Abstract
Cystic fibrosis (CF) is diagnosed in the first years of life. There are only two reports in the literature of adult patients with unusual presentation of newly diagnosed CF. We report here an adult patient apparently in a good health, who presented with serious hypokalaemia and metabolic alkalosis as the only abnormalities, who, through a fortuitous event, was tested by additional means for seemingly unrelated conditions that led to evidence of signs typical of CF, which was then confirmed by genetic analyses. This is the first adult patient in Italy with newly diagnosed CF. As unexplained hypokalaemia in an apparently healthy adult is very rare and has now been shown to represent an uncommon presentation of CF, physicians must take these facts into account when determining an appropriate imaging, biochemical work-up and genetic analyses to arrive at a diagnosis.
Keywords: cystic fibrosis, hypokalaemia
Background
Cystic fibrosis (CF) is generally diagnosed in the first few years of life, with respiratory or digestive symptoms usually leading to the diagnosis. However, it has been reported that about 4% of cases are not identified until adulthood [1, 2]. There are only two reports in the literature of adult patients with unusual presentation of newly diagnosed CF [3, 4]. This article reports the case of an adult patient apparently in good health, who presented with serious hypokalaemia and metabolic alkalosis after a very stressful physical exertion. Through a fortuitous event, he was tested by additional means for seemingly unrelated conditions that revealed signs typical of CF, which was then confirmed by genetic analyses.
Case report
In the summer of 2011, a 34-year-old Caucasian male farmer presented at the emergency room of a local hospital complaining about generalized weakness. He had been working outdoors in a very hot climate and sweated profusely. Clinical examination and electrocardiogram were unremarkable, and blood pressure was 123/78 mmHg. Routine blood tests were normal with the exception of severe hypokalaemia (2.2 mmol/L). After rehydration through i.v. infusion of 1 L of saline supplemented with 40 mmol of potassium chloride, the patient was discharged. One year later, the patient again presented with the same vague symptoms. Clinical examination and blood tests were unremarkable except for the recurrence of hypokalaemia (2.0 mmol/L). Following correction of hypokalaemia via intravenous infusion of 1 L of saline supplemented with 80 mmol of potassium chloride, the patient was referred to our nephrology clinic.
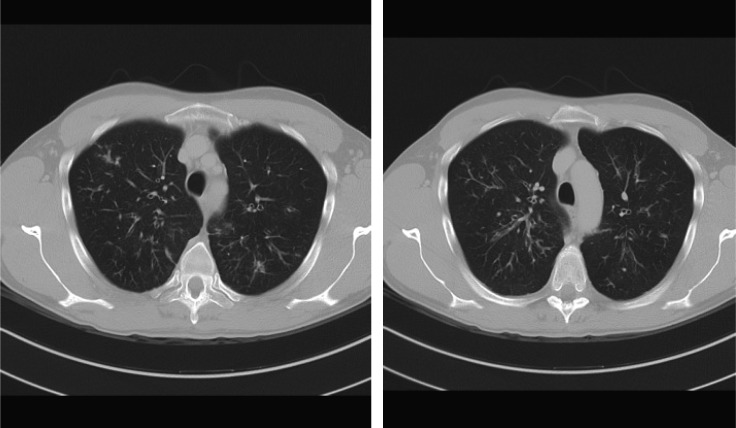
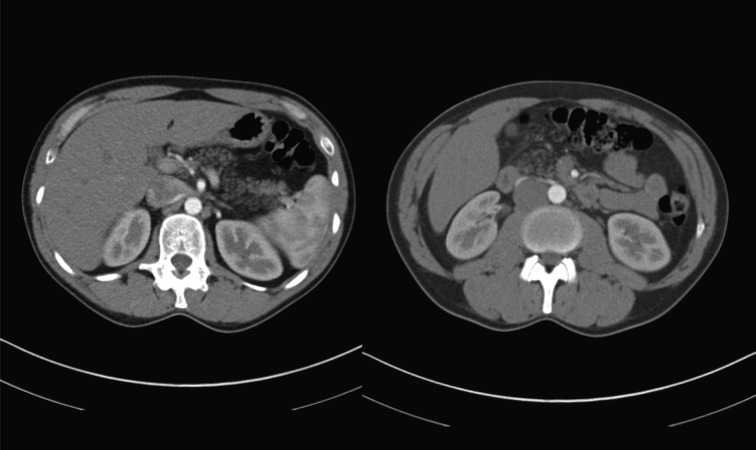
Upon admission to the Padova Nephrology Clinic, the patient had normal haematology results, normal renal function, Na 138 mmol, K 3.2 mmol, Mg 0.84 mmol, UNa 126 mmol/L, UK 5 mmol/L, UCl 10 mmol/L, urinary pH 7.5, HCO3− 30 mmol, anion GAP 15 mmol, aldosterone 187 pmol (normal range 21–415), cortisol 397 nmol (138–690) and plasma renin activity 3 μg/L/h (normal range 0.20–3.3). The chest X-ray was normal. Clinical history ruled out all of the common causes of hypokalaemia including vomiting, diarrhoea and use of diuretics. During his stay in our clinic, plasma K was normal, and the patient was going to be discharged without diagnostic explanation for the episodes of hypokalaemia. However, immediately prior to discharge, the patient complained about a colicky pain located in the left abdomen. Given the absence of clinical findings, an abdominal computerized tomography (CT) scan was ordered which was extended to include the chest given the presence of cough noted in this patient. The CT scan showed clear chest CF-related signs: ‘tree in bud’ pattern in both lungs along with bronchiectasis (Figure 1). The CT results for the abdomen showed that the pancreas was completely replaced by adipose tissue, again a pathognomic sign of CF (Figure 2). The patient then underwent a chloride sweat test, which was highly positive 138 mEq/L, (positive test >60 mEq/L).
Fig. 1.
CT scan showing ‘tree in bud’ aspect in both lungs.
Fig. 2.
CT scan showing adipose tissue replacement in the pancreas.
As CF also affects male sexual function [5], semen analysis was done and showed azoospermia, although the ultrasound of the scrotum showed the presence of vas deferens.
Genetic analysis revealed two heterozygous mutations of the Cystic Fibrosis transmembrane conductans regulator (CFTR) gene: the heterozygous deletion of exons 17a-18 and mutation of 2789+5 G→A. The patient's findings and symptoms suggest that this combination represents ‘mild’ mutations that result in a quantitative reduction in functional CFTR protein or normal dosing of functionally impaired CFTR protein.
Discussion
Around 90% of patients with CF are identified within the first 8 years of life, with 60% diagnosed before their first birthday [1, 2]. Respiratory and digestive symptoms and electrolytic abnormalities including metabolic alkalosis are a common clinical picture leading to the diagnosis of CF. Although it is estimated that about 4% of patients with CF will not be identified until adulthood, only two cases of adult patients with newly diagnosed CF with uncommon presentation have been reported in literature [3, 4]. To our knowledge, this case represents the first adult patient in Italy with newly diagnosed CF.
Systematic screening for CF in northeastern Italy was introduced in 1973 using incompletely digested proteins in the meconium as a marker [6], with subsequent changes to incorporate improvements/advances in the field of CF; current testing being based on a nongenetic test (immunoreactive trypsinogen, IRT) followed by genetic screening of those positive to IRT. Our patient therefore might have missed the screening or might represent a false-negative screening result, which is not unexpected given that the presumed initial CF neonatal screening test, IRT, has a sensitivity of under 95%.
The patient's presentation was, however, unusual given that a generalized weakness and hypokalaemia with metabolic alkalosis were the only definite abnormalities.
The majority of adult patients with CF have biochemical evidence of exocrine pancreatic insufficiency, and >80% of them do not release enough pancreatic enzymes into the gastrointestinal tract to support normal digestion [7]. Fatty replacement of the pancreas in CF is thought to result from protein plug obstruction of the acinar duct [8]. Interestingly, in patients with CF and severe pancreatic insufficiency, a large majority of CF patients have clinically apparent pancreatic exocrine insufficiency and, to lesser extent (30–50%), endocrine insufficiency [9, 10]. They are also thought to have more severe forms of lung disease and higher sweat chloride concentrations [11]. That chest radiographs, in our patient, were uninformative is perhaps explained by their relative insensitivity in the evaluation of the pulmonary status in patients with CF while CT has been shown to be superior [12]. In CF, the abnormally low airway mucus water content decreases mucous clearance resulting in mucous plugging of the airways and an increased incidence of bacterial airway infection. The resulting bronchial wall inflammation progresses to bronchiectasis and bronchiolar secretions that result in a tree-in-bud CT pattern. The nature and heterozygous pattern of the mutations found in our patient likely underpin his minimally overt symptoms and their clinical manifestation only after very stressful physical activity resulting in copious sweat production.
The pathophysiology of hypokalaemia in patients with CF has been attributed to secondary hyperaldosteronism [3] in response to the dehydration and loss of chloride through the sweat. However, the normal plasma potassium level upon the patient's potassium level correction before his presentation to our clinic may have masked any hyperaldosteronism, preventing this from providing a basis for a diagnosis. The diagnosis of CF came instead from a fortunate circumstance, i.e. the complaint of colicky abdominal pain in combination with a cough, the investigation of which finally provided the data necessary to arrive at a diagnosis. Therefore, as unexplained hypokalaemia in an apparently healthy adult is rare and has now been shown to represent an uncommon presentation of CF, a physician must take these facts into account when determining an appropriate imaging, biochemical work-up and genetic analysis to arrive at a diagnosis in these types of cases.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Southern KW, Munck A, Pollitt R, et al. ECFS CF Neonatal Screening Working Group. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6:57–65. doi: 10.1016/j.jcf.2006.05.008. doi:10.1016/j.jcf.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Bethesda, MD: Cystic Fibrosis Foundation: 2002. Cystic Fibrosis Foundation: Patient Registry 2001 Annual Data Report. [Google Scholar]
- 3.Bates C, Baum M, Quigley R. Cystic fibrosis presenting with hypokalemia and metabolic alkalosis in a previously healthy adolescent. J Am Soc Nephrol. 1997;8:352–355. doi: 10.1681/ASN.V82352. [DOI] [PubMed] [Google Scholar]
- 4.Davé S, Honney S, Raymond J, et al. An unusual presentation of cystic fibrosis in an adult. Am J Kidney Dis. 2005;45:e41–e44. doi: 10.1053/j.ajkd.2004.11.009. doi:10.1053/j.ajkd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Stahl PJ, Schlegel PN. Genetic evaluation of the azoospermic or severely oligozoospermic male. Curr Opin Obstet Gynecol. 2012;24:221–228. doi: 10.1097/GCO.0b013e3283558560. doi:10.1097/GCO.0b013e3283558560. [DOI] [PubMed] [Google Scholar]
- 6.Pederzini F, Armani P, Barbato A, et al. Newborn screening for cystic fibrosis. The methods compared on 229,626 newborns tested in 8 years in the Veneto Region. It J Pediatr. 1983;9:44–57. [Google Scholar]
- 7.Borgo G, Mastella G, Gasparini P, et al. Pancreatic function and gene deletion F508 in cystic fibrosis. J Med Genet. 1990;27:665–669. doi: 10.1136/jmg.27.11.665. doi:10.1136/jmg.27.11.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tjon A, Tham RTO, Heyerman HGM, et al. Cystic fibrosis: MR imaging of the pancreas. Radiology. 1991;179:183–186. doi: 10.1148/radiology.179.1.2006275. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich CF, Chichakli M, Hirche TO, et al. Sonographic findings of the hepatobiliary-pancreatic system in adult patients with cystic fibrosis. Ultrasound Med. 2002;21:409–416. doi: 10.7863/jum.2002.21.4.409. [DOI] [PubMed] [Google Scholar]
- 10.Soyer P, Spelle L, Pelage JP, et al. Cystic fibrosis in adolescents and adults: fatty replacement of the pancreas—CT evaluation and functional correlation. Radiology. 1999;210:611–615. doi: 10.1148/radiology.210.3.r99mr08611. [DOI] [PubMed] [Google Scholar]
- 11.Kubesh P, Dörk T, Wulbrand U, et al. Genetic determinants of airways colonization with Pseudomonas aeruginosa in cystic fibrosis. Lancet. 1993;341:189–193. doi: 10.1016/0140-6736(93)90062-l. doi:10.1016/0140-6736(93)90062-L. [DOI] [PubMed] [Google Scholar]
- 12.Helbich TH, Heinz-Peer G, Eichler I, et al. Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology. 1999;213:537–544. doi: 10.1148/radiology.213.2.r99nv04537. [DOI] [PubMed] [Google Scholar]