Abstract
Background
Maintenance of the optimal fluid status in hemodialysis patients is still a challenging task in daily clinical practice. A bioelectric impedance technique has been applied for evaluation of hydration status in the dialysis population in recent years, but serial observations of its role in clinical dry weight determination are lacking. In this study, serial follow-up data of a body composition monitor based on bioimpedance spectroscopy (BCM-BIS) applied in dialysis patients were analyzed to define the technique's feasibility, precision and possible role in dry weight determination.
Methods
BCM-BIS was applied monthly to 194 hemodialysis patients for 6 months. Intra-patient precision was analyzed. Bland–Altman analysis and repeated-measures analysis of variance (ANOVA) were used to define the relationship between the dry weights determined by BCM-BIS and by clinical judgment.
Results
The coefficients of variation (CVs) of fluid parameters were <5%. Serial changes in dry weight differences were compared in groups with different post-dialysis hydration status and dry weight differences decreased gradually. Bland–Altman analysis revealed that the range of these differences was significantly narrower towards the latter part of the study. The upper limit of agreement with 95% confidence interval (CI) was 1.47 L and the lower limit was −3.02 L.
Conclusions
BCM-BIS is precise and can be easily applied in the clinical setting. Discrepancy between the dry weights determined by BCM-BIS and by clinical judgment significantly decreased during the study. It is sensitive in dry weight determination, especially for those patients with obvious over-hydration (OH) by BCM-BIS. Patients with post-dialysis OH results beyond some critical values (>1.5 L or <−3 L) should be closely monitored.
Keywords: bioimpedance spectroscopy, body composition monitor, haemodialysis
Introduction
Background
Fluid status is one of the key factors influencing the quality of life and life expectancy in the dialysis population [1]. Because clinical assessment of hydration status can be time consuming and requires specific clinical skills [2], various strategies have been proposed to help reach the optimal fluid status in dialysis patients but each has its own limitation [3]. A body composition monitor based on bioimpedance spectroscopy (BCM-BIS) with modified fluid and physiological models was developed in recent years to detect the presence of excessive fluid in dialysis patients [4, 5]. Due to its non-invasive and easy-to-perform characteristics, it has been increasingly applied as a bedside tool to evaluate patients' fluid status in many dialysis facilities worldwide and its use in the clinical setting is increasing.
Purpose
Most published studies reporting the results of BCM-BIS use delineated the relationship between BCM-BIS results and basic clinical findings such as blood pressure (BP) from cross-sectional data [6]. One observational study found the degree of over-hydration (OH) determined by BCM-BIS associated with a significantly increased relative risk of death, whereas in the same population inter-dialysis weight gain was not unambiguously associated with death hazard [7]. However, data of long-term application of this tool in chronic stable dialysis patients to evaluate its clinical feasibility and stability are lacking. Moreover, intra-patient precision and the relationship between the post-dialytic post-dialysis hydration status measured by BCM-BIS and clinically determined dry weight have not been examined. Dry weight determination is one of the more challenging aspects of clinical assessment in dialysis patients, and a convenient, accurate and objective tool for exploring the euvolemic status of these patients would be welcome. The simplicity of use and the provision of immediate results of estimates of excessive fluid status by BCM-BIS [8, 9] raised our hopes and prompted this study. The ABISAD (Application of Bioimpedance Spectroscopy in Asian Dialysis Patients) project commenced in July 2011 when BCM-BIS became available and began to be applied in several collaborating dialysis facilities in Taiwan, Republic of China. In this first phase of the ABISAD project (this study), we focus on the possible benefits of this innovative tool with its objective assessment of fluid status in regular application in hemodialysis patients. The study aims to assess the feasibility and any possible technical obstacles during practice, the relationship of clinical parameters with BCM-BIS measurement results and evaluation of its clinical reliability and its role in dry weight determination. In the second phase, an interventional study will be designed using data from the Phase I study. Patients from additional dialysis facilities will be randomized to the BCM-BIS or control group to assess the long-term effects of fluid control on clinical outcomes.
Materials and methods
Subjects
Chronic stable adult (age >18) hemodialysis patients of An-Shin and Tai-Shin dialysis centers in Taiwan were enrolled in the study since July 2011. Patients with mechanical valves, pacemakers, coronary artery stents or implanted metallic devices were excluded. A total of 1005 BCM measurements were performed in 194 incident and prevalent patients and 133 patients completed six serial monthly BCM measurements between July and December 2011.
Instruments
The body composition monitor (BCM: Fresenius Medical Care, Bad Homburg, Germany) was used for all the measurements. The basic principle of this machine is the use of the BIS which determines the fluid content of the body by measuring the serial values of electric impedance by applying small microAmp electric currents at 50 different frequencies between 5 and 1000 kHz. These impedance data were used to obtain the amount of total body water (TBW) and extracellular water (ECW) through a special fluid model [8] and then to obtain the amount of excessive fluid through a physiological body composition model [9]. The parameters related to the fluid status measured by BCM-BIS are as follows:
Total body water [unit liter (L)]
Extracellular water [unit liter (L)]
Intracellular water [ICW, unit liter (L)]
Extracellular to ICW ratio (EIR)
Pre-dialysis OH [unit liter (L)]
Relative pre-dialysis OH [OHR, defined as OH divided by normally hydrated ECW (OH/ECW), unit percentage (%)]
Post-dialysis OH [OHp, defined as OH minus the ultrafiltration volume (OH − UFV), unit liter (L)]
Relative post-dialysis OH [OHRp, defined as OHp divided by ECW (OHp/ECW), unit percentage (%)]
Cole–Cole plot: a graphic output depicts the raw data of the measurement together with the optimized model function in the impedance plane during measurement; smooth dome shape usually indicates the good quality of measurement and other bizarre contours may appear when the measurements fail.
Measurement quality: a numerical indicator appears in the same screen with the Cole–Cole plot during measurement; a quality value close to 100% (usually > 90%) indicates successful measurement and a very low quality value is usually accompanied with failed measurement.
In this study, in order to analyze the possible different clinical presentations and different sequential changes in BCM data in patients with different hydration status, patients were divided into several pre-dialysis or post-dialysis hydration groups. According to the pre-dialysis data, they were grouped into Group A (OH < 2.5 L) and Group B (OH ≥ 2.5 L). According to the post-dialysis data, they were grouped into Group 1 (OHp ≥ 1.1 L), Group 2 (−1.1 L < OHp < 1.1 L) and Group 3 (OHp ≤ −1.1 L). The rationale for using these ranges came from the data of previous BCM studies in hemodialysis patients. OH > 2.5 L, which was termed as severe OH, is associated with a higher mortality rate [7]. The range between −1.1 and 1.1 L was established from a previous study based on a population of 1247 healthy Caucasian controls; −1.1 and 1.1 L were the 10th and 90th percentiles of this healthy distribution [10].
Some terms related to dry weight used in this study are defined as follows:
Dry weight determined by clinical judgment (DWclinical): equal to pre-dialysis body weight (BW) minus predetermined UFV (BW − UFV). By definition, this is the lowest weight a patient can tolerate without developing symptoms or hypotension. This weight is clinically determined after evaluating clinical parameters in the intra-dialysis sessions or during the inter-dialysis periods. Symptoms and signs suggesting possible fluid aberrance include recent repeated episodes of intra-dialysis hypotension, hypertension, muscle cramping, need for nursing intervention, premature ending of dialysis sessions and severe post-dialysis thirst.
Dry weight (normal hydration weight) determined by BCM-BIS (DWbcm): calculated by BCM-BIS from the result of BW minus OH (BW − OH).
Dry weight difference (DWdiff): defined as DWclinical minus DWbcm (DWclinical− DWbcm).
Procedures
BCM-BIS measurement was performed in each of the 194 patients enrolled in the study monthly before the midweek dialysis session. The measurement was performed by a specific member of staff who had completed a training course in the BCM-BIS technique. All the electrode pads were of single use and the test was repeated if an incomplete Cole–Cole plot or poor test quality were found. Inputs to the BCM include age, sex, body height, pre-dialysis BW, BP and predetermined UFV for that dialysis session. Concomitant biochemical data, including blood cell counts, nutritional parameters and lipid profiles, were checked before the midweek dialysis session in the same month. After the tests, the results are transferred to a personal computer via a card reader and manipulated in the computer through specific software (Fluid Management Tool: FMT), which produces various graphic outputs and from which the data can be exported in Excel format for further analysis. Post-dialysis BP was input into FMT after the dialysis session ended.
Statistics
The modified Bland–Altman analysis [11] was used to compare the difference of dry weights determined by clinical judgment and by BCM-BIS. Repeated-measures analysis of variance (ANOVA) was used to compare the serial mean of repeated-measured numerical data. One-way ANOVA was used to compare the mean of numerical data in different categorical groups. All the statistical tests and graphs were analyzed and produced by SAS 9.2 through the interface of SAS enterprise 4.2 and R version 2.13.1 Patched (2011-07-22 r56481) from The R Foundation for Statistical Computing.
Results
Basic demographic data
There were 103 females (53.09%) and 91 males (46.91%) enrolled in this study. The average age was 61.55 ± 12.10 years (mean ± SD) with average pre-dialysis weight 58.87 ± 11.11 kg and average height 1.60 ± 0.08 m. The average dialysis vintage was 7.71 ± 5.86 years. Ninety-five cases (48.97%) had diabetes mellitus. All the participants were chronic stable outpatients; acutely unstable patients such as those with sepsis, obvious heart failure or acute lung edema were not included in the study.
Clinical feasibility
The BCM-BIS tests were performed without problems during the 6-month study period. During this period, there was a total of 220 incident and prevalent patients in the centers and 194 of them underwent at least one measurement after informed consent. Twenty-six patients (11.82%) did not receive the test for various reasons, which included pacemaker implantation (4), coronary stents (9), artificial joints and internal fixations (17), hand tremor due to cerebrovascular accident (CVA) (1), contagious skin disease (1), patient refusal to wait for the test (11) (some patients have multiple reasons). One patient had repeated measurements with final success in single month due to the problem of greasy skin. The other patient had repeated test failures which were suspected to result from his very coarse and uneven skin. The measurement time was fast with average measurement time 100.41 ± 52.18s per test. Nearly all the tests were completed in single measurement with good results which were judged by high measurement quality (average quality 94.96 ± 3.59%) and acceptable Cole–Cole plot contour. Including the time needed for supine rest for at least 5 min before the test, the total time for each test was <10 min. Thus, it was smoothly applied before dialysis without delay in dialysis schedule and accepted by most patients.
Baseline fluid status data from BCM-BIS
From 194 baseline measurements, the average pre- and post-dialysis hydration status represented by OH, OHp, OHR and OHRp is summarized in Table 1. Data on TBW, ICW, ECW and EIR are also shown.
Table 1.
Summary statistics of hydration status data in 194 baseline measurements (pctl: percentile, 95% CI)
| Variable | Mean ± SD | Range | N | 10th pctl | 25th pctl | 50th pctl | 75th pctl | 90th pctl | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| OH (L) | 2.03 ± 1.36 | −1.40–7.00 | 194 | 0.50 | 1.10 | 1.80 | 2.80 | 3.80 | 1.84–2.22 |
| OHp (L) | −0.41 ± 1.60 | −5.20–5.00 | 194 | −2.10 | −1.40 | −0.45 | 0.50 | 1.70 | −0.63 to −0.18 |
| OHR (%) | 13.64 ± 8.16 | −7.00 to 37.00 | 194 | 4.20 | 8.50 | 12.90 | 18.50 | 24.70 | 12.49–14.80 |
| OHRp (%) | −3.09 ± 10.61 | −36.60 to 33.60 | 194 | −15.60 | −9.60 | −3.50 | 3.60 | 10.40 | −4.59 to −1.58 |
| TBW (L) | 29.92 ± 6.28 | 15.70–47.70 | 194 | 22.10 | 25.70 | 28.95 | 34.50 | 38.60 | 29.03–30.81 |
| ICW (L) | 15.25 ± 3.89 | 6.3028.20 | 194 | 10.80 | 12.70 | 14.70 | 17.70 | 20.60 | 14.70–15.80 |
| ECW (L) | 14.66 ± 2.74 | 8.10–21.40 | 194 | 11.30 | 12.60 | 14.50 | 16.60 | 18.20 | 14.28–15.05 |
| EIR | 0.99 ± 0.15 | 0.69–1.48 | 194 | 0.81 | 0.88 | 0.97 | 1.09 | 1.19 | 0.96–1.01 |
In order to detect the possible relationships between patients' hydration status and their clinical presentations, we compared the different clinical parameters in patient groups with different pre-dialysis (Group A and Group B) or post-dialysis (Group 1, Group 2 and Group 3) hydration status, as described in the ‘Instruments’ section. The results are summarized in Table 2. Before dialysis, 35.05% patients had OH ≥ 2.5 L. Except higher UFV and lower serum creatinine in this group (Group B), no other clinical parameters were different between patients from Groups A and B. After dialysis, 32.99% patients belong to Group 3 by BCM-BIS with OHp ≤ −1.1 L and 14.95% patients belong to Group 1 with OHp ≥ 1.1 L. Contrary to pre-dialysis, several clinical parameters were significantly different in patients with OHp ≤ −1.1 L (Group 3), compared with the other two groups. Patients in Group 3 were younger, had higher pre-dialysis BW, higher body mass index (BMI), higher UFV and better nutrition markers (albumin, creatinine and hemoglobin). Additionally, the patients in Group 1 exhibited a rise in BP after dialysis which was not seen for the other groups. Systolic BP, no matter before (SBPpre) or after (SBPpost) dialysis, was not significantly different in groups with different hydration status.
Table 2.
The relationship between clinical parameters and different hydration statusa
| Grouped by OH (n = 194) |
Grouped by OHp (n = 194) |
||||
|---|---|---|---|---|---|
| OH < 2.5 L | OH ≥ 2.5 L | −1.1 L < OHp < 1.1 L | OHp ≥ 1.1 L | OHp ≤ −1.1 L | |
| 64.95% (n = 126) | 35.05% (n = 68) | 52.06% (n = 101) | 14.95% (n = 29) | 32.99% (n = 64) | |
| Age | 60.79 ± 12.9 | 62.96 ± 10.4 | 63.04 ± 11.26 | 65.79 ± 11.24 | 57.27 ± 12.67** |
| DM (%) | 44.44% | 57.35% | 49.50% | 48.28% | 48.44% |
| SBPpre (mmHg) | 135 ± 19 | 137 ± 19 | 134 ± 18 | 139 ± 23 | 137 ± 18 |
| SBPpost (mmHg) | 127 ± 18 | 132 ± 20 | 127 ± 19 | 136 ± 23 | 127 ± 15 |
| SBPdrop (mmHg) | 10.45 ± 20.74 | 3.48 ± 29.33 | 9.45 ± 23.98 | −12.70 ± 21.42** | 17.71 ± 17.70 |
| BW (kgw) | 58.18 ± 11.19 | 60.16 ± 10.92 | 57.44 ± 10.36 | 57.02 ± 11.17 | 61.98 ± 11.74* |
| UFV (L) | 2.28 ± 1.07 | 2.73 ± 1.38* | 2.07 ± 0.88 | 1.73 ± 0.79 | 3.33 ± 1.29*** |
| Albumin | |||||
| (g/L) | 40.4 ± 3.80 | 39.4 ± 5.0 | 39.9 ± 4.10 | 38.0 ± 5.10 | 41.1 ± 3.80* |
| (g/dL) | (4.04 ± 0.38) | (3.94 ± 0.5) | (3.99 ± 0.41) | (3.80 ± 0.51) | (4.11 ± 0.38)* |
| Creatinine | |||||
| (µmol/L) | 823.00 ± 153.82 | 762.01 ± 183.87* | 782.34 ± 156.47 | 730.18 ± 205.97 | 863.67 ± 149.40** |
| (mg/dL) | (9.31 ± 1.74) | (8.62 ± 2.08)* | (8.85 ± 1.77) | (8.26 ± 2.33) | (9.77 ± 1.69)** |
| Hemoglobin | |||||
| (g/L) | 107.6 ± 13.8 | 102.9 ± 18.6 | 106.1 ± 13.6 | 98.5 ± 17.7 | 108.7 ± 17.3* |
| (g/dL) | (10.76 ± 1.38) | (10.29 ± 1.86) | (10.61 ± 1.36) | (9.85 ± 1.77) | (10.87 ± 1.73)* |
| BMI (kg/m2) | 22.86 ± 3.7 | 22.96 ± 3.41 | 22.39 ± 3.52 | 22.40 ± 3.03 | 23.92 ± 3.78* |
aFisher's exact test for DM percentage comparison; one-way ANOVA using the post-hoc Bonferroni test for other numerical variables. Abbreviations are explained in the text. Data are indicated in SI units. Albumin, creatinine and hemoglobin are additionally followed by the conventional units in brackets.
*P < 0.05. **P < 0.01. ***P < 0.001.
Intra-patient precision
In order to assess the reliability of BCM-BIS, the coefficient of variation (CV) calculated from the mean and standard deviation (SD) of six measurements of TBW, ICW, ECW and EIR in each patient was obtained from 133 patients with complete 6-month follow-up. (Table 3) Although many physiological changes may have occurred during this period, the CV of measurements of these four parameters were less than or close to 5%, which was acceptable for clinical use.
Table 3.
Average CV of TBW, ICW, ECW and EIR, calculated from the CV of six measurements of every single patient
| Variable | Mean ± SD | Range | N | 95% CI |
|---|---|---|---|---|
| CV of TBW (%) | 3.07 ± 2.02 | 0.52–11.57 | 133 | 2.72–3.41 |
| CV of ICW (%) | 3.94 ± 3.00 | 0.99–18.26 | 133 | 3.42–4.45 |
| CV of ECW (%) | 3.86 ± 1.94 | 1.17–14.08 | 133 | 3.53–4.19 |
| CV of EIR (%) | 4.91 ± 2.75 | 1.52–22.23 | 133 | 4.44–5.38 |
Serial follow-up data for change in DWdiff (OHp)
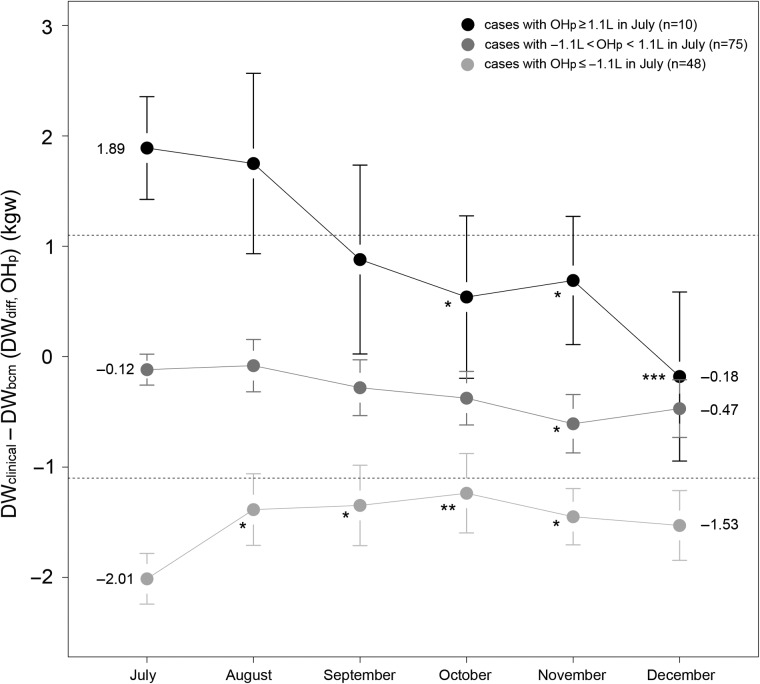
For 133 patients with complete 6 months of measurements, the serial change in DWdiff (OHp) with time was compared with within-subjects repeated-measures ANOVA in three different post-dialysis hydration groups as above (Group 1: OHp ≥ 1.1 L, Group 2: −1.1 L < OHp < 1.1 L and Group 3: OHp ≤ −1.1 L). The average OHp approached the acceptable range (between −1.1 and 1.1 L) from 1.89 L (95% CI: 1.42–2.36) to −0.18 L (−0.95–0.59) in Group 1 and from −2.01 (−2.24 to −1.78) to −1.53 L (−1.85 to −1.21) in Group 3 although it was still ≤−1.1 L. The sequential OHp was significantly different from OHp in July in these two groups (Figure 1).
Fig. 1.
Monthly change of DWdiff (OHp) in three different groups. The DWdiff approached the acceptable range (−1.1 L < OHp < 1.1 L) for the group with initial OHp ≥1.1 L (Group 1) and the group with initial OHp ≤−1.1 L (Group 3). Data were expressed as mean (dot) and 95% CI (bar). The comparisons of means were performed using within-subjects repeated-measures ANOVA. *P < 0.05, **P < 0.01 and ***P < 0.001 if the sequential means were different from data of July of the same group. The actual mean values from July to December are shown.
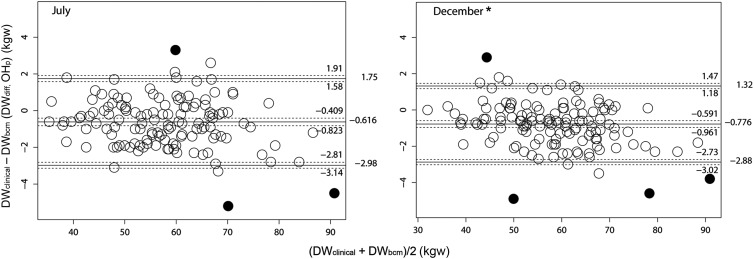
Serial Bland–Altman analysis to compare DWclinical and DWbcm
To compare the serial relationship between the dry weight determined by clinical methods and by BCM-BIS, modified Bland–Altman analyses [11] were done monthly from July to December 2012. The mean DWdiff (OHp) was persistently below zero, but the range of the limits of agreement significantly decreased from July to December. Data of July and December are shown in Figure 2. In December, the upper limit of agreement with 95% CI was 1.47 L and the lower limit was −3.02 L.
Fig. 2.
Modified Bland–Altman analyses as suggested by Ludbrook [11] were used in method comparison for the dry weight determined by clinical symptoms (DWclinical) and by BCM-BIS (DWbcm) in July and December. Outliers (black dots) were marked and excluded from calculation by bivariate boxplot analysis with bvbox () function in R. The mean values of DWdiff (middle solid line) with limits of agreement (upper and lower solid lines) are shown with their 95% CIs (dash lines). The actual values were also shown on the right. The range of limits of agreement in November and December was significantly narrower than the range in July, August and September due to significantly lower upper limits. Only data of July and December are shown (*n = 133 for each panel).
Discussion
This is the first study to demonstrate the relationship between the dry weight determined by clinical evaluation and the ‘normally hydrated’ weight (physiological dry weight) estimated by BCM-BIS from serial follow-up data. Using serial measurements in the same patients, we wanted to delineate whether this tool can be used as a practical bedside ultrafiltration guide. Such information would not be obtained by an analysis of cross-section data at a specific time point. This higher measurement frequency (once per month) when compared with previous studies in the literature provided data and relationships not evident previously [12]. Before July 2011, physicians determined the dry weight mainly by assessment of intra-dialysis and inter-dialysis symptoms and signs. We anticipated that the more objective measurement of hydration by BCM-BIS would provide additional insights into volume management. Indeed, patients who were inadequately ultrafiltrated to meet the BCM-BIS target (Group 1 in Figure 1) gradually approached the ‘acceptable hydration’ range by December, 2011. For those with initial ‘over ultrafiltrated’ (Group 3 in Figure 1), the DWdiff (OHp) also approached the acceptable range, although remaining somewhat <−1.1 L. Although preliminary (and to be tested in the Phase II study) these data suggest that BCM-BIS provides a more sensitive measure of hydration than the standard clinical practice. Even though some patients were symptomless at the time BCM-BIS detected a high OHp, their dry weight could still be reduced in the following months. From the results of Group 3 (OHp persistently <−1.1 L in November and December), combined with the OHp data in Table 2 (95% CI of OHp: −0.63 to −0.18 L), we can speculate that because DWbcm represents a kind of normal hydration physiological ‘dry weight’, most dialysis patients could tolerate more fluid removal to reach a relatively dehydrated condition than the normal physiological status without obvious clinical aberrance during the dialysis session. This may also be considered as a kind of adaptation to decrease the possibility of fluid overload during the inter-dialysis period. Supported by the evidence from Table 2, patients who were over ultrafiltrated to the degree of dehydration as assessed by BCM-BIS and who appeared to be clinically more tolerant to this were younger and well-nourished (Table 2).
In order to define a clearer role of BCM-BIS in dry weight determination, we used Bland–Altman analysis in sequential 6 months to delineate the differences between the dry weight determined by BCM-BIS and the weight already determined by clinical assessment at the time point. Towards the latter part of the study (November and December), the range of dry weight differences became significantly narrower than the range in the earlier months. This change came mainly from the decrease in the possible maximal ‘under-ultrafiltrated’ value (In December, the upper 95% confidence limit of upper limits of agreement was 1.47 L, lower than 1.58, 1.94, 1.83 and 1.48 L in July, August, September and October).
The accuracy of BCM-BIS for the measurements of body water has already been validated in comparison with the gold standard of other body composition measurement methods [13]. This study revealed the long-term intra-subject precision of this tool with an acceptable CV of <5%. Practically speaking, this tool has also been found to be easily used, fast and highly feasible in a clinical setting.
Although it is still not possible to conclude that we can determine the dry weight simply by BCM-BIS without clinical information in this study, this tool indeed revealed some pitfalls when the dry weight was determined by clinical symptoms or signs alone (such as systemic BP). The relationship between the dry weight determined clinically and the ‘normally hydrated’ dry weight determined by BCM-BIS is clearly delineated. The evidence that the dry weight difference became less obvious near the study end also demonstrated the sensitivity of this equipment, especially for those inadequately ultrafiltrated patients. The relative constant upper (around 1.5 L) and lower (around −3 L) range defined by 95% CI of dry weight discrepancy during the follow-up period provide a very useful reference value for a general judgment of BCM result. Those patients with BCM results outside this range are very likely to be over-hydrated or dehydrated because <5% of stable patients have this result and should be closely monitored.
In conclusion, BCM-BIS is a promising tool that can be easily applied in bedside practice in assistance to clinical dry weight determination and monitoring, especially in patients with BCM-BIS results revealing inadequately ultrafiltrated or results beyond some critical values. In the second phase of the ABISAD study, more participants will be enrolled in a randomized design to answer additional questions, such as the suitable frequency of BCM-BIS application and its possible long-term beneficial effects on clinical morbidities and mortality.
Acknowledgements
Special thanks is given to Dr Feidhlim Woods for his detailed revision of this paper, to Dr Michael Etter for his precious opinions and also to G. M. Irene Feng for her efforts to organize the proceeding of the whole project.
Conflict of interest statement
None declared.
References
- 1.Dorhout Mees EJ, Ozbasli C, Akcicek F. Cardiovascular disturbances in hemodialysis patients: the importance of volume overload. J Nephrol. 1995;8:71–78. [Google Scholar]
- 2.Chazot C, Charra B, Vo Van C, et al. The Janus-faced aspect of dry weight. Nephrol Dial Transplant. 1999;14:121–124. doi: 10.1093/ndt/14.1.121. doi:10.1093/ndt/14.1.121. [DOI] [PubMed] [Google Scholar]
- 3.Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol. 1999;10:392–403. doi: 10.1681/ASN.V102392. [DOI] [PubMed] [Google Scholar]
- 4.Wabel P, Moissl U, Chamney P, et al. Towards improved cardiovascular management: the necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant. 2008;23:2965–2971. doi: 10.1093/ndt/gfn228. doi:10.1093/ndt/gfn228. [DOI] [PubMed] [Google Scholar]
- 5.Devolder I, Verleysen A, Vijt D, et al. Body composition, hydration and related parameters in hemodialysis versus peritoneal dialysis patients. Perit Dial Int. 2010;30:208–214. doi: 10.3747/pdi.2008.00284. doi:10.3747/pdi.2008.00284. [DOI] [PubMed] [Google Scholar]
- 6.Passaure J, Petrov H, Schleser A, et al. Evaluation of clinical dry weight assessment in hemodialysis patients using bioimpedance spectroscopy: a cross-sectional study. Nephrol Dial Transplant. 2009;1:1–7. doi: 10.1093/ndt/gfp517. [DOI] [PubMed] [Google Scholar]
- 7.Wizemann V, Wabel P, Chamney P, et al. The mortality risk of overhydration in hemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–1579. doi: 10.1093/ndt/gfn707. doi:10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921–933. doi: 10.1088/0967-3334/27/9/012. doi:10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- 9.Chamney PW, Wabel P, Moissl UM, et al. A whole-body model to distinguish excess fluid from hydration of major body tissues. Am J Clin Nutr. 2007;85:80–89. doi: 10.1093/ajcn/85.1.80. [DOI] [PubMed] [Google Scholar]
- 10.Wieskotten S, Heinke S, Wabel P, et al. Modell-basierte Erkennung von Mangelern ährungmittels Bioimpedanzspektroskopie [Model-based Identification of Malnutrition via Bioimpedance Spectroscopy] At Automa-tisierungstechnik. 2007;10:531–538. doi:10.1524/auto.2007.55.10.531. [Google Scholar]
- 11.Ludbrook J. Confidence in Altman–Bland plots: a critical review of the method of differences. Clin Exp Pharmacol Physiol. 2010;37:143–149. doi: 10.1111/j.1440-1681.2009.05288.x. doi:10.1111/j.1440-1681.2009.05288.x. [DOI] [PubMed] [Google Scholar]
- 12.Machek P, Jirka T, Moissl U, et al. Guided optimization of fluid status in hemodialysis patients. Nephrol Dial Transplant. 2010;25:538–544. doi: 10.1093/ndt/gfp487. doi:10.1093/ndt/gfp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wabel P, Chamney P, Moissl U, et al. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009;27:75–80. doi: 10.1159/000167013. doi:10.1159/000167013. [DOI] [PMC free article] [PubMed] [Google Scholar]