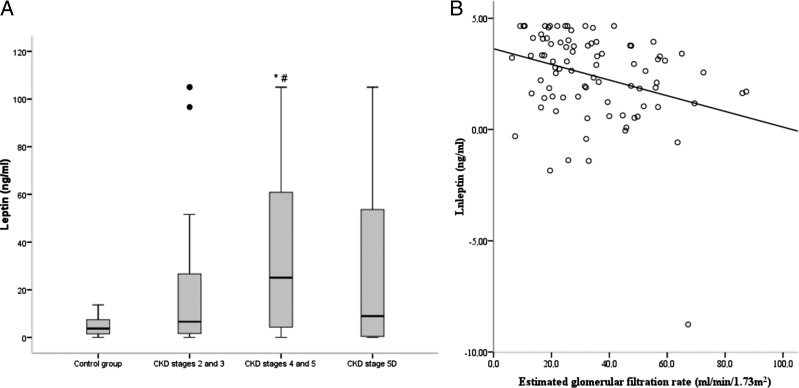
In comparison with control data from healthy subjects, the CKD population had higher mean plasma leptin levels. When the analysis was restricted to pre-dialysis patients, we observed a significant, inverse correlation between leptin levels and the GFR (Figure 1).
Fig. 1.
(A) Serum leptin levels as a function of the CKD stage (*#P < 0.05 when comparing CKD stages 2 and 3 with stages 4 and 5, and when comparing the control group with CKD stages 4 and 5). (B) The linear relationship between serum leptin levels and the estimated GFR, for CKD stages 2–5 (n = 96, r2 = −0.304, P = 0.003). Control group with apparently normal healthy subjects (n = 10).
Tables 1 and 2 show the baseline characteristics of 142 patients (mean age = 67 ± 12) as a function of the median leptin level (11.42 ng/mL). Patients with leptin levels ≥11.42 ng/mL were more likely to be at a later CKD stage, to be female and to have a higher waist circumference, BMI, ACS and higher insulin, CRP, total and low density lipoprotein (LDL) cholesterol, triglycerides and PTH levels but lower TAdip levels.
Table 1.
Clinical and demographic characteristics of the study populationa
| All (n = 142) | Leptin |
P | ||
|---|---|---|---|---|
| <11.42 ng/mL (n = 71) | ≥11.42 ng/mL (n = 71) | |||
| Age, years | 67 ± 12 | 65 ± 13 | 69 ± 12 | 0.06 |
| Male gender, n (%) | 86 (60) | 56 (79) | 30 (42) | 0.001 |
| BMI(kg/m2) | 28 ± 6 | 25 ± 5 | 31 ± 6 | 0.001 |
| Waist circumference (cm) | 100 ± 15 | 93 ± 13 | 106 ± 14 | 0.001 |
| Presence of CVD, n (%) | 45 (32) | 20 (28) | 25 (35) | 0.471 |
| ACS on CT (%) | 3.0 ± 3.0 (1.85) | 2.5 ± 2.6 (1.58) | 3.5 ± 3.3 (2.32) | 0.111 |
| Bone mineral density (HU) | 130.4 ± 48.2 | 132 ± 47 | 128 ± 50 | 0.448 |
| Smoking habit, n (%) | 17 (12) | 14 (20) | 3 (4) | 0.004 |
| Diabetes mellitus, n (%) | 60 (42) | 26 (37) | 34 (48) | 0.234 |
| Systolic arterial pressure (mmHg) | 153 ± 26 | 150 ± 25 | 156 ± 27 | 0.226 |
| Diastolic arterial pressure (mmHg) | 81 ± 12 | 81 ± 13 | 81 ± 12 | 0.948 |
| CKD stage, n (%) | 0.004 | |||
| 2 | 12 (8) | 10 (14) | 2 (3) | |
| 3 | 37 (26) | 20 (28) | 17 (24) | |
| 4 | 37 (26) | 13 (18) | 24 (34) | |
| 5 | 10 (7) | 4 (6) | 6 (8) | |
| 5D | 46 (32) | 24 (34) | 22 (31) | |
aData are expressed as the mean ± SD or, for binary variables, the number (frequency). Between-group comparisons were made using the non-parametric Mann–Whitney test for continuous variables and Fisher's exact test for categorical variables.
ACS, Aortic calcification score; BMI, body mass index; CVD, cardiovascular disease; CKD, chronic kidney disease; CT, computed tomography; HU, Hounsfield units.
Table 2.
Biochemical characteristics of the study populationa
| All (n = 142) | Leptin | P-value | ||
|---|---|---|---|---|
| <11.42 ng/mL (n = 71) | ≥11.42 ng/mL (n = 71) | |||
| GFRb, mL/min/1.73 m2 | 35 ± 19 | 40.42 ± 20.71 | 30.05 ± 15.76 | 0.016 |
| Leptin, ng/mL | 29.58 ± 36.1 (11.42) | 2.79 ± 2.98 (1.82) | 56.38 ± 34.15 (43.57) | NA |
| Total adiponectin, µg/mL | 10.56 ± 5.91 | 11.57 ± 6.42 | 9.38 ± 5.14 | 0.031 |
| HMW adiponectin, µg/mL | 5.50 ± 4.24 | 6.29 ± 4.81 | 4.72 ± 3.46 | 0.027 |
| Insulin, µU/mL | 29.3 ± 23.5 | 20.6 ± 17.8 | 38.1 ± 25.3 | 0.001 |
| Triglycerides, mMol/L | 2.03 ± 1.3 | 1.57 ± 0.8 | 2.46 ± 1.5 | <0.0001 |
| Total cholesterol, mMol/L | 4.9 ± 1.1 | 4.60 ± 1.15 | 5.12 ± 1.11 | 0.009 |
| LDL cholesterol, mMol/L | 2.62 ± 0.9 | 2.46 ± 0.8 | 2.78 ± 0.9 | 0.04 |
| CRP, mg/L | 11.2 ± 24 (3.5) | 9.5 ± 19.3 (2.12) | 12.9 ± 27.8 (4.26) | 0.01 |
| Interleukin-6, pg/mL | 5.25 ± 7.9 | 4.18 ± 4.21 | 6.24 ± 10.11 | 0.183 |
| Calcium, mMol/L | 2.29 ± 0.18 | 2.26 ± 0.19 | 2.31 ± 0.17 | 0.101 |
| Phosphate, mMol/L | 1.28 ± 0.46 | 1.33 ± 0.53 | 1.24 ± 0.37 | 0.212 |
| Intact PTH, pg/mL | 137 ± 137 (85) | 116 ± 138 (78) | 159 ± 135 (121) | 0.01 |
| Albumin, g/L | 37.4 ± 6.4 | 36.9 ± 7.2 | 37.9 ± 5.5 | 0.506 |
| Haemoglobin, g/dL | 12.1 ± 1.7 | 12.24 ± 1.81 | 11.94 ± 1.66 | 0.48 |
aData are expressed as the mean ± SD and, for variables with a non-Gaussian distribution, the median.
PTH, parathyroid hormone; CRP, C-reactive protein; GFR, glomerular filtration rate; HMW, high-molecular weight; LDL, low density lipoprotein.
bCalculated for patients at CKD stages 2–5 (n = 96).
We found that leptin was positively correlated with age (r² = 0.206, P = 0.014), waist circumference (r2 = 0.547, P = 0.001), BMI (r2 = 0.651, P = 0.001), CRP (r2 = 0.231, P = 0.006), interleukin-6 (r2 = 0.214, P = 0.016), insulin (r2 = 0.428, P = 0.001), triglycerides (r2 = 0.298, P = 0.001), platelets (r2 = 0.175, P = 0.037) and PTH (r2 = 0.305, P = 0.001) and negatively correlated with TAdip (r2 = −0.192, P = 0.022), HMWAdip (r2 = −0.165, P = 0.05) and GFR (estimated by CysC in pre-dialysis patients only) (r² = −0.304, P = 0.003). In terms of vascular measurements, there was a positive correlation between the leptin level and the ACS (r2 = 0.167, P = 0.05). Leptin was not significantly associated with coronary calcification or bone mineral density.
Table 3 shows the mean serum leptin, TAdip, HMWAdip and insulin levels as a function of the MS score. As the MS score increased, we observed a gradual but significant increase in leptin and insulin levels. Patients with an MS score of 1 differed significantly from those with a score of 0 in terms of the mean TAdip and HMWAdip values. The same was true when comparing an MS score of 1 with an MS score of 2–4. It is noteworthy that none of the patients had the highest possible MS score of 5.
Table 3.
Adipokines and insulin levels as a function of the MS score
| MS score |
P-value | |||||
|---|---|---|---|---|---|---|
| 0 (n = 6) | 1 (n = 25) | 2 (n = 47) | 3 (n = 39) | 4 (n = 25) | ||
| GFR-epia | 48.1 ± 16.1 | 35.2 ± 24.7 | 36.5 ± 18.5 | 36.9 ± 18.8 | 25.6 ± 10.4b,c,d | |
| Leptin | 8.5 ± 16.5 | 9.5 ± 22.3 | 23.1 ± 34.4e | 42 ± 37b,c,e | 47.3 ± 38.4b,c,e | <0.001 |
| TAdip | 8.8 ± 6.1 | 14.3 ± 6.5b | 10.5 ± 5.5e | 8.8 ± 4e | 10 ± 6.9e | 0.006 |
| HMWAdip | 4.3 ± 4.1 | 7.8 ± 4.6b | 5.3 ± 3.6e | 4.1 ± 2.4e | 5.8 ± 6e | 0.014 |
| Insulin | 14.8 ± 9.9 | 17.1 ± 13.8 | 25.8 ± 22.2e | 37.2 ± 26.6b,c,e | 38.9 ± 22.9b,c,e | <0.001 |
aCalculated for patients at CKD stages 2–5 (n = 96). For units, please see Table 2.
b0 versus 1, 3, 4, P < 0,05.
c2 versus 3, 4, P < 0,05.
d3 versus 4, P < 0,05.
e1 versus 2, 3, 4, P < 0,05;
GFR, glomerular filtration rate; TAdip, total adiponectin; HMWAdip, high-molecular weight adiponectin.
We performed a multivariate, stepwise, linear regression analysis in order to identify independent determinants of (i) plasma leptin levels and (ii) the MS score (Table 4). Only BMI, insulin, MS score and PTH were independent predictors of (log-normalized) serum leptin levels (P = 0.002, 0.016, 0.028 and 0.017, respectively). Only leptin, insulin and TAdip were independent predictors of the MS score (P = 0.05, 0.04 and 0.04, respectively).
Table 4.
Multivariate stepwise linear regression: determinants of serum leptin levels (log-normalized) and the MS score
| Items | Β (95% CI) | P-value |
|---|---|---|
| Leptin levels (log-normalized)a | ||
| BMI | 0.294 (0.041–0.182) | 0.002 |
| Insulin | 0.189 (0.002–0.033) | 0.028 |
| MS score | 0.233 (0.088–0.839) | 0.016 |
| PTH | 0.198 (0.001–0.005) | 0.017 |
| MS scoreb | ||
| Leptin | 1.012 (1.000–1.024) | 0.05 |
| Insulin | 1.020 (1.001–1.040) | 0.04 |
| TAdip | 0.925 (0.856–0.999) | 0.04 |
aR² for the model = 0.324. BMI: bone mass index; PTH: parathyroid hormone; MS: metabolic syndrome. Variables included in the model: age (per 1 year increment), bone mass index (per 1 kg/m2 increment), triglycerides (per 1 mmol/L increment), CRP (per 1 mg/L increment), insulin (per 1 μU/mL increment), ACS (per 1% increment), total adiponectin (per 1 μg/mL increment), CKD stage (per 1 stage increment), parathyroid hormone (per 1 pg/mL increment) and metabolic syndrome score (per 1 score increment).
bR² for the model = 0.290. TAdip: total adiponectin. Variables included in the model: haemoglobin (per 1 g/dL increment), interleukin-6 (per 1 pg/mL increment), leptin (per 1 ng/mL increment), insulin (per 1 μU/mL increment), albumin (per 1 g/dL increment), total adiponectin (per 1 μg/mL increment) and CKD stage (per one stage increment).
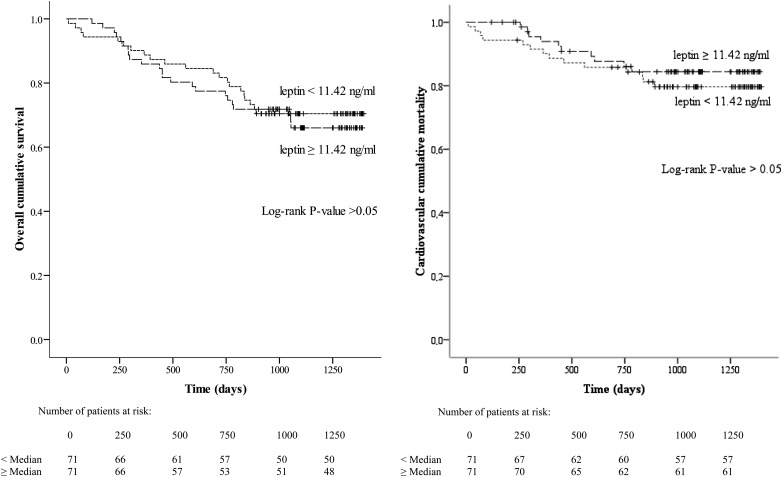
During the follow-up period (mean duration: 969 ± 374 days; median duration: 1058; range: 10–1396), 44 patients died (24 of whom died from CVD). In the cohort as a whole, a leptin level of ≥11.42 ng/mL was not a significant predictor of overall mortality, cardiovascular mortality or cardiovascular events (log-rank comparison between curves: P > 0.05) (Figure 2). Similarly, serum insulin and TAdip did not have predictive values (data not shown). These same findings were observed when considering only pre-dialysis patients or only non-MI (nMI) patients (log-rank comparison between curves: P > 0.05).
Fig. 2.
Kaplan–Meier estimates of the probability of (i) overall cumulative survival and (ii) cardiovascular cumulative mortality for all patients, as a function of the median leptin level (P > 0.05, in the log-rank comparison between curves).
Lastly, we observed a significantly higher overall mortality rate in MI patients (n = 35) than in nMI patients (n = 107) (with 16 and 28 deaths, respectively; log-rank comparison between curves: P = 0.02). However, these two groups did not differ significantly in terms of the median (range) leptin level [16.14 (1.91–53.5) versus 10.29 (1.78–43.1), respectively; P > 0.05]. Also, leptin did not predict mortality even in nMI patients.
Discussion
Our study confirmed that leptin levels are elevated in CKD patients and that these levels rise progressively with decreasing GFR. Leptin levels were significantly and positively correlated with inflammatory markers (interleukin-6 and CRP) and factors closely related to MS (waist circumference, triglycerides and insulin). A gradual but statistically significant increase in leptin and insulin levels was observed as the MS score increased; a multivariate, stepwise, linear regression analysis confirmed that leptin was an independent predictor of MS. Moreover, we found that PTH was an independent determinant of plasma leptin levels, together with well-known classical determinants of leptin levels (such as BMI or insulin). However, we failed to demonstrate that leptin was a significant predictor of the clinical outcome in stage 2–5D CKD patients.
The relationship between leptin and inflammation in CKD was first characterized about 15 years ago [24]. Leptin promotes a Th1-mediated immune response and upregulates the production of inflammatory cytokines in human monocytes [25]. Persistent inflammation is a common state in CKD and is associated with an adverse outcome [26]. Furthermore, insulin resistance (an important factor in MS) is also associated with elevated levels of pro-inflammatory cytokines [27]. Our data confirmed that leptin was positively correlated with inflammatory markers and was an independent predictor of MS.
Some authors have suggested that leptin is an independent component of MS. However, there are few reliable data on the association between leptin and MS in general and in CKD in particular [13, 14, 28]. To the best of our knowledge, the present study is the first to show that leptin is an independent predictor of MS in patients at different CKD stages. We were not surprised to find that the insulin level was also an independent predictor of MS, in view of the close relationship between insulin resistance and MS.
Our findings raise an important issue because leptin may be considered as a potential, independent therapeutic target in CKD patients, along with the other components of MS (such as blood pressure or triglyceride levels). From an interventional standpoint, it is known that leptin levels can be reduced by haemodiafiltration or the administration of retinoic acid, vitamin D3 or antibody against the leptin receptor [29–31]. However, the long-term effects of this intervention have not been studied. This issue does not prove the causality and remains at the level of hypothesis with the need for further studies.
Our study also provided information on determinants of plasma leptin levels: BMI, insulin, the MS score and PTH levels were found to be independent predictors. It is known that the BMI (as a proxy for fat mass) is correlated with leptin levels [32]. High insulin also appears to increase free leptin; hence, the combination of an elevated fat mass with insulin resistance may contribute to an elevation of serum leptin levels [33]. In view of the involvement of adipose tissue and insulin in the regulation of leptin, it is reasonable to assume that leptin and MS are closely interrelated. The significance of this relationship was confirmed by our multivariate stepwise regression analysis: leptin appeared to have a role in MS and vice versa.
An interesting finding from the present study is that PTH was one independent determinant of leptin levels along the CKD stages. A variety of reports have suggested the existence of a relationship between PTH and leptin; however, the causal nature of the relationship has not been established. In leptin-deficient, obese mice, leptin administration increased PTH levels [34]. Bolland et al. [35] observed that fat mass is an important determinant of PTH levels in postmenopausal woman. Furthermore, Maetani et al. [36] showed that variance in PTH levels is related to leptin levels. In the general population, obesity is also associated with higher PTH levels [37, 38] as well as in patients with CKD [39, 40]. Furthermore, measurements of body composition suggest that the high PTH associated with an elevated BMI is directly related to the higher adiposity of these individuals [37]. These findings suggest that fat mass may (through leptin) lead to alterations in the parathyroid glands. If leptin is able to regulate PTH, there must be (from an endocrine viewpoint) a feedback loop by which PTH affects leptin levels. Taniguchi et al. [41] demonstrated that PTH-(1-34) causes lipolysis after binding to receptors distinct from traditional β-adrenergic receptors. One hypothesis is the presence of the PTH receptor in fat tissue. Also, patients with increased production of PTH due to primary hyperparathyroidism experienced influences in gene regulation in fat tissue, which may result in altered adipose tissue function and release of pathogenic factors that increase the risk of CVD [42]. However, evidence of the independent regulation of leptin by PTH is currently lacking and further investigation must address this issue.
In the present study, we found that leptin levels were positively correlated with well-known markers of CVD (such as waist circumference, triglycerides, ACS and even MS). However, we could not show that leptin was a predictor of cardiovascular events and overall and cardiovascular mortality during the follow-up period. This finding is acceptable since in the general population studies, thousands of patients were necessary to show weak associations between plasma leptin and outcomes [6]. On the other hand, this is the first time that the role of leptin in outcomes has been reported in patients at different CKD stages (including early-stage patients).
In the literature, there are few data in favour of a consistent, positive link between high serum leptin levels and mortality in both non-CKD patients and CKD patients [6, 7, 43, 44]. In contrast, Scholze et al. [10] found a relationship between low leptin levels and mortality. In light of these observations, one could hypothesize that low leptin levels may indicate malnutrition—which would act as a faster killer than obesity or CVD. However, although we found that MI patients in the present study had a higher overall mortality rate, they did not differ significantly from non-MI patients in terms of leptin levels. Moreover, leptin was not a significant predictor of overall mortality, cardiovascular mortality and cardiovascular events when we restricted our analysis to non-MI patients. Hence, our results do not suggest that leptin is a marker of malnutrition. In a second hypothesis, other factors such as endothelial dysfunction or inflammation may have a greater influence than leptin on the outcomes of patients at various CKD stages [45]. Leptin's impact on CVD in CKD patients is a complex phenomenon and, in view of the scarcity of literature data, requires further investigation.
Our study has at least two limitations. The relatively small size of our cohort may have had an effect on our analyses of the major outcomes. Furthermore, we used the diabetes mellitus status (and not fasting glucose levels) to calculate the MS score. In contrast, the study's major strength relates to the enrolment of patients at different CKD stages.
In conclusion, we demonstrated that plasma leptin levels are elevated in CKD patients and that these levels rise progressively as the GFR decreases. Leptin levels were significantly and positively correlated with pro-inflammatory markers (interleukin-6 and CRP) and factors closely related to MS (waist circumference, triglycerides and insulin). We found a significant, independent link between plasma leptin levels and MS, and PTH. However, leptin levels did not adequately predict mortality in the present cohort of patients at different CKD stages. Additional research is needed to (i) better understand leptin's involvement in MS and in the regulation of PTH and (ii) establish whether pharmacological intervention could reduce leptin levels and improve clinical outcomes.
Conflict of interest statement
None declared.
References
- 1.Vanholder R, Massy Z, Argiles A, et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- 2.Vanholder R, de Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 3.Parhami F, Tintut Y, Ballard A, et al. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 4.Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J Med Sci. 2001;47:141–150. [PubMed] [Google Scholar]
- 5.Singhal A, Farooqi IS, Cole TJ, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–1924. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 6.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 7.Reilly MP, Iqbal N, Schutta M, et al. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3872–3878. doi: 10.1210/jc.2003-031676. [DOI] [PubMed] [Google Scholar]
- 8.Iribarren C, Husson G, Go AS, et al. Plasma leptin levels and coronary artery calcification in older adults. J Clin Endocrinol Metab. 2007;92:729–732. doi: 10.1210/jc.2006-1138. [DOI] [PubMed] [Google Scholar]
- 9.Couillard C, Lamarche B, Mauriège P, et al. Leptinemia is not a risk factor for ischemic heart disease in men. Prospective results from the Quebec cardiovascular study. Diabetes Care. 1998;21:782–786. doi: 10.2337/diacare.21.5.782. [DOI] [PubMed] [Google Scholar]
- 10.Scholze A, Rattensperger D, Zidek W, et al. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity. 2007;15:1617–1622. doi: 10.1038/oby.2007.191. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Kuwae N, Wu DY, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 12.de Mutsert R, Snijder MB, van der Sman-de Beer F, et al. Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol 2007. 18:967–974. doi: 10.1681/ASN.2006091050. [DOI] [PubMed] [Google Scholar]
- 13.Leyva F, Godsland IF, Ghatei M, et al. Hyperleptinemia as a component of a metabolic syndrome of cardiovascular risk. Arterioscler Thromb Vasc Biol. 1998;18:928–933. doi: 10.1161/01.atv.18.6.928. [DOI] [PubMed] [Google Scholar]
- 14.Lee MC, Lee CJ, Ho GJ, et al. Hyperleptinemia positively correlated with metabolic syndrome in renal transplant recipients. Clin Transplant. 2010;24:124–129. doi: 10.1111/j.1399-0012.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 15.Stenvinkel P, Heimbürger O, Lönnqvist F. Serum leptin concentrations correlate to plasma insulin concentrations independent of body fat content in chronic renal failure. Nephrol Dial Transplant. 1997;12:1321–1325. doi: 10.1093/ndt/12.7.1321. [DOI] [PubMed] [Google Scholar]
- 16.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Executive summary of the third report of the National Cholesterol Education Program (NCEP) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications: Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3.418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 20.Zureik M, Temmar M, Adamopoulos C, et al. Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J Hypertens. 2002;20:85–93. doi: 10.1097/00004872-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 22.Kauppila LI, Polak JF, Cupples LA, et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 23.Raggi P, James G, Burke SK, et al. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res. 2005;20:764–772. doi: 10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 24.Heimbürger O, Lönnqvist F, Danielsson A, et al. Serum immunoreactive leptin concentration and its relation to the body fat content in chronic renal failure. J Am Soc Nephrol. 1997;8:1423–1430. doi: 10.1681/ASN.V891423. [DOI] [PubMed] [Google Scholar]
- 25.Lord GM, Matarese G, Howard JK, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 26.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 27.Marette A. Mediators of cytokine-induced insulin resistance in obesity and other inflammatory settings. Curr Opin Clin Nutr Metab Care. 2002;5:377–383. doi: 10.1097/00075197-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Tsai JP, Tsai CC, Liu HM, et al. Hyperleptinaemia positively correlated with metabolic syndrome in hemodialysis patients. Eur J Intern Med. 2011;22:105–109. doi: 10.1016/j.ejim.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Oh KH, Chin HJ, et al. Effective removal of leptin via hemodiafiltration with on-line endogenous reinfusion therapy. Clin Nephrol. 2009;72:442–448. doi: 10.5414/cnp72442. [DOI] [PubMed] [Google Scholar]
- 30.Menendez C, Lage M, Peino R, et al. Retinoic acid and vitamin D3 powerfully inhibit in vitro leptin secretion by human adipose tissue. J Endocrinol. 2000;170:425–431. doi: 10.1677/joe.0.1700425. [DOI] [PubMed] [Google Scholar]
- 31.Fazeli M, Zarkesh-Esfahani H, Wu Z, et al. Identification of a monoclonal antibody against the leptin receptor that acts as an antagonist and blocks human monocyte and T cell activation. J Immunol Methods. 2006;312:190–200. doi: 10.1016/j.jim.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 33.Lewandowski K, Randeva HS, O'Callaghan CJ, et al. Effects of insulin and glucocorticoids on the leptin system are mediated through free leptin. Clin Endocrinol (Oxf) 2001;54:533–539. doi: 10.1046/j.1365-2265.2001.01243.x. [DOI] [PubMed] [Google Scholar]
- 34.Matsunuma A, Kawane T, Maeda T, et al. Leptin corrects increased gene expression of renal 25-hydroxyvitamin D3–1 alphahydroxylase and 24-hydroxylase in leptin-deficient, ob/ob mice. Endocrinology. 2004;145:1367–1375. doi: 10.1210/en.2003-1010. [DOI] [PubMed] [Google Scholar]
- 35.Bolland MJ, Grey AB, Ames RW, et al. Fat mass is an important predictor of parathyroid hormone levels in postmenopausal women. Bone. 2006;38:317–321. doi: 10.1016/j.bone.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Maetani M, Maskarinec G, Franke AA, et al. Association of leptin, 25-hydroxyvitamin D, and parathyroid hormone in women. Nutr Cancer. 2009;61:225–231. doi: 10.1080/01635580802455149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snijder MB, van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 38.Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromso study. Eur J Endocrinol. 2004;151:167–172. doi: 10.1530/eje.0.1510167. [DOI] [PubMed] [Google Scholar]
- 39.Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Obesity is associated with secondary hyperparathyroidism in men with moderate and severe chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1024–1029. doi: 10.2215/CJN.01970507. [DOI] [PubMed] [Google Scholar]
- 40.Saab G, Whaley-Connell A, McFarlane SI, et al. Obesity is associated with increased parathyroid hormone levels independent of glomerular filtration rate in chronic kidney disease. Metabolism. 2010;59:385–389. doi: 10.1016/j.metabol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi A, Kataoka K, Kono T, et al. Parathyroid hormone-induced lipolysis in human adipose tissue. J Lipid Res. 1987;5:490–494. [PubMed] [Google Scholar]
- 42.Christensen MH, Dankel SN, Nordbø Y, et al. Primary hyperparathyroidism influences the expression of inflammatory and metabolic genes in adipose tissue. PLoS One. 2011;6:e20481. doi: 10.1371/journal.pone.0020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diez JJ, Iglesias P, Fernández-Reyes MJ, et al. Serum concentrations of leptin, adiponectin and resistin, and their relationship with cardiovascular disease in patients with end-stage renal disease. Clin Endocrinol. 2005;62:242–249. doi: 10.1111/j.1365-2265.2005.02207.x. [DOI] [PubMed] [Google Scholar]
- 44.Beberashvili I, Sinuani I, Azar A, et al. Longitudinal study of leptin levels in chronic hemodialysis patients. Nutr J. 2011;10:68. doi: 10.1186/1475-2891-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550–556. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]