Abstract
Recent studies have shown that long noncoding RNAs (lncRNAs) play pivotal roles in the initiation and progression of cancer, including esophageal squamous cell carcinoma (ESCC). The lncRNA HOX transcript antisense RNA (HOTAIR) was reported to be dysregulated and correlated with the progression of ESCC. However, the biological role and the underlying mechanism of HOTAIR in the development of ESCC remain unclear. Herein, we found that HOTAIR was aberrantly upregulated in ESCC cells and that HOTAIR depletion inhibited proliferation and led to G1 cell cycle arrest in ESCC cells. Besides, we found that HOTAIR acted as an endogenous sponge to downregulate miR-1 expression by directly binding to miR-1. Furthermore, HOTAIR overturned the effect of miR-1 on the proliferation and cell cycle profile in ESCC cells, which involved the derepression of cyclin D1 (CCND1) expression, a target of miR-1. Taken together, our study elucidated a novel HOTAIR /miR-1/CCND1 regulatory axis in which HOTAIR acted as a competing endogenous RNA by sponging miR-1 and upregulated CCND1 expression, thereby facilitating the tumorigenesis of ESCC. Investigation of this lncRNA/miRNA/mRNA pathway may contribute to a better understanding of ESCC pathogenesis and facilitate the development of lncRNA-directed therapy against this disease.
Introduction
Esophageal cancer is one of the most common and lethal malignancies in the word, with the disease ranking eighth by global occurrence and sixth by global mortality rate among all cancer types [1]. Histologically, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) comprise more than 90% of esophageal cancer [2]. ESCC is the most frequent subtype of esophageal cancer in Eastern countries, causing more than 400,000 deaths each year [3]. Despite the wide application of chemotherapy, radiation therapy, and, if possible, esophagogastric resection, the overall survival (OS) for ESCC remains unsatisfying, with reported 5-year OS rates of less than 15% [4]. Therefore, it is imperative to quickly identify clinically applicable biomarkers for ESCC prognosis and to understand the crucial molecular mechanisms associated with this prevalent form of cancer.
Long noncoding RNAs (lncRNAs), which range in size from several hundred base pairs to tens of thousands of base pairs with lack of protein-coding capability, have recently attracted significant attention in delineating the complex mechanisms underlying malignant processes such as carcinogenesis, invasion, metastasis, and drug resistance. Although only a small percentage of functional lncRNAs have been well characterized to date, they have been shown to control every level of the gene expression program, like chromatin modification, transcription, and posttranscriptional processing [5]. The discovery of lncRNAs and the elucidation of the functions revealed a novel biological pathway involved in the regulation of gene expression in cancer. Recent studies have demonstrated that several lncRNAs, such as HOTAIR [6], POU3F3 [4], PlncRNA-1 [7], etc., are aberrant in ESCC, opening up a potential avenue for understanding the occurrence and development of ESCC.
Recently, a new regulatory circuitry has been identified in which lncRNAs can cross-talk with mRNAs through competition for shared miRNA-response elements. In this case, lncRNAs may function as competing endogenous RNAs (ceRNAs), namely, miRNA sponges or antagomirs, to downregulate the expression and activities of miRNAs, thereby modulating the derepression of miRNA targets and imposing an additional level of posttranscriptional regulation. The ceRNA-miRNA-mRNA regulatory interactions maintain the overall activity and functional balance of gene networks in a cell, and any perturbation of this system may lead to pathological processes such as cancer [8]. Thus, the complex mechanism underlying ceRNAs in cells is a fresh perspective for researchers to explore the language of RNA molecules and gene expression networks in tumorigenesis.
The HOX transcript antisense RNA (HOTAIR) is a ~2.2-kb lncRNA expressed from the HOXC locus, which was discovered as a repressor of the HOXD genes [9]. Subsequent studies suggested that HOTAIR is significantly overexpressed in a variety of tumors and associated with proliferation and metastasis of these tumors [10], [11]. Clinically, overexpression of HOTAIR in primary tumors is identified as a powerful outcome predictor for eventual metastasis and death [12], [13], [14]. Recent papers have reported that HOTAIR was aberrantly upregulated in ESCC and that HOTAIR expression was found to be an independent prognostic factor in ESCC patients [15], [16]. Nevertheless, the overall biological function and molecular mechanism of HOTAIR in ESCC carcinogenesis are far from being fully elucidated.
In this study, we determined HOTAIR levels in ESCC tissues and cells and investigated the effect of HOTAIR on cell proliferation and cell cycle of ESCC cells in vitro. Additionally, mechanistic analysis revealed that HOTAIR may serve as a ceRNA to upregulate the expression of CCND1 (cyclin D1) by competing for miR-1, thus promoting cell proliferation and regulating cell cycle in ESCC cells. These findings elucidated the first evidence for the cros-talk between HOTAIR, miR-1, and CCND1, shedding new light on the diagnosis and therapy for esophageal cancer.
Materials and Methods
Clinical Samples
Thirty-two pairs of ESCC tissues and the corresponding nontumor fresh specimens were obtained from the First Affiliated Hospital of Zhengzhou University. They were snap-frozen in liquid nitrogen and stored at −80°C immediately after resection. The protocol was approved by the Ethics Committee of Zhengzhou University, and all patients provided written informed consent for the utilization of the tissue samples in this study.
Cell Culture and Transfection
Six ESCC cell lines (KYSE30, KYSE140, KYSE150, KYSE180, KYSE410, and KYSE510) were purchased from the German Culture Collection (DSMZ, Braunschweig, Germany). Human normal esophageal epithelium cells (HEEpiC) were obtained from ScienCell (Carlsbad, CA). These cells were grown in RPMI 1640 (Biowhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) at 37°C under 5% CO2 and saturated moisture.
For investigating the role of HOTAIR, KYSE30 and KYSE510 cells were transfected with either siRNAs targeting HOTAIR (si-HOTAIR) or scrambled negative controls (si-NC; GenePharma, Shanghai, China). For investigating the relationship between HOTAIR and miR-1, HOTAIR cDNA and miR-1 mimics (GenePharma) were cloned into the mammalian expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA) individually or in combination, and then the vectors were transfected into cells. For the function study of CCND1, KYSE30 and KYSE510 cells were transfected with either si-CCND1 or si-NC. All transfection reactions were performed by using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from cultured cells using the Trizol reagent (Invitrogen, Carlsbad, CA) and reversely transcribed into cDNAs by using the Prime-Script one-step RT-PCR kit (TAKARA, Dalian, China). HOTAIR and CCND1 expression levels were determined by using PrimeScript RT-PCR kits (Takara Biochemicals, Kyoto, Japan), and glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. The expression level of miR-1 was determined using TaqMan miRNA assay (Applied Biosystems, Foster City, CA), and U6 snRNA was used as an endogenous control. All reactions were performed using the 7000 Sequence Detection System (Applied Biosystems). Comparative quantification was determined using the 2–∆∆Ct method.
Cell Proliferation Assay
Cell growth viability was measured using a Cell Counting Kit-8 (CCK8; Dojindo, Tokyo, Japan) following the manufacturer's instructions. Briefly, cells were seeded in the 96-well plate, and cell growth viability was detected every 24 hours. Absorbance was recorded at 450 nm by using a microplate reader (ELx 800; Bio-Tek Instruments Inc., Winooski, VT).
Cell Cycle Analysis
The cells were harvested, washed with PBS, and fixed with 70% ethanol at −20°C for 24 hours. The propidium iodide staining was conducted by using the Cell Cycle and Apoptosis Analysis Kit (Beyotime, Jiangsu, China). Then, cell cycle distribution was analyzed by BD LSRII Flow cytometry system with FACSDiva software (BD Biosciences, San Jose, CA).
Western Blot Analysis
The cultured cells were harvested and lysed using RIPA buffer (Sigma-Aldrich, St. Louis, MO). The protein concentration was quantified by using BCA Protein Assay Kit (Pierce Biotechnology Inc., Rockford, IL). The equivalent amounts of protein were separated by 10% SDS polyacrylamide gel electrophoresis and transfected to prewetted PVDF membranes. The membranes were then blocked in 5% nonfat milk for 1 hour and incubated with antihuman CCND1 (Abcam, Cambridge, MA) and β-actin antibody (Santa Cruz Biotech, Santa Cruz, CA) at 4°C overnight. On the following day, the membranes were washed and incubated with HRP-conjugated secondary antibody for 2 hours. Protein bands were detected using ImageQuant LAS 4000 and ECL Plus detection reagents (Santa Cruz Biotech).
Luciferase Assay
Cells were cotransfected with either empty vector or miR-1 and pMIR-Report Luciferase vector comprising 3′UTR of CCND1, wild-type, or mutant HOTAIR fragment. At 48 hours posttransfection, cells were harvested, and luciferase activity was measured using the dual-luciferase reporter assay system (Promega, Madison WI).
RNA Immunoprecipitation (RIP) Assay
RNA immunoprecipitaion assay was performed by using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit and the Ago2 antibody (Millipore, Billerica, MA) in accordance with the manufacturer's instructions. Briefly, KYSE30 cells were lysed in RIP lysis buffer, and the cell lysate was coimmunoprecipitated with anti-Ago2 antibody. Normal mouse IgG was used as a negative control, and anti-snRNP70 was used as a positive control. Then, proteinase K was added to digest the proteins. Immunoprecipitated RNA was isolated, and HOTAIR and miR-1 were detected by qRT-PCR.
Immunohistochemical Staining
The streptavidin-peroxidase staining method was performed to detect the CCND1 expression in ESCC tissues. The sections from tumor specimens were dewaxed in xylene and dehydrated with descending ethanol. After antigen retrieval in a microwave oven, the sections were treated with 3% hydrogen peroxide for 15 minutes to block the endogenous peroxide and rinsed with PBS. Then, the samples were incubated with primary antibody anti-CCND1 (Sigma, St. Louis, MO) at 4°C overnight and incubated with the secondary antibody conjugated with biotin. After washing, the streptavidin-peroxidase was added, and the blot was visualized by using diaminobenzidine tetrahydrochloride. The counterstaining of the samples was carried out by hematoxylin. The negative control was the substitution of PBS for primary antibody. Staining intensity was assessed microscopically by two pathologists who were blinded to details regarding patient background as absent staining = score 0, weak staining = score 1, moderate staining = score 2, and strong staining = score 3. The extent of staining was scored according to the percentage of the positively stained cells in 5 to 10 fields (0%-100%). The immunohistochemistry score was calculated by multiplying the intensity score with the extent of staining as described previously [17].
Statistical Analysis
All experiments were repeated three independent times, and all data were presented as mean ± standard deviation. Statistical analyses and plotting graphs were performed on the GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA). The differences between groups were examined by Student's t test, and the value of *P < .05, **P < .01, or ***P < .001 was considered statistically significant.
Results
The Knockdown of HOTAIR Repressed Proliferation and Led to G1 Cell Cycle Arrest in ESCC Cells
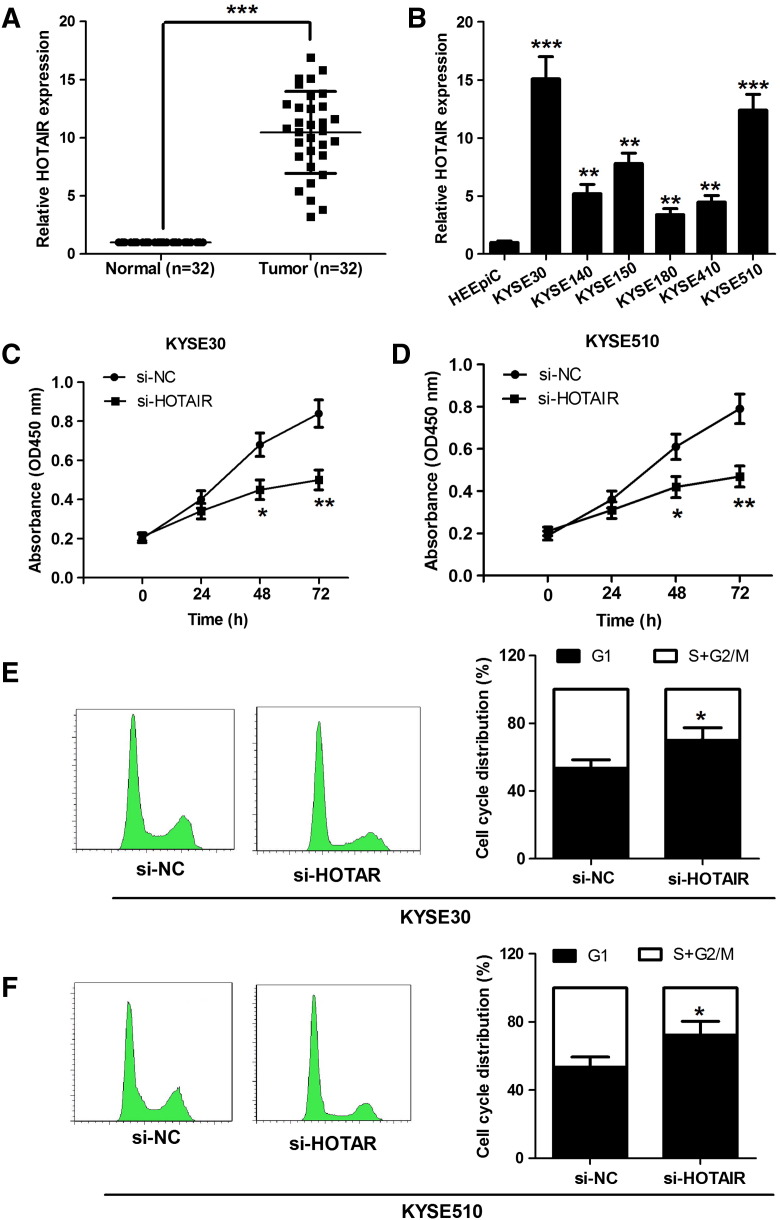
HOTAIR expression levels were assessed in 32 pairs of ESCC tissues and the corresponding nontumor fresh specimens by qRT-PCR analysis. We found that HOTAIR was obviously upregulated in ESCC tissues compared with that in nontumor tissues (Figure 1A). We next examined the expression level of HOTAIR in six ESCC cell lines (KYSE30, KYSE140, KYSE150, KYSE180, KYSE410, and KYSE510) and human normal esophageal epithelium cell line HEEpiC by qRT-PCR. As illustrated in Figure 1B, compared with HEEpiC, ESCC cells had a significant high HOTAIR expression status, especially in KYSE30 and KYSE510 cell lines. To understand the significance of HOTAIR upregulation in ESCC cells, we transfected KYSE30 and KYSE510 cells with si-HOTAIR or si-NC. Then, these cells were used to perform proliferation and cell cycle distribution detection. The data obtained from CCK8 assay showed that the knockdown of HOTAIR significantly inhibited ESCC cell proliferation (Figure 1, C and D). Flow cytometry analysis showed that the percentage of G1 phase cells increased in the KYSE30 and KYSE510 cells when they were introduced with si-HOTAIR (Figure 1, E and F).
Figure 1.
HOTAIR silencing inhibits cell proliferation and induces G1 cell cycle arrest in ESCC cells. (A) HOTAIR expression levels were assessed in 32 pairs of ESCC tissues and the corresponding nontumor fresh specimens by qRT-PCR analysis. (B) The data obtained from qRT-PCR show that, compared with HEEpiC, ESCC cells have a significantly high HOTAIR expression status, especially in KYSE30 and KYSE510 cell lines. (C and D) The data obtained from CCK8 assay show that the knockdown of HOTAIR significantly inhibits ESCC cell proliferation. (E and F) Flow cytometry analysis show that the percentage of G1 phase cells increases in the KYSE30 and KYSE510 cells when they are introduced with si-HOTAIR. *P < .05, **P < .01, and ***P < .001.
HOTAIR Reduced miR-1 Expression in ESCC Cells
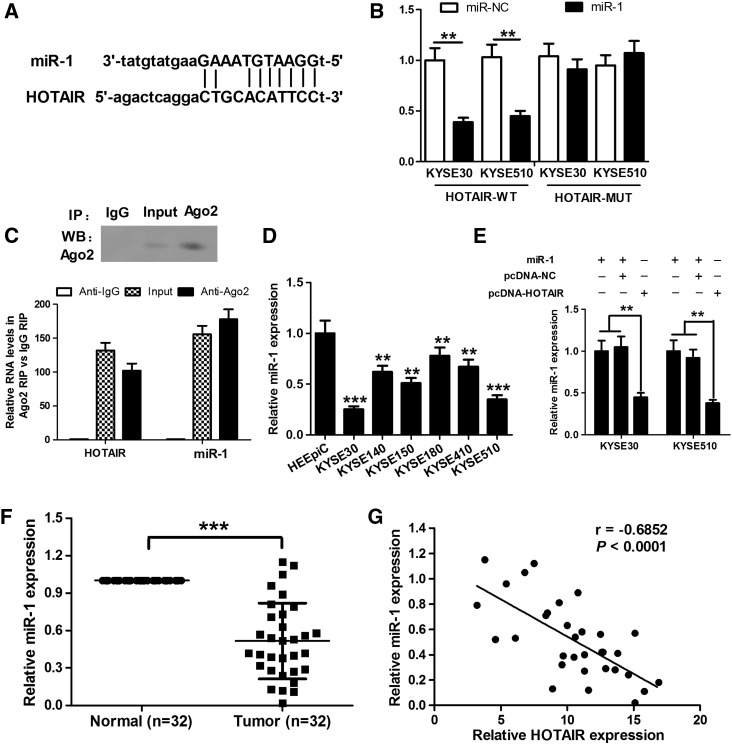
Many lncRNAs contain motif with sequence complementary to miRNAs and have an inhibition effect on miRNAs expression and activity, thereby playing a role in tumorigenesis and cancer progression. To investigate whether HOTAIR has a similar mechanism in ESCC, we performed the bioinformatics analysis of miRNA recognition sequences on HOTAIR by the online software starBase v2.0. The bioinformatics analysis showed that HOTAIR mRNA contained binding sequences complementary to miR-1 seed regions (Figure 2A). For further confirmation, we performed the dual-luciferase reporter assay. We subcloned the wild-type sequence of HOTAIR (HOTAIR-WT) or its mutant sequence (HOTAIR-MUT) into the pMIR luciferase reporter and then cotransfected with miR-1 mimics or miR-NC into KYSE30 and KYSE510 cells. The results showed that overexpression of miR-1 significantly reduced the luciferase activity of the pMIR luciferase reporter containing HOTAIR-WT but not the reporter containing HOTAIR-MUT (Figure 2B). It is well known that miRNAs may modulate their targets through forming RNA-induced silencing complex (RISC). Furthermore, recent data have shown that lncRNAs can act as molecular sponges to modulate the miRNAs activity by associating with RISC [17]. Therefore, we performed RIP assay on KYSE30 cell extracts using antibodies against Ago2, a key component of the RISC complex, to investigate whether both HOTAIR and miR-1 might be in the RISC complex. The results confirmed that the Ago2 antibody precipitated the Ago2 protein from KYSE30 cell extracts (Figure 2C, upper panel). Moreover, HOTAIR and miR-1 levels in immunoprecipitates were determined by qRT-PCR. As expected, HOTAIR and miR-1 levels were both significantly high in Ago2 pellets relative to control IgG immunoprecipitates (Figure 2C, lower panel). Accordingly, these results suggest that HOTAIR is present in Ago2-containing RISC, likely through association with miR-1, which was consistent with bioinformatic analysis and luciferase assays. Next, we determined miR-1 expression in six ESCC cell lines and human normal esophageal epithelium cells HEEpiC. As shown in Figure 2D, the decrease of miR-1 level was very evident in ESCC cells compared with HEEpiC, especially in KYSE30 and KYSE510 cell lines, which showed an opposite result to HOTAIR expression. Furthermore, we cloned HOTAIR cDNA into pcDNA3.1 vector and cotransfected into KYSE30 and KYSE510 cell cells with miR-1 mimics, and then detected miR-1 expression by qRT-PCR. As shown in Figure 2E, the overexpression of HOTAIR resulted in the decrease of miR-1 expression. Then, we correlated HOTAIR expression with the presence of miR-1 in ESCC tissues. As expected, miR-1 expression was also downregulated in ESCC tissues, and a negative correlation was observed between HOTAIR and miR-1 expression levels in ESCC tissues (r = −0.6852, P < .0001; Figure 2, F and G). Taken together, these data revealed that HOTAIR can decrease miR-1 expression.
Figure 2.
HOTAIR suppresses miR-1 expression in ESCC cells. (A) The bioinformatics analysis shows that HOTAIR mRNA contains binding sequences complementary to miR-1 seed regions. (B) Dual-luciferase reporter assay shows that overexpression of miR-1 significantly reduces the luciferase activity of the pMIR luciferase reporter containing the HOTAIR-WT but not the reporter containing the HOTAIR-MUT. (C) RIP assay shows that Ago2 antibody precipitates the Ago2 protein from KYSE30 cell extracts (upper panel). The data derived from qRT-PCR show that HOTAIR and miR-1 levels are both significantly high in Ago2 pellets relative to control IgG immunoprecipitates (lower panel). (D) qRT-PCR assay shows that ESCC cells, especially KYSE30 and KYSE510 cells, have lower miR-1 level than HEEpiC. (E) The overexpression of HOTAIR suppresses miR-1 expression. (F) qRT-PCR assay shows that miR-1 expression was downregulated in ESCC tissues compared with the corresponding nontumor specimens. (G) The correlation analysis shows a negative correlation between HOTAIR and miR-1 expression levels in ESCC tissues (n = 32). **P < .01 and ***P < .001.
HOTAIR Relieved the Inhibitory Effects of miR-1 on ESCC Cells
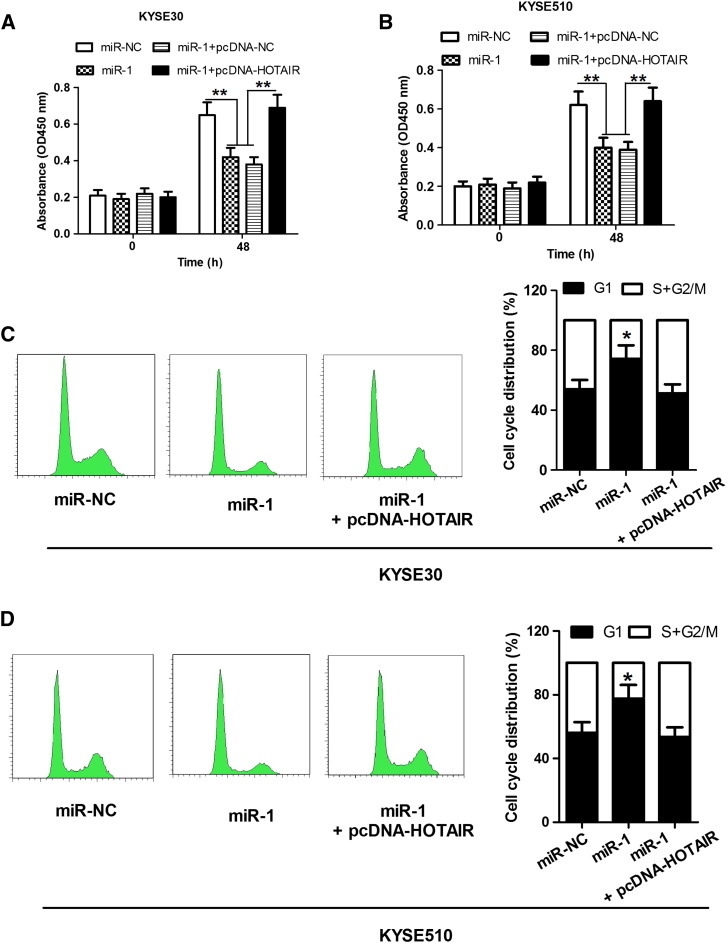
In view of the inhibitory effect of HOTAIR on miR-1 expression in ESCC cells, we further investigated whether HOTAIR had the same effect on the function of miR-1. We performed cell proliferation detection and found that the increase of miR-1 level significantly reduced cell viability in KYSE30 and KYSE510 cells. However, the treatment of miR-1 + pcDNA-HOTAIR abolished the inhibitory effect induced by miR-1 (Figure 3, A and B). In addition, we also performed the detection of cell cycle profile. The results showed that the increase of miR-1 level induced the G1 cell cycle arrest in KYSE30 and KYSE510 cells and that the treatment of miR-1 + pcDNA-HOTAIR relieved the G1 cell cycle arrest induced by miR-1 (Figure 3, C and D). These data showed that miR-1, acting as a tumor suppressor gene, inhibits cell proliferation and induces G1 cell cycle arrest in ESCC cells, whereas HOTAIR can overturn these inhibitory effects.
Figure 3.
HOTAIR relieves the inhibitory effects of miR-1 on ESCC cells. (A and B) CCK8 assays show that the increase of miR-1 level significantly reduces cell viability in KYSE30 and KYSE510 cells, and the treatment of miR-1 + pcDNA-HOTAIR abolishes the inhibitory effect induced by miR-1. (C and D) Flow cytometry analysis show that the increase of miR-1 level induces the G1 cell cycle arrest in KYSE30 and KYSE510 cells; inversely, the treatment of miR-1 + pcDNA-HOTAIR relieves the G1 cell cycle arrest induced by miR-1. *P < .05 and **P < .01.
HOTAIR Worked in ESCC Cells by Controlling the miR-1 Target, CCND1
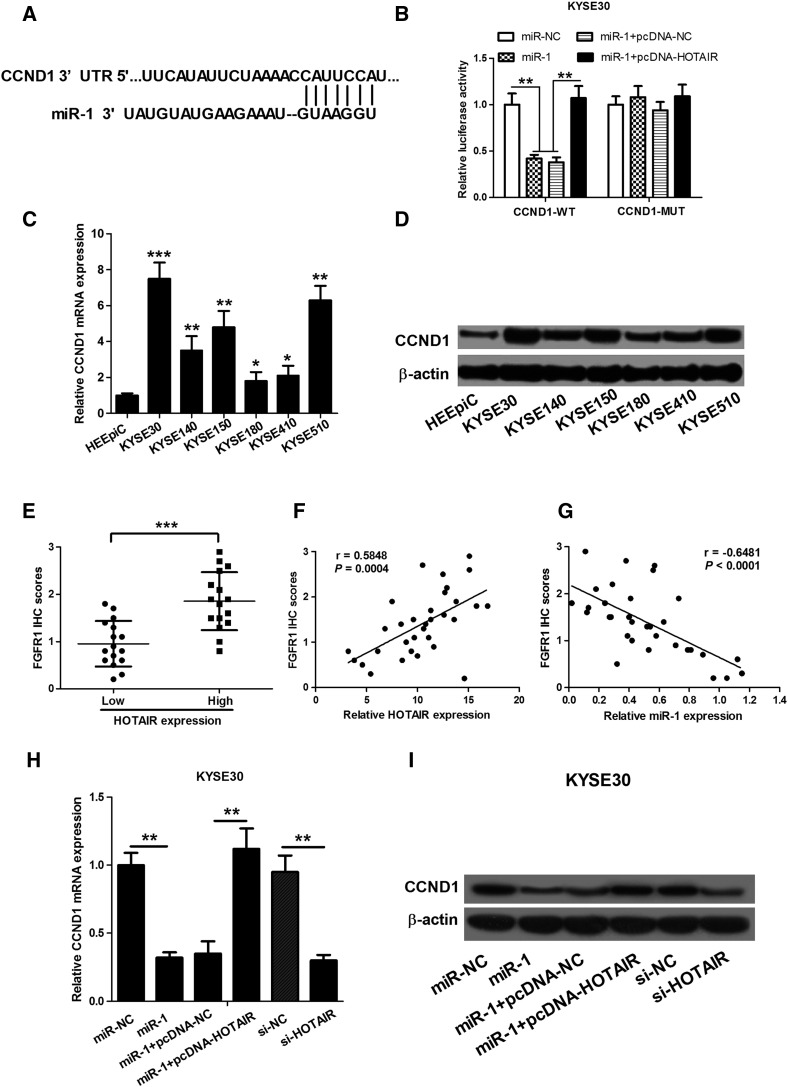
We further investigated the molecular mechanisms by which HOTAIR and miR-1 exert regulatory effects on ESCC cell proliferation and cell cycle distribution. We performed bioinformatics-based target prediction analysis by TargetScan and found that CCND1 is a potential target of miR-1 (Figure 4A). Then, dual-luciferase reporter assay was performed to identify whether the 3′UTR of CCND1 mRNA is a binding target of miR-1. The wild-type 3′UTR of CCND1 (CCND1-WT) or the corresponding mutant type was subcloned into the pMIR luciferase reporter and cotransfected with miR-NC, miR-1 mimics, miR-1 + pcDNA-HOTAIR, or miR-1 + pcDNA-NC into KYSE30 cells. The results showed that the increase of miR-1 level reduced the luciferase activity of the pMIR luciferase reporter containing CCND1-WT but not mutant reporter, confirming that CCND1 was a target of miR-1. However, the treatment of miR-1 + pcDNA-HOTAIR restored the luciferase activity of the pMIR luciferase reporter containing CCND1-WT but not mutant reporter as compared with miR-1 and miR-1 + pcDNA-NC groups (Figure 4B).
Figure 4.
HOTAIR works in ESCC cells by controlling the miR-1 target, CCND1. (A) Bioinformatics-based target prediction analysis shows that CCND1 is a potential target of miR-1. (B) Dual-luciferase reporter assay shows that the increase of miR-1 level reduces the luciferase activity of the pMIR luciferase reporter containing CCND1-WT, but not mutant reporter, and the treatment of miR-1 + pcDNA-HOTAIR restores the luciferase activity of the pMIR luciferase reporter containing CCND1-WT, but not mutant reporter, as compared with miR-1 and miR-1 + pcDNA-NC groups. (C and D) qRT-PCR and Western blot assay show that ESCC cells, especially KYSE30 and KYSE510 cells, have a higher level of CCND1 mRNA and protein than HEEpiC. (E) The immunohistochemistry shows that CCND1 levels in high-HOTAIR ESCC tissues were significantly higher than those in low-HOTAIR ESCC tissues. (F) The correlation analysis shows that CCND1 levels were positively correlated with HOTAIR expression in ESCC tissues. (G) The correlation analysis shows that CCND1 levels were inversely correlated with miR-1 expression in ESCC tissues. (H and I) Overexpression of miR-1 or knockdown of HOTAIR in KYSE30 cells markedly induces the expression of CCND1 mRNA and protein expression, and the treatment of miR-1 + pcDNA-HOTAIR regains the expression of CCND1 mRNA and protein. *P < .05, **P < .01, and ***P < .001.
Next, we detected the expression of CCND1 mRNA and protein in six ESCC cell lines and human normal esophageal epithelium cell line HEEpiC by qRT-PCR and Western blot. As shown in Figure 4, C and D, the increase of CCND1 mRNA and protein level was very obvious in ESCC cells compared with HEEpiC, especially in KYSE30 and KYSE510 cell lines, which showed an opposite result to miR-1 expression and a same result to HOTAIR expression. We also detected the CCND1 protein levels in ESCC tissues with low and high HOTAIR expression, and the median value of HOTAIR level was used to distinguish the two groups. As shown in Figure 4E, CCND1 levels in high-HOTAIR ESCC tissues were significantly higher than that in low-HOTAIR ESCC tissues. Moreover, CCND1 levels were positively correlated with HOTAIR expression in ESCC tissues (r = 0.5848, P = .0004; Figure 4F). However, CCND1 levels were inversely correlated with miR-1 expression in ESCC tissues (r = −0.6481, P < .0001; Figure 4G).
In addition, we detected the expression of CCND1 mRNA and protein in KYSE30 cells transfected with miR-NC, miR-1 mimics, miR-1 + pcDNA-HOTAIR, miR-1 + pcDNA-NC, si-HOTAR, or si-NC using qRT-PCR and Western blot. As shown in Figure 4, H and I, overexpression of miR-1 or knockdown of HOTAIR in KYSE30 cells markedly induced the expression of CCND1 mRNA and protein expression. However, the treatment of miR-1 + pcDNA-HOTAIR regained the expression of CCND1 mRNA and protein. These results showed that HOTAIR regulates the derepression of CCND1 by binding miR-1.
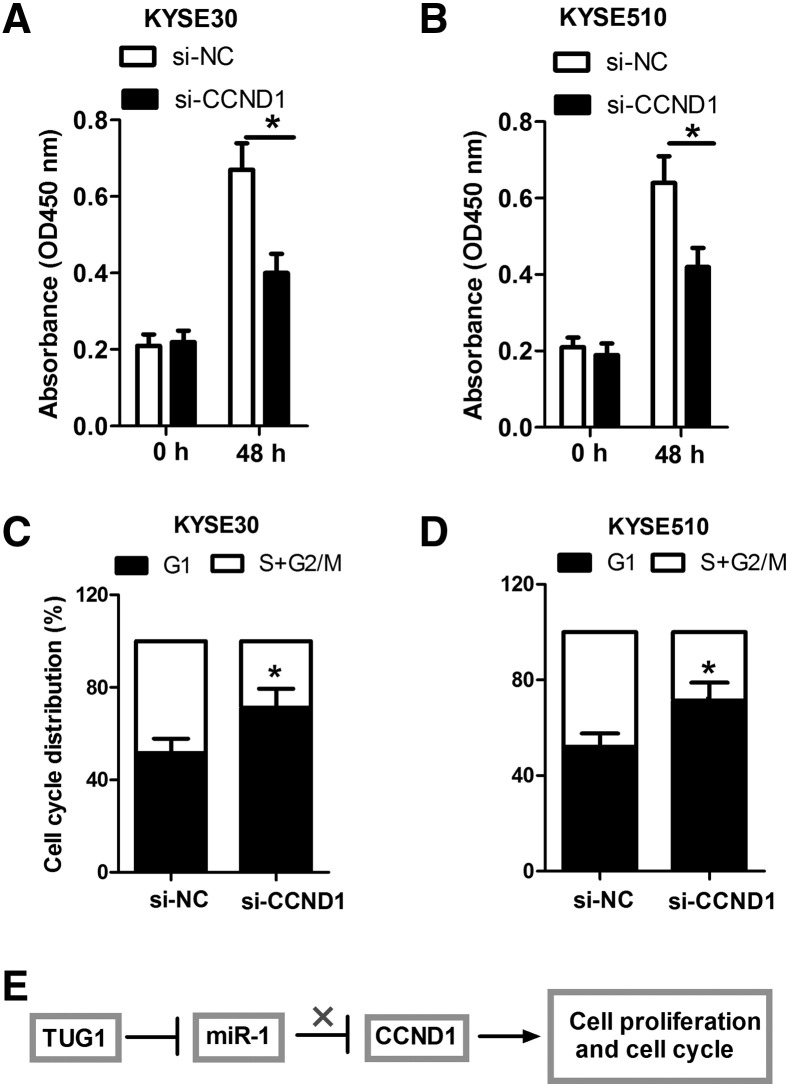
The CCND1 Silencing Inhibited Proliferation and Led to G1 Cell Cycle Arrest in ESCC Cells
To further investigate whether HOTAIR and miR-1 affected ESCC cell growth by regulating the expression of CCND1, we silenced CCND1 expression in KYSE30 and KYSE510 cells (data not shown) and then performed cell proliferation and cell cycle distribution detection. The results showed that the knockdown of CCND1 inhibited cell proliferation and induced G1 cell cycle arrest in YSE30 and KYSE510 cells (Figure 5, A–D). Taken together, these results elucidate a novel HOTAIR-miR-1-CCND1 pathway regulatory axis in which HOTAIR regulates the derepression of CCND1 by binding miR-1, therefore facilitating the tumorigenesis of ESCC (Figure 5E).
Figure 5.
CCND1 silencing inhibited proliferation and led to G1 cell cycle arrest in ESCC cells. (A and B) The knockdown of CCND1 inhibits cell proliferation in YSE30 and KYSE510 cells. (C and D) The knockdown of CCND1 induces G1 cell cycle arrest in YSE30 and KYSE510 cells. (E) Model diagram indicating the regulation mechanism of HOTAIR in ESCC. *P < .05.
Discussion
In this study, we measured the profile of HOTAIR in 32 paired cancerous and noncancerous tissue samples, as well as 6 ESCC cell lines and a normal esophageal epithelium cells (HEEpiC). Then, we tested the function of HOTAIR in ESCC cells by performing loss-of-function approaches. The data showed that HOTAIR was upregulated in ESCC tissues and cells. Furthermore, HOTAIR depletion suppressed cell proliferation and induced cell cycle arrest in KYSE30 and KYSE510 cells in vitro. These results were in line with the recent evidence that high expression level of HOTAIR was associated with proliferation, colony formation, and migratory capacity of ESCC cells [15], [16]. Our findings indicate that HOTAIR acts as an oncogene or exerts an active role to modulate multiple oncogenic properties in ESCC cells, providing a novel prognostic marker and therapeutic target for ESCC.
Recently, the lncRNA-miRNA-mRNA regulatory network has been widely identified, where lncRNA functions as ceRNA to interfere with miRNAs at posttranscriptional level, thus leading to derepression of miRNA target genes [18]. For example, HOTAIR may function as a ceRNA to block the expression of miR-331-3p and induce activation of its target gene HER2 in gastric cancer [19]. Moreover, lncRNA PTEN-P1 could sponge miR-19b and miR-20a, thereby modulating derepression of PTEN tumor suppressor in various malignancies including prostate cancer, glioblastoma, and melanoma, and the deregulation in this network induces tumorigenesis [20], [21]. Based on these findings, we speculated that lncRNA HOTAIR may participate in this system and act as a ceRNA in ESCC, and searched for potential interactions between HOTAIR and miRNA. To confirm this speculation, we performed bioinformatic analysis, RIP, and luciferase assays. As expected, we found that HOTAIR contains complementary base pairing with miR-1 and could directly bind to the predicted miRNA-response element of miR-1. In addition, results from qRT-PCR analysis showed that miR-1 expression was inversely correlated with HOTAIR level in ESCC tissues and cells. Moreover, ectopic overexpression of miR-1 could inhibit cell proliferation and induce cell cycle arrest in ESCC cells, whereas HOTAIR overturned these inhibitory effects of miR-1 on ESCC cells. Thus, HOTAIR may serve as an endogenous sponge to inhibit both the expression and function of miR-1.
Using online tools, we found CCND1 was a potential target of miR-1. So, we next focused on CCND1 to investigate whether HOTAIR-related function of miR-1 could result in the derepression of its mRNA targets. CCND1, which belongs to the family of D-type cyclin, is a common oncogene of human cancers, including breast cancer, lymphomas, gliomas, nasopharyngeal carcinoma, etc. [22], [23], [24]. CCND1 exerts carcinogenic action by promoting G1-S progression to regulate cell cycle [25]. In ESCC, elevated levels of CCND1 have been identified and predicate an unfavorable overall survival of surgically treated esophageal cancer patients [26], [27]. Here, we observed that there were an inverse correlation between the levels of CCND1 mRNA or protein expression and the levels of miR-1 expression in ESCC tissues and cells, and a positive correlation between CCND1 and HOTAIR expression. Then, luciferase assays confirmed that CCND1 was a direct target of miR-1 and that HOTAIR could abate the miR-1–induced repressing activity on 3′-UTR of CCND1, as well as mRNA and protein levels of HOTAIR. Moreover, CCND1 deletion could inhibit cell proliferation and G1-S transition in vitro. Therefore, CCND1 may coexpress with HOTAIR in ESCC cells, and the HOTAIR-miR-1-CCND1 regulatory network might be biologically significant in ESCC tumorigenesis.
All together, the findings presented in this study highlights that HOTAIR was determined as an oncogene by promoting proliferation and G1-S progression in ESCC cell lines. Mechanistic analysis revealed a HOTAIR-miR-1-CCND1 pathway regulatory network in which HOTAIR modulated the derepression of CCND1 by directly sponging miR-1, thereby promoting ESCC tumorigenesis. Moreover, identification the ceRNA activity of HOTAIR undoubtedly allows us to better understand the pathogenesis and development of ESCC and provides an lncRNA-directed target for this deadly disease.
Footnotes
Conflict of Interest: The authors declared that no conflict of interest exists.
References
- 1.Enzinger P.C., Mayer R.J. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Daly J.M., Fry W.A., Little A.G., Winchester D.P., McKee R.F., Stewart A.K., Fremgen A.M. Esophageal cancer: results of an American College of Surgeons patient care evaluation study. J Am Coll Surg. 2000;190(5):562–572. doi: 10.1016/s1072-7515(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P., Hulshof M., Van Lanschot J., Steyerberg E., Henegouwen M.B., Wijnhoven B., Richel D., Nieuwenhuijzen G., Hospers G., Bonenkamp J. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Tong Y.-S., Wang X.-W., Zhou X.-L., Liu Z.-H., Yang T.-X., Shi W.-H., Xie H.-W., Lv J., Wu Q.-Q., Cao X.-F. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer. 2015;14(1):1. doi: 10.1186/1476-4598-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Wu Z., Mei Q., Guo M., Fu X., Han W. Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Brit J Cancer. 2013;109(8):2266–2278. doi: 10.1038/bjc.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C.-M., Wu Q.-Q., Li S.-Q., Chen F.-J., Tuo L., Xie H.-W., Tong Y.-S., Ji L., Zhou G.-Z., Cao G. Upregulation of the long non-coding RNA PlncRNA-1 promotes esophageal squamous carcinoma cell proliferation and correlates with advanced clinical stage. Dig Dis Sci. 2014;59(3):591–597. doi: 10.1007/s10620-013-2956-7. [DOI] [PubMed] [Google Scholar]
- 8.Ergun S., Oztuzcu S. Oncocers: ceRNA-mediated cross-talk by sponging miRNAs in oncogenic pathways. Tumor Biol. 2015;36(5):3129–3136. doi: 10.1007/s13277-015-3346-x. [DOI] [PubMed] [Google Scholar]
- 9.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spizzo R., Almeida M.I., Colombatti A., Calin G.A. Long non-coding RNAs and cancer: a new frontier of translational? Oncogene. 2012;31(43):4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y., Zhang L., Wang Y., Li H., Ren X., Wei F., Yu W., Wang X., Zhang L., Yu J. Long noncoding RNA HOTAIR involvement in cancer. Tumor Biol. 2014;35(10):9531–9538. doi: 10.1007/s13277-014-2523-7. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z.-H., Wang X.-L., Tang H.-M., Jiang T., Chen J., Lu S., Qiu G.-Q., Peng Z.-H., Yan D.-W. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep. 2014;32(1):395–402. doi: 10.3892/or.2014.3186. [DOI] [PubMed] [Google Scholar]
- 13.Nie Y., Liu X., Qu S., Song E., Zou H., Gong C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104(4):458–464. doi: 10.1111/cas.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Wang Y., Yu J., Dong R., Qiu H. A high level of circulating HOTAIR is associated with progression and poor prognosis of cervical cancer. Tumor Biol. 2015;36(3):1661–1665. doi: 10.1007/s13277-014-2765-4. [DOI] [PubMed] [Google Scholar]
- 15.Lv X.-B., Lian G.-Y., Wang H.-R., Song E., Yao H., Wang M.-H. Long noncoding RNA HOTAIR is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F.J., Sun M., Li S.Q., Wu Q.Q., Ji L., Liu Z.L., Zhou G.Z., Cao G., Jin L., Xie H.W. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog. 2013;52(11):908–915. doi: 10.1002/mc.21944. [DOI] [PubMed] [Google Scholar]
- 17.Wang F., Ying H.-Q., He B.-S., Pan Y.-Q., Deng Q.-W., Sun H.-L., Chen J., Liu X., Wang S.-K. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6(10):7899. doi: 10.18632/oncotarget.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia T., Liao Q., Jiang X., Shao Y., Xiao B., Xi Y., Guo J. Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci Rep. 2014;4 doi: 10.1038/srep06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X.-h., Sun M., Nie F.-q., Ge Y.-b., Zhang E.-b., Yin D.-d., Kong R., Xia R., Lu K.-h., Li J.-h. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13(1):1. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu G., Yao W., Gumireddy K., Li A., Wang J., Xiao W., Chen K., Xiao H., Li H., Tang K. Pseudogene PTENP1 functions as a competing endogenous RNA to suppress clear-cell renal cell carcinoma progression. Mol Cancer Ther. 2014;13(12):3086–3097. doi: 10.1158/1535-7163.MCT-14-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poliseno L., Salmena L., Zhang J., Carver B., Haveman W.J., Pandolfi P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessendorf S., Schwaenen C., Kohlhammer H., Kienle D., Wrobel G., Barth T.F., Nessling M., Möller P., Döhner H., Lichter P. Hidden gene amplifications in aggressive B-cell non-Hodgkin lymphomas detected by microarray-based comparative genomic hybridization. Oncogene. 2003;22(9):1425–1429. doi: 10.1038/sj.onc.1206297. [DOI] [PubMed] [Google Scholar]
- 23.Xie L., Xu L., He Z., Zhou W., Wang L., Zhang L., Lan K., Ren C., Liu W., Yao K. Identification of differentially expressed genes in nasopharyngeal carcinoma by means of the Atlas human cancer cDNA expression array. J Cancer Res Clin. 2000;126(7):400–406. doi: 10.1007/pl00008488. [DOI] [PubMed] [Google Scholar]
- 24.Büschges R., Weber R.G., Actor B., Lichter P., Collins V.P., Reifenberger G. Amplification and expression of cyclin D genes (CCND1 CCND2 and CCND3) in human malignant gliomas. Brain Pathol. 1999;9(3):435–442. doi: 10.1111/j.1750-3639.1999.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tashiro E., Tsuchiya A., Imoto M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007;98(5):629–635. doi: 10.1111/j.1349-7006.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarbia M., Stahl M., Fink U., Heep H., Dutkowski P., Willers R., Seeber S., Gabbert H.E. Prognostic significance of cyclin D1 in esophageal squamous cell carcinoma patients treated with surgery alone or combined therapy modalities. Int J Cancer. 1999;84(1):86–91. doi: 10.1002/(sici)1097-0215(19990219)84:1<86::aid-ijc16>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Sunpaweravong P., Sunpaweravong S., Puttawibul P., Mitarnun W., Zeng C., Baron A.E., Franklin W., Said S., Varella-Garcia M. Epidermal growth factor receptor and cyclin D1 are independently amplified and overexpressed in esophageal squamous cell carcinoma. J Cancer Res Clin. 2005;131(2):111–119. doi: 10.1007/s00432-004-0610-7. [DOI] [PubMed] [Google Scholar]