Abstract
Secondary oxalosis causing acute kidney injury (AKI) has been widely reported in native kidneys but its occurrence in allograft kidneys is relatively uncommon. We present three patients with acute kidney allograft dysfunction secondary to tubular oxalate microcrystal deposits confirmed on allograft biopsy in the setting of acute gastrointestinal dysfunction. These three patients presented with AKI that was preceded by episodes of ongoing diarrhea ranging from 10 to 90 days. All patients were on vitamin C and/or multivitamin supplementation. Two of the three patients needed long-term renal replacement therapy with the third patient recovering his kidney function after 2 months. The risks versus benefits of vitamin C supplementation in renal transplant patients should be carefully evaluated especially in the setting of gastrointestinal dysfunction.
Keywords: AKI, allograft, secondary oxalosis, transplant, vitamin C
Introduction
Kidney allograft failure has a significant impact on patient morbidity and mortality [1]. Secondary oxalosis (or increased accumulation of oxalic acid due to increased intake or decreased excretion) affecting the allograft kidney is an uncommon cause of acute kidney injury (AKI). To the best of our knowledge, there are only two previous case reports of secondary oxalosis in renal transplant patients [2, 3]. Here, we present three cases with acute allograft dysfunction and moderate-to-severe tubular calcium oxalate microcrystal deposits on allograft biopsy.
Case history
The Institutional Review Board at the University of Iowa Hospital and Clinics (IRB) waived the need for an informed consent.
Case 1
This is a 78-year-old male with a history of a kidney transplant for end-stage kidney disease (ESKD) secondary to glomerulonephritis. He presented with a 10-day history of nausea, vomiting, abdominal cramps, watery diarrhea and AKI. His baseline serum creatinine level prior to the admission was 2.5 mg/dL (221 μmol/L). He also reported a weight loss of ∼3.6 kg since the diarrhea started. He denied fevers or chills. A brief summary of his laboratory values is shown in Table 1. His maintenance immunosuppression consisted of cyclosporine 50 mg orally twice daily, mycophenolate mofetil (MMF) 500 mg per oral twice daily, prednisone 5 mg orally once daily and a 500 mg tablet of vitamin C daily.
Table 1.
Summary of laboratory values on admission
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Laboratory results on admission | |||
| Sodium, mEq/L | 121 (121 mmol/L) | 131 (131 mmol/L) | 141 (141 mmol/L) |
| Potassium, mEq/L | 5.8 (5.8 mmol/L) | 3.8 (3.8 mmol/L) | 4.0 (4.0 mmol/L) |
| Chloride, mEq/L | 83 (83 mmol/L) | 115 (115 mmol/L) | 111 (111 mmol/L) |
| Bicarbonate, mEq/L | 11 (11 mmol/L) | 8.5 (8.5 mmol/L) | 13 (13 mmol/L) |
| Blood urea nitrogen, mg/dL | 145 (51.8 mmol/L) | 58 (20.7 mmol/L) | 70 (24.99 mmol/L) |
| Serum creatinine, mg/dL | 10.2 (901.6 μmol/L) | 4.1 (362.4 μmol/L) | 5.3 (468.5 μmol/L) |
| Urine analysis | |||
| Protein | 2+ | 1+ | Trace |
| Blood | 1+ | Trace | Negative |
| Bacteria | Few | Few | Negative |
| Other laboratory tests | |||
| CMV-PCR | Non-reactive | Non-reactive | Non-reactive |
| Cyclosporine levels | 113 ng/mL | 41 ng/mL | N/a |
| Tacrolimus levels | N/a | N/a | 7.8 ng/mL |
| Kidney ultrasound | No hydronephrosis or renal artery stenosis | No hydronephrosis or renal artery stenosis | No hydronephrosis or renal artery stenosis |
The vitals on admission were unremarkable. On examination, the patient appeared chronically ill and had mild tenderness over the kidney allograft located in the left lower quadrant without any bruits. The rest of the physical examination was unremarkable.
He was started on hemodialysis for 2 hours on the first day. In view of complex clinical presentation and multiple possible confounders, a kidney biopsy was performed.
The biopsy showed moderate-to-severe calcium oxalate tubular microcrystal deposition associated with mild-to-moderate interstitial fibrosis and tubular atrophy. Vitamin C supplementation was stopped and the patient remains on intermittent hemodialysis.
Case 2
This is a 59-year-old male with a history of a kidney transplant for ESKD of uncertain etiology. His immediate post-transplant baseline creatinine level was 2.2 mg/dL (194.4 μmol/L) which prompted multiple kidney biopsies in the first year to evaluate for acute rejection.
On admission, he presented with AKI and intermittent watery diarrhea for the past 12 months and 45.5 kg weight loss. After an extensive workup for his diarrhea including colonoscopy, the presumed etiology appeared to be related to the use of mycophenolic acid (MPA). His pertinent laboratory findings are summarized in Table 1. His maintenance immunosuppressive medications included cyclosporine 75 mg orally twice daily, MPA 360 mg orally twice daily and prednisone 5 mg orally once daily. In addition, he continued to take a 500 mg tablet of vitamin C every day.
The vitals and the physical examination on admission were unremarkable. The worsening AKI prompted a kidney allograft biopsy. The biopsy findings were consistent with chronic transplant glomerulopathy with moderate-to-severe calcium oxalate deposits associated with mild-to-moderate interstitial inflammation.
In spite of stopping the vitamin C, the patient had progressive worsening of the kidney function and has remained on hemodialysis.
Case 3
This is a 40-year-old male status post kidney pancreas transplant for ESKD due to type 1 diabetes mellitus. His baseline serum creatinine post-transplant was 1.5 mg/dL (132.6 μmol/L).
He presented with a 6-week history of nausea, vomiting, diarrhea and flu-like symptoms. His serum creatinine upon admission was 5.4 mg/dL (477.3 μmol/L). His maintenance immunosuppression consisted of tacrolimus 2 mg orally twice daily, MMF 500 mg orally three times daily and prednisone 2.5 mg orally daily. Additional medications included multivitamin one tablet orally daily (with vitamin C content of 60 mg) and an additional 500 mg of vitamin C every day. The vitals and the physical examination on admission were unremarkable. His pertinent laboratory findings are summarized in Table 1. Due to a concern for allograft rejection (in the setting of rise in serum creatinine levels, amylase and lipase), a kidney biopsy was performed.
Biopsy findings were consistent with mild interstitial fibrosis and tubular atrophy with moderate-to-severe calcium oxalate deposits within the tubules.
He was advised to stop the multivitamin and the vitamin C tablet. Over a period of 2 months, his serum creatinine level improved to 2.4 mg/dL (212.2 μmol/L).
Discussion
Oxalate is a metabolic end product that is excreted essentially unchanged in the urine after absorption in the gastrointestinal tract. Urinary oxalate is largely derived from the endogenous metabolism of glycine, glycolate, hydroxyproline and vitamin C [4]. Hyperoxalosis from any cause converges on a common final pathophysiological pathway of supersaturation of the renal tubular fluid leading to the precipitation of oxalate crystals in the renal interstitium creating an interstitial nephritis, macrophage recruitment and surge in inflammatory mediators ultimately leading to tubular atrophy and AKI [5]. Secondary oxalosis in native kidneys is well documented and can cause AKI resulting in a rapid loss of kidney function [6–9].
Isolated tubular deposits of oxalate crystals are not a rare finding in the normal or failing kidney at any stage, and even in the transplanted kidney. Isolated oxalate crystals do not imply renal damage, although oxalate deposits in kidney grafts have a negative effect on long-term renal function [10, 11]. In contrast, abundant tubular or interstitial deposits of calcium oxalate are highly suggestive of a hyperoxaluric condition in the native or transplanted kidney.
Enteric dysfunction is a documented cause of secondary oxalosis. The differential diagnosis for diarrhea in the post-transplant patient includes medications (immunosuppressive and non-immunosuppressive), bacterial/viral enteric infections (e.g. Shigella spp., Escherichia coli, Cytomegalovirus, Campylobacter spp., Clostridium difficile) and bacterial overgrowth syndromes [12]. Normally, intestinal calcium binds to oxalates and is excreted as calcium oxalate in the stool, thereby limiting the absorption into the systemic circulation to ∼10–12% of the enteric oxalates. In malabsorption syndromes, steatorrhea, post-gastric bypass surgery, the enteric calcium chelates with fatty acids and is not biochemically available to bind oxalates, thereby increasing systemic absorption [12] Gastrointestinal complications especially diarrhea is common in patients on immunosuppression especially MMF [13]. Gastrointestinal epithelial cells are partially dependent on the de novo purine synthesis pathway for replication and regeneration, which is inhibited by MMF and its active metabolite. Thus, MMF can lead to fluid malabsorptions and diarrhea, which could be a precipitating factor for secondary oxalosis [13]. The three patients reported here were on MMF/MPA as part of their immunosuppressive regimen. Replacement of MMF with an alternative medication could be an appropriate adjunct to therapy in the setting of concern for secondary oxalosis [14].
The causative role of alterations in oxalate metabolizing bacterial flora in the gut and hyperoxaluria is also gaining significance [15, 16]. Common intestinal commensals such as Oxalobacter formigenes can metabolize enteric oxalates and play a crucial role in oxalate homeostasis. If this homeostasis is disturbed, oxalates can be absorbed to a greater extent, thereby increasing the renal excretion and risk for calcium oxalate precipitation and deposits. Decreased enteric activity of Oxalobacter sp. may not be enough by itself to result in life-threatening hyperoxaluria, but it may be an important contributing factor in patients with multiple confounding risk factors such as diarrhea, vitamin C intake and pancreatitis.
We postulate that one other potential factor for precipitating secondary oxalosis is vitamin C (not prescribed and even in normal doses) as this was present in all of our patients. All other potential causes for secondary oxalate nephropathy (ethylene glycol, orlistat and methoxyflurane) were ruled out. Vitamin C has been increasingly prescribed for a number of indications ranging from the common cold to anemia management in dialysis patients. Clinicians often underestimate the role of vitamin C in hyperoxalosis [17]. Vitamin C, being a water-soluble vitamin, offers a false sense of safety and although in the vast majority of cases, daily vitamin C supplementation may not be of concern, one has to be cautious in patients with established risk factors for kidney disease. In addition, patients may receive vitamin C as part of total parenteral nutrition. Previously reported cases with series in liver transplant patients suggest that even normal doses of vitamin C supplementation (≤500–1000 mg/day) may contribute to oxalosis in the presence of additional risk factors such as a diarrheal illness [18].
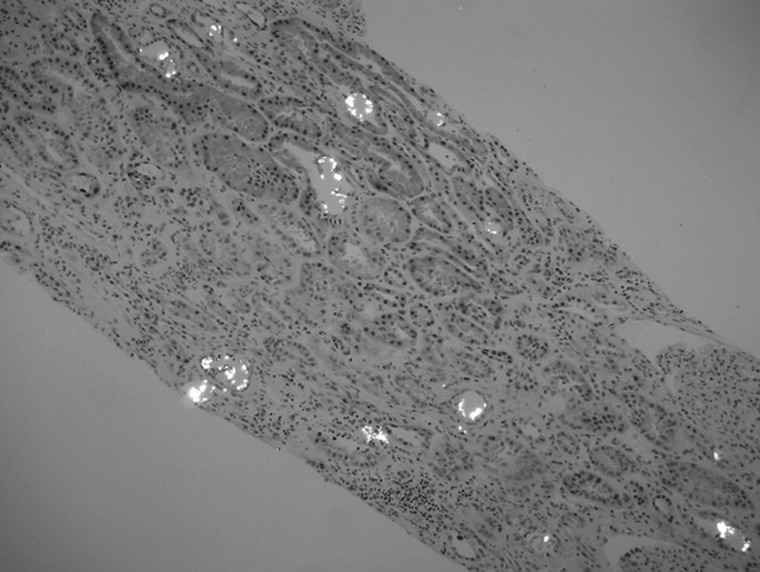
The calcium oxalate crystal appears as a colorless crystal predominantly present in the distal tubules and shows bright white polarization characteristics (Figure 1). The presence of oxalate crystals can be overlooked as these are colorless and are visible only on the hematoxylin and eosin stained slides. In addition, they are dissolved away and not readily visible with the other special stains routinely used for kidney biopsy such as periodic acid Schiff, trichrome and silver stains. Polarization microscopy does make them visible in a spectacular fashion and readily demonstrates the true extent.
Fig. 1.
Intratubular calcium oxalate microcrystals showing bright white polarization (hematoxylin and eosin stain, 100× magnification).
These cases illustrate the importance of recognizing the presence of oxalate crystals as a distinct etiology of renal allograft dysfunction. Excluding precipitating causes such as vitamin C while ensuring adequate fluids and pancreatic enzyme replacement for steatorrhea could be helpful in preventing oxalate nephropathy.
Conflict of interest statement
None declared.
References
- 1.Marcen R, Teruel JL. Patient outcomes after kidney allograft loss. Transplant Rev. 2008;22:62–72. doi: 10.1016/j.trre.2007.09.005. doi:10.1016/j.trre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Cuvelier C, Goffin E, Cosyns JP, et al. Enteric hyperoxaluria: a hidden cause of early renal graft failure in two successive transplants: spontaneous late graft recovery. Am J Kidney Dis. 2002;40:E3. doi: 10.1053/ajkd.2002.33934. doi:10.1053/ajkd.2002.33934. [DOI] [PubMed] [Google Scholar]
- 3.Jahromi A. Acute renal failure secondary to oxalosis in a recipient of a simultaneous kidney–pancreas transplant: was mycophenolate the cause? Nephrol Dial Transplant. 2008;23:2409–2411. doi: 10.1093/ndt/gfn194. doi:10.1093/ndt/gfn194. [DOI] [PubMed] [Google Scholar]
- 4.Hagler L, Herman RH. Oxalate metabolism. Am J Clin Nutr. 1973;26:758–765. doi: 10.1093/ajcn/26.6.758. [DOI] [PubMed] [Google Scholar]
- 5.Rathi S, Kern W, Lau K. Vitamin C-induced hyperoxaluria causing reversible tubulointerstitial nephritis and chronic renal failure: a case report. J Med Case Rep. 2007;1:155. doi: 10.1186/1752-1947-1-155. doi:10.1186/1752-1947-1-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill P, Karim M, Davies DR, et al. Rapidly progressive irreversible renal failure in patients with pancreatic insufficiency. Am J Kidney Dis. 2003;42:842–845. doi: 10.1016/s0272-6386(03)00948-x. doi:10.1016/S0272-6386(03)00948-X. [DOI] [PubMed] [Google Scholar]
- 7.Mandell I, Krauss E, Millan JC. Oxalate-induced acute renal failure in Crohn's disease. Am J Med. 1980;69:628–632. doi: 10.1016/0002-9343(80)90479-9. doi:10.1016/0002-9343(80)90479-9. [DOI] [PubMed] [Google Scholar]
- 8.Wharton R, D'Agati V, Magun AM, et al. Acute deterioration of renal function associated with enteric hyperoxaluria. Clin Nephrol. 1990;34:116–121. [PubMed] [Google Scholar]
- 9.Wong K, Thomson C, Bailey RR, et al. Acute oxalate nephropathy after a massive intravenous dose of vitamin C. Aust N Z J Med. 1994;24:410–411. doi: 10.1111/j.1445-5994.1994.tb01477.x. doi:10.1111/j.1445-5994.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 10.Bagnasco SM, Mohammed BS, Mani H, et al. Oxalate deposits in biopsies from native and transplanted kidneys, and impact on graft function. Nephrol Dial Transplant. 2009;24:1319–1325. doi: 10.1093/ndt/gfn697. doi:10.1093/ndt/gfn697. [DOI] [PubMed] [Google Scholar]
- 11.Pinheiro HS, Camara NO, Osaki KS, et al. Early presence of calcium oxalate deposition in kidney graft biopsies is associated with poor long-term graft survival. Am J Transplant. 2005;5:323–329. doi: 10.1111/j.1600-6143.2004.00684.x. doi:10.1111/j.1600-6143.2004.00684.x. [DOI] [PubMed] [Google Scholar]
- 12.Maes B, Hadaya K, De Moor B, et al. Severe diarrhea in renal transplant patients: results of the DIDACT study. Am J Transplant. 2006;6:1466–1472. doi: 10.1111/j.1600-6143.2006.01320.x. doi:10.1111/j.1600-6143.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- 13.Davies NM, Grinyo J, Heading R, et al. Gastrointestinal side effects of mycophenolic acid in renal transplant patients: a reappraisal. Nephrol Dial Transplant. 2007;22:2440–2448. doi: 10.1093/ndt/gfm308. doi:10.1093/ndt/gfm308. [DOI] [PubMed] [Google Scholar]
- 14.Hiel AL, Tintillier M, Cuvelier C, et al. Acute renal failure secondary to oxalosis in a recipient of a simultaneous kidney-pancreas transplant: was mycophenolate the cause? Nephrol Dial Transplant. 2009;24:326. doi: 10.1093/ndt/gfn578. doi:10.1093/ndt/gfn578. [DOI] [PubMed] [Google Scholar]
- 15.Sidhu H, Hoppe B, Hesse A, et al. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet. 1998;352:1026–1029. doi: 10.1016/S0140-6736(98)03038-4. doi:10.1016/S0140-6736(98)03038-4. [DOI] [PubMed] [Google Scholar]
- 16.Sidhu H, Schmidt ME, Cornelius JG, et al. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol. 1999;10:S334–S340. [PubMed] [Google Scholar]
- 17.Auer BL, Auer D, Rodgers AL. Relative hyperoxaluria, crystalluria and haematuria after megadose ingestion of vitamin C. Eur J Clin Invest. 1998;28:695–700. doi: 10.1046/j.1365-2362.1998.00349.x. doi:10.1046/j.1365-2362.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- 18.Lefaucheur C, Hill GS, Amrein C, et al. Acute oxalate nephropathy: a new etiology for acute renal failure following nonrenal solid organ transplantation. Am J Transplant. 2006;6:2516–2521. doi: 10.1111/j.1600-6143.2006.01485.x. doi:10.1111/j.1600-6143.2006.01485.x. [DOI] [PubMed] [Google Scholar]