Abstract
PURPOSE: Human papillomavirus (HPV) type 16 is one of the major etiologic factors of cervical cancer. Our study aims to investigate the potentiality of the antiviral clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated Cas9 system (CRISPR/Cas9) targeting the E6 and E7 oncogenes of HPV16 as a potential chemosensitizer of cisplatin (cis-diaminedichloroplatinum II; CDDP) for cervical cancer. METHODS: Specifically, the therapeutic efficacy of combination of CDDP and HPV16 E6 + E7-CRISPR/Cas9 was assessed in cervical cancer cells and cervical cancer xenograft models. RESULTS: In vitro experiments showed that long-term exposure of SiHa cells to the HPV16 E6 + E7-CRISPR/Cas9 induced apoptosis, and its pro-apoptosis effect became more obvious when combined with CDDP. In vivo study found the efficacy of the combination of HPV16 E6 + E7-CRISPR/Cas9 and CDDP were superior to either of the treatments in term of apoptosis induction and metastasis inhibition. CONCLUSION: Collectively, our results suggested that HPV16 E6 + E7-CRISPR/Cas9 could be an effective sensitizer of CDDP chemotherapy in cervical cancer.
Introduction
Cervical cancer is one of the most common types of gynecological malignancies worldwide. There are 450,000 new cases and approximately 233,000 deaths per year caused by cervical cancer. Infection with high-risk human papillomaviruses (HPV), such as type 16 and 18, is a major cause of cervical cancer [1], [2], [3], [4].
The E6 and E7 proteins encoded by HPV play major roles in the development and maintenance of malignancy in cervical cancer [5], [6]. The E6 viral oncoprotein binds to wide-type tumor suppressor p53, while E7 binds to the retinoblastoma (RB) family of tumor suppressor proteins and disrupts RB/E2F complexes, thereby driving cell division. Therefore, the E6 and E7 oncogenes represent ideal targets for gene therapy of cervical cancer.
In previous studies, RNAi technique is used to inhibit gene expression involved in various human diseases. Several therapeutic strategies including the application of shRNAs targeting E6 oncogene presented inhibitory effect on cervical cancer cell growth [7], [8]. Recently, CRISPR/Cas9 has been developed as a novel therapeutic strategy and has entered into clinical trials [9]. As the first report of inhibition of tumor growth derived from cervical cancer cells with a mixture of CRISPR/Cas9 targeting HPV gene [10], we demonstrate that the CRISPR/Cas9 specific to HPV16 oncogenes including targeting the E6, E7-transcript can effectively, specifically and stably suppress E6 and E7 expression in cervical cancer and inhibit the cancer cell growth. These results suggest that CRISPR/Cas9 targeting HPV key oncogenes as a new strategy for cervical cancer and other HPV-associated cancer therapy.
CDDP is one of the commonly used first-line chemotherapy agents for various cancers. CDDP-based chemotherapy is an important treatment option for patients with unresectable recurrent or metastatic cervical cancer [11], [12]. CDDP inhibits HPV E6/E7 expression [13], and allows p53 to escape from E6-mediated degradation, thereby leading to the accumulation of p53 in the nucleoli of HeLa cells to induce apoptosis [14]. Combination of CDDP and radiotherapy restores p53 function and enhances the radiosensitivity of HPV-16-positive SiHa cells [15]. Unfortunately, the severe side effects limit the clinical use of high-dose CDDP, and the development of resistance poses another major obstacle for long-term use of CDDP [16], [17]. Establishment of new therapeutic strategies either sensitizing cancer cell to CDDP or relieving the side effects of CDDP will undoubtedly benefit cancer patients.
Here, we test the ability of HPV16 E6/E7-CRISPR/Cas9 to sensitize HPV16 positive cervical cancer cell SiHa to CDDP in vitro and in vivo, and find that long-term combinatory exposure to E6 + E7- CRISPR/Cas9 and CDDP exert synergistic cytotoxicity and antitumor effects both on SiHa cells and in xenograft mouse models of cervical cancer.
Material and Methods
Plasmids
The hCas9 expression vector was a gift from Xingxu Huang (Addgene plasmid # 44,758) [18], and gRNA cloning vectors was a gift from George Church (Addgene plasmid # 41,824) [19]. The plasmids were prepared by using the Qiagen Endofree Plasmid Kit (Qiagen; Hilden, Germany).
Construction of gRNA Expression Plasmids
gRNA expression plasmids were constructed according to manufacturer's protocol [10]. Briefly, to prepare a 100-bp dsDNA insert fragment containing the target sequence (20 bp) and a protospacer-adjacent motif (PAM) sequence, we used a set of oligonucleotides and generated the fragment using T4 PNK (NEB; Ipswich, MA, USA). The dsDNA fragments were purified and inserted into the BbsI site of a gRNA cloning vector with T4 DNA ligase (NEB; Ipswich, MA, USA). Detailed BLAST searching of human and murine genomes was carried out to identify potential off target binding of HPV gRNAs. Two sets of oligonucleotides were designed. All oligonucleotides were synthesized and purified by Sangon Biotech Co. (Shanghai, China). The sgRNAs specific to HPV16 E6 gene were 5′-CACCGCAACAGTTACTGCGACGTG-3′, and 5′-AAACCACGTCGCAG TAACTGTTGC-3′. The sgRNAs specific to HPV16 E7 gene were 5′-CACCGACACGTAGACATTCGTACTT-3′, and 5′-AAACAAGTACGAAT GTCTACGTGT-3′ [10].
Cell Culture and Transfection
The human cervical cancer cell lines SiHa and C33-A were used. SiHa cells contain a single copy of HPV16 integrated in the chromosome and express the E6 and E7 oncogenes [20], whereas C33-A cells were negative for HPV. Cells were maintained in Dulbecco's modified Eagle's minimal essential medium (DMEM), supplemented with 10% fetal calf serum (Hyclone; Logan, UT, USA) at 37°C in a humidified atmosphere containing 5%CO2. SiHa cells were seeded into 6-well plates and grew until 60% to 80% confluency, after which they were transfected with 1 μg of hCas9 expression vector and 1 μg of gRNA expression vector using Lipofectamine2000 (Invitrogen; Carlsbad, CA, USA) according to the manufacturer's instructions. Cells were harvested two days after transfection.
CDDP Treatment and Combinatory Treatment with CRISPR/Cas9 Transfection
CDDP was obtained from Sigma-Aldrich (St. Louis, MO, USA). For CDDP + CRISPR/Cas9 combination treatment, cells were exposed to CRISIR/Cas9 and subsequently exposed to CDDP after washing with phosphate buffered saline (PBS).
Real-Time RT-PCR
For quantitative real-time RT-PCR, SiHa cells were transfected with CRISPR/Cas9 as previously described. Total RNA was extracted with TRIzol reagent (Invitrogen; Carlsbad, CA, USA), the mRNA expression of E6 and E7 was determined by RT-PCR [21]. GAPDH was used as an internal reference. The PCR primers were as follows, E6 forward, 5′-AATGTTTCAGGACCCACAGG-3′; E6 reverse, 5′-TCAGGACACAGTGGCTTTTG-3′; E7 forward, 5′-ATGCATGGAGATACACCTACATTGC-3′; E7 reverse, 5′-ACAATTCCTA GTGTGCCCAT TAACA-3′. In total, three independent experiments were performed.
Western Blot
Western blotting was performed as previously described [10]. Primary antibodies used were mouse monoclonal anti-p53 and anti-GAPDH, and goat polyclonal anti-p21 (Santa Cruz Biotechnologies; Santa Cruz, CA, USA). GAPDH was used as internal control for protein loading and analysis.
Flow Cytometry Analysis
After single-agent or combination therapy, apoptotic cells were stained with Annexin V-fluorescence isothiocyanate (FITC) and propidium iodide (PharMingen; San Diego, CA, USA) according to the manufacturer's instructions and apoptotic cells were quantified by flow cytometry. All experiments were performed in triplicate, and repeated three times.
In Vivo Xenografts
Pathogen-free female BALB/C nude mice (aged 4–5 week, SPF grade and weighing 18–20 g) were purchased from the center of experimental animal, the Academy of Military Medical Science (Beijing, China). All animal experiments and protocols were performed strictly in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animals were kept and the experiments were performed in accordance with committee's criteria for the care and use of laboratory animals. Approval for animal experiments was obtained from the institutional animal welfare committee.
SiHa cells (1×107) in 0.2 ml of serum-free medium were injected subcutaneously into the right flanks of the mice. Three weeks after the injection of the cells, mice were randomly divided into four groups (eight mice per group): the empty vector group, CDDP-treated group, E6 + E7-treated group, E6/E7-CRISPR/Cas9-treated group. CRISPR/Cas9 and gRNA plasmids were delivered into mice using the hydrodynamic tail vein injection method [22]. Briefly, 40 μg Cas9 and 20 μg E6-gRNA and/or 20 μg E7-gRNA, dissolved in 1.5 ml Ringer's solution (147 mM NaCl, 4 mM KCl and 1.13 mM CaCl2), were rapidly injected into the tail vein. On the day after the CRISPR/Cas9 injection, CDDP (4 mg/kg body weight) was intraperitoneally injected. In animals treated with CDDP alone, CDDP was intraperitoneally injected every 7-day intervals. In addition, tumor size was measured with digital calipers and volumes were calculated as length × width × height. Mice were killed on day 21 (day 0 was set as the first injection occurred), and the tumor samples were evaluated by real time PCR, western blot, and flow cytometry.
Statistical Analysis
The statistical significance between groups was determined by the Mann–Whitney U test. P < .05 was considered statistically significant. All statistical analyses were performed using the SPSS software (SPSS, Chicago, IL, USA).
Results
Inhibition of HPV16 E6 and E7 Expression by CRISPR/Cas9
HPV16 E6- and E7-CRISPR/Cas9 were transfected into SiHa cells to examine the knocking down efficiency. Real-time RT-PCR showed that E6-CRISPR/Cas9 knocked down HPV16-E6 and -E7 genes expression by about eight times relative to negative control, which was similar to the results in our previous article [10]. These recombinant plasmids were then used in the following experiments.
The Anti-Viability Effect of HPV16 E6/E7-CRISPR/Cas9
HPV16 E6- and E7-CRISPR/Cas9 was transfected into HPV16 positive (SiHa) and HPV16 negative (C33-A) cancer cells, respectively, to observe its effect on cell viability. As shown in Figure 1, both HPV16 E6- and E7-CRISPR/Cas9 severely compromised viability of SiHa cells, while they had minor effect on C33A cell viability. These results suggested that HPV16 E6- and E7-CRISPR/Cas9 exerted specific anti-viability activity on HPV16 positive cells.
Figure 1.
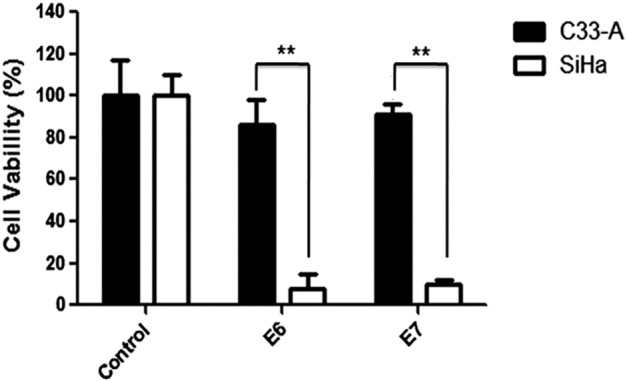
The specificity and activity on cell proliferation of HPV16-E6/E7- CRISPR/Cas9. Transfection of HPV-16-E6/E7-CRISPR/Cas9 did not affect proliferation of HPV-negative C33-A cervical cancer cells, while the proliferation was severely inhibited by transfection of either HPV-16-E6-CRISPR/Cas9 or HPV-16-E7-CRISPR/Cas9. The figures are representative of three independent experiments. Data represent the mean of three impendent experiments ± SE. The asterisk represents statistically significant difference compared with negative control (*P < .05; **P < .01).
Cytotoxicity of HPV16 E6/E7- CRISPR/Cas9 and In Vitro Synergistic Effect of HPV16 E6/E7-CRISPR/Cas9 and CDDP
According to conventional gynecologic practice, 33 μM of CDDP could strongly reduce HPV16 E6 oncogene expression in HPV-positive cancer cells [13], this concentration of CDDP was therefore used in the following experiments. To assess the cytotoxicity of HPV16 E6/E7 CRISPR/Cas9, SiHa cells were exposed to HPV16 E6/E7 CRISPR/Cas9 and 33 μM of CDDP for 7 days, respectively. As shown in Figure 2, the most outstanding cytotoxicity was observed in cells transfected by E6 + E7 CRISPR/Cas9 among other treatments. The additive cytotoxicity of HPV16 E6/E7 CRISPR/Cas9 and CDDP was further examined, by which cells were exposed to 33 μM of CDDP for 4 days followed by HPV16 E6/E7-CRISPR/Cas9 transfections on subsequent days. Cells exposed to CDDP combined with E6 + E7-CRISPR/Cas9 suffered the most significant cell growth inhibition compared to cells exposed to CDDP alone. Of note, short-term (less than 3 days) exposure of SiHa cells to CDDP combined with HPV16 E6/E7 CRISPR/Cas9 exhibited no synergistic cytotoxicity (data not shown).
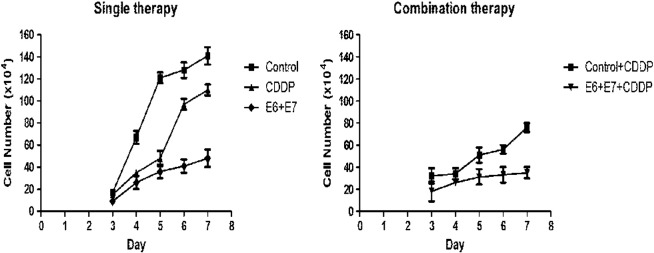
Figure 2.
Cytotoxic effect of single or combinatory treatment of CDDP, HPV16 E6/E7-CRISPR/Cas9 on SiHa cells. (A) Compared with negative control cells, HPV16 E6/E7-CRISPR/Cas9 exerted most significant cytotoxic effect on SiHa cells. (B) The cytotoxicity of CDDP was strengthened when combined with HPV16 E6/E7-CRISPR/Cas9. Data represent the mean of three impendent experiments ± SE. The asterisk represents statistically significant difference compared with negative control (*P < .05; **P < .01).
Effect of HPV16 E6- and E7-CRISPR/Cas9 in Combination with CDDP on Apoptosis and Cell Recovery
To assess the effect of CDDP and E6/E7-CRISPR/Cas9 treatment on cell apoptosis, adherent cells were collected and protein were extracted after 6-day exposure and the expression of p53 and p21 were analyzed by western blot. As shown in Figure 3A, apoptotic proteins P53 and P21 were enhanced in E6 + E7-CRISPR/Cas9-transfected cells compared with that of CDDP-treated cells.
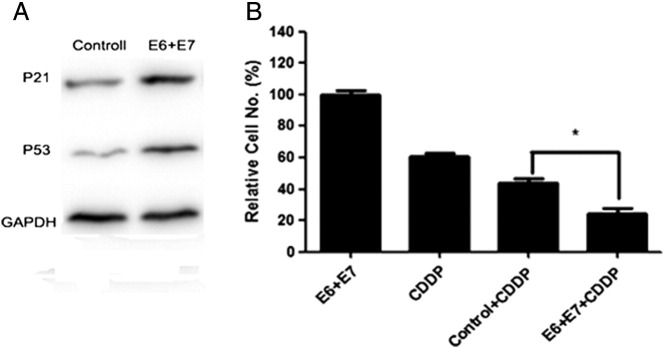
Figure 3.
The effect of CDDP and HPV16 E6/E7-CRISPR/Cas9 on cell apoptosis and recovery. (A) Western blot results showed that HPV16 E6/E7-CRISPR/Cas9 caused the most significant increase of P53 and P21 expression.(B) Cell recovery assay showed that CDDP and HPV16 E6/E7-CRISPR/Cas9 co-treatment most significantly inhibited cell recovery ability. The figures are representative of three independent experiments. Data represent the mean of three impendent experiments ± SE. The asterisk represents statistically significant difference compared with negative control (*P < .05; **P < .01).
Cells exposed to long-term (7 days) single or combinatory treatment of CDDP and E6/E7-CRISPR/Cas9 were subsequently incubated for 7 days in normal media. Cells recovery was most slow from exposure to HPV16 E6 + E7-CRISPR/Cas9 + CDDP among other treatment (Figure 3B).
Synergistic Inhibition of In Vivo Tumor Growth by Combination Therapy with HPV16 E6/E7-CRISPR/Cas9 and CDDP
The inhibitory efficacy of HPV16 E6/E7-CRISPR/Cas9 and CDDP co-treatment on cervical cancer growth was further examined in xenograft mice models. Compared with CDDP single treatment, HPV16 E6/E7-CRISPR/Cas9 and CDDP co-administration significantly restrained tumor growth as illustrated by much smaller tumor volume in mice co-treated with HPV16 E6 + E7 + CDDP(Figure 4A and B). And the mRNA level of HPV16 E6 and E7 was decreased in tumor tissues of co-treated mice relative to that of CDDP single treated mice (Figure 4C and D). These data confirmed that HPV16 E6 + E7-CRISPR/Cas9 could potentiate the therapeutic efficacy of CDDP for cervical cancer.
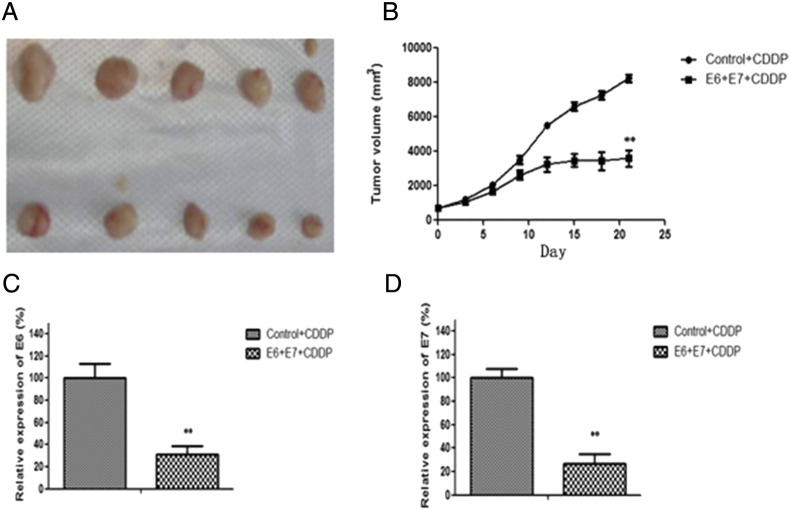
Figure 4.
Synergistic inhibition of CDDP and HPV16 E6/E7-CRISPR/Cas9 co-treatment on xenograft tumor growth. (A) Mice were killed at day 21 after first injection, tumor were removed and photographed.; (B) The volumes of xenograft tumors were measured and plotted at indicated time points after treatment.(C-D) The total RNA was isolated from tumortissues, and the expression of E6 and E7were detected by real-time RT-PCR. Data represent the mean of three impendent experiments ± SD. The asterisk represents statistically significant difference compared with negative control (*P < .05; **P < .01).
The Inhibitory Effect of HPV16 E6/E7-CRISPR/Cas9 and CDDP Co-Treatment on In Vivo Cancer Cell Apoptosis and Lung Metastasis
To elucidate the inhibitory mechanism of HPV16 E6/E7-CRISPR/Cas9 and CDDP co-treatment on xenograft tumor growth, the expression of apoptosis-associated protein including P53, Bax and Bcl-2 in xenografts was detected using western blot. Compared to CDDP-treated mice, pro-apoptosis proteins P53 and Bax were elevated while anti-apoptosis protein Bcl-2 was reduced in tumor specimens of HPV16 E6/E7-CRISPR/Cas9 and CDDP co-treated-mice (Figure 5A). And flow cytometry analysis of xenografts showed a significant increase of apoptotic cells in tumor specimens of HPV16 E6/E7-CRISPR/Cas9 and CDDP co-treated-mice relative to CDDP-treated mice (Figure 5B). Finally, histopathological examination found more atypical nuclei in the lungs of CDDP -treated-mice than that in the HPV16 E6/E7-CRISPR/Cas9 and CDDP co-treated-mice, indicating the enhanced inhibitory effect of HPV16 E6/E7-CRISPR/Cas9 and CDDP co-treatment on lung metastasis of cervical cancer cells (Figure 6).
Figure 5.
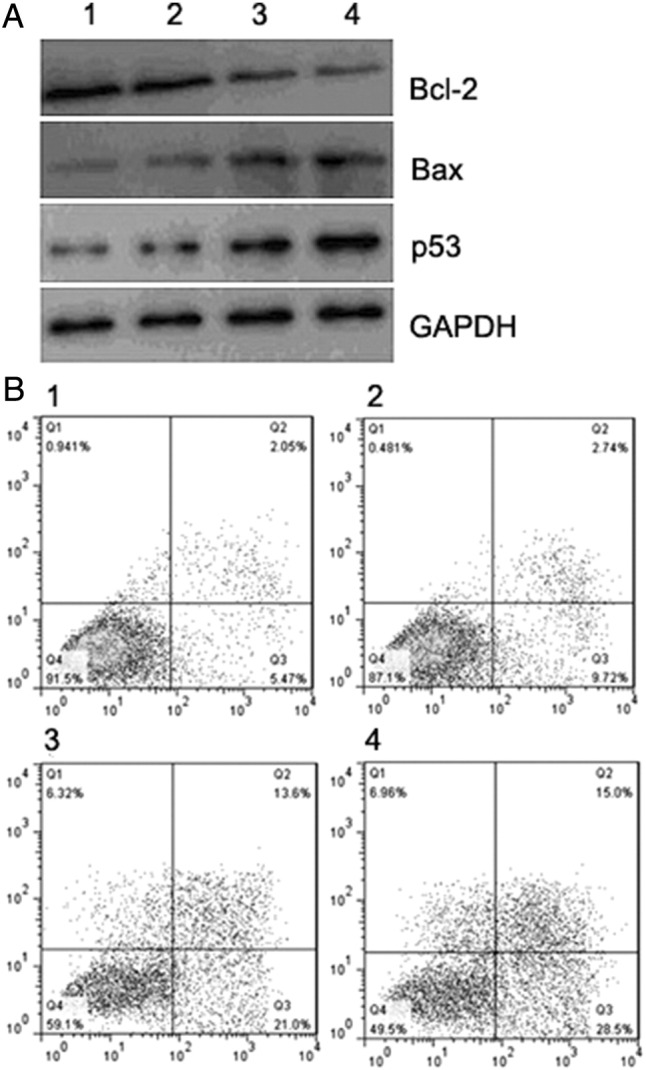
Proapoptotic effect of CDDP combined with HPV16 E6/E7-CRISPR/Cas9 in xenograft tumor tissues. (A) Western blot results showed that CDDP and HPV16 E6/E7-CRISPR/Cas9 co-treated xenograft tumor tissues presented the most significant increase of Bax and P53 and decrease of Bcl-2. (B) Apoptosis assay by flow cytometry using Annexin V and PI staining showed that CDDP and HPV16 E6/E7-CRISPR/Cas9 cotreatment produced a significant increase in percentage of apoptotic cells. (1, Control-treated; 2 CDDP-treated; 3, E6 + E7 CRISPR/Cas9-treated; 4, E6 + E7 + CDDP-treated).
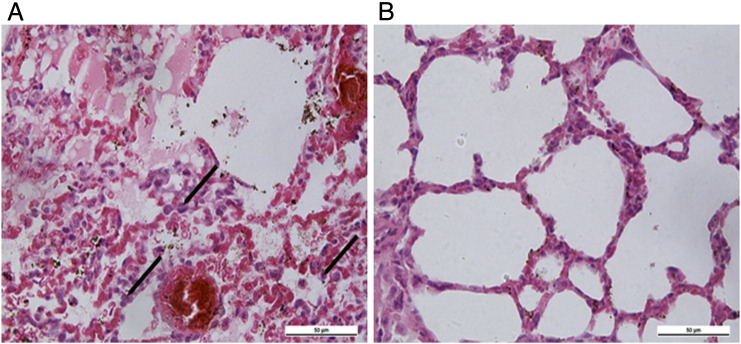
Figure 6.
Analysis of lung metastasis using H&E staining. H&E staining of lung tissue sections from control + CDDP-treated mice (A) and HPV16 E6 + E7 CRISPR/Cas9 + CDDP-treated mice (B) indicated that HPV16 E6 + E7 CRISPR/Cas9 + CDDP-treatment inhibited lung metastasis of cervical cancer cells.
Discussion
Cervical cancer is the second most common cancer in women worldwide though well-organized screening has been expanded. The majority of cases are caused by infection of high-risk types of human papillomaviruses which express the E6 and E7 oncogenes [23], [24]. Studies have demonstrated that the expression of these oncogenes is indispensable for tumor development and maintenance of malignant phenotypes. Development of novel strategies targeting E6 and E7 oncogenes will be a kind of addition to the current therapeutic arsenal against cervical cancer with high risk HPV infection.
Recently, CRISPR/Cas9 has been developed as a promising new tool to suppress gene expression [25], [26]. Tumor cells are typically resistant to growth suppressive signals and apoptosis, and in HPV-infected cancer cells the viral oncogenes E6 and E7 aggravated this kind of resistance. In our previous study, CRISPR/Cas9 specific to HPV16 E6 and E7 oncogenes were designed to effectively knockdown E6 and E7 expression along with accumulation of p53 and p21 protein, and finally result in remarkably inhibition of proliferation of cervical cancer in vitro and in vivo. Here, transfection of HPV16 E6/E7-CRISPR/Cas9 caused significant suppression of cell growth in HPV16-positive SiHa cervical cancer cells, whereas they had no effect in HPV-negative C33-A cells, demonstrating the effectiveness and specificity of these CRISPR/Cas9 systems. CDDP is the most active anticancer agent available for clinical treatment of cervical cancer, but its administration is severely hindered by the occurrence of insensitivity and resistance. We then aimed to investigate whether HPV16 E6/E7-CRISPR/Cas9 could be used as a CDDP sensitizer for cervical cancer treatment. In our studies, exposure of cells to either E6 + E7 specific CRISPR/Cas9 with CDDP significantly inhibited cell growth in vitro, suggesting that CRISPR/Cas9 targeting E6 + E7 could function as CDDP-sensitizers. However, short-term exposure of cervical cancer cells to E6 + E7-CEISPR/Cas9 combined with CDDP showed no synergistic cytotoxicity. Contrarily, long-term E6 and E7 gene silence increased the sensitivity of SiHa cells to CDDP and induced substantial apoptosis. E6 + E7 specific CEISPR/Cas9 was a more potent sensitizer of CDDP, which was similar to the result of the superiority of the E6 + E7-specific CEISPR/Cas9 at inducing cellular senescence. Published data suggested that the functional restoration of wild-type TP53, which induced the regression of cervical carcinomas, could be achieved either by inhibition of E6 + E7 expression or by treatment with CDDP. Therefore, the combination of CDDP and HPV16 E6/E7-CRISPR/Cas9 might have a synergistic effect on the restoration of TP53 and be a more effective therapy for cervical cancer than either treatment alone. This assumption about the chemosensitizing activity of HPV16 E6 + E7-CRISPR/Cas9 was validated in vitro and in vivo, and the mechanisms of the synergistic action of HPV16 E6 + E7-CEISPR/Cas9 and CDDP were at least associated with induction of apoptosis.
Tumor suppressor p53 is a powerful anti-tumor molecule that is often inactivated in various cancers [27]. In the present study, transfection of HPV16 E6 + E7-CRISPR/Cas9 plasmids strongly increased p53 protein level. P53 promotes cell apoptosis via transcriptionally activating genes promoting cell death. We found that the expression of Bax was greatly increased while Bcl-2 was diminished along with P53 elevated in vitro and in vivo after transfection of HPV16 E6 + E7-CRISPR/Cas9 plasmids. Consistent with these observations, HPV16 E6/E7- CRISPR/Cas9 and CDDP co-treatment caused robust apoptosis and tumor growth suppression compared to negative controls.
This is the first report of HPV16 E6/E7-CRISPR/cas9 as an effective sensitizer for CDDP chemotherapy in cervical cancer. We have demonstrated that HPV16 E6/E7-CRISPR/Cas9 could effectively and specifically coordinate with CDDP for HPV16 positive cervical cancer. Notably, CDDP and HPV16 E6/E7-CRISPR/Cas9 co-treatment could inhibit lung metastasis of subcutaneous xenograft, while CDDP alone could not prevent lung dissemination. These results indicated that a combination therapy involving cisplatin and HPV16 E6/E7-CRISPR/Cas9 could be advantageous in improving prognosis, which of cervical cancer patients encouraged further investigation of the potentiality of HPV16 E6/E7-CRISPR/Cas9 as a therapeutic agent for cervical cancer.
In conclusion, we validated the therapeutic synergy of HPV16 E6/E7- CRISPR/Cas9 and CDDP for HPV16-positive cervical cancer with in vitro and in vivo study models, promising HPV16 E6/E7-CRISPR/Cas9 as a potential chemosensitizer for CDDP. Further study about associations and synergies between HPV16 E6/E7-CRISPR/Cas9 and other chemo-therapeutics will produce new options for cervical cancer treatment and improve the clinical outcome of cervical cancer patients.
Disclosure Statement
The authors declare no conflicts of interest.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (Grant No. 81602295 to Shuai Zhen).
Contributor Information
Wen-Juan Luo, Email: luowj@mail.xjtu.edu.cn.
Le Zhao, Email: zhaole2@mail.xjtu.edu.cn.
References
- 1.Cogliano V., Baan R., Straif K., Grosse Y., Secretan B., El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N., Bosch F.X., de Sanjose S., Herrero R., Castellsague X., Shah K.V., Snijders P.J., Meijer C.J. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Zur Hausen H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 4.De Villiers E.M., Fauquet C., Broker T.R., Bernard H.U., zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura A., Nakahara T., Ueno K., Sasaki S., Yoshida S.K. Requirement of E7 oncoprotein for viability of HeLa cells. Microbes Infect. 2006;8:984–993. doi: 10.1016/j.micinf.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Kim D.H., Rossi J.J. Strategies for silencing human disease using RNA interference. Genetics. 2006;8:173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 8.Yamato K., Fen J., Kobuchi H., Nasu Y., Yamada T., Nishihara T., Ikeda Y., Kizaki M., Yoshinouchi M. Induction of cell death in human papillomavirus 18-positive cervical cancer cells by E6 siRNA. Cancer Gene Ther. 2006;13:234–241. doi: 10.1038/sj.cgt.7700891. [DOI] [PubMed] [Google Scholar]
- 9.Zhen S., Hua L., Yun H.L., Gao L.-C., Fu J., Wan D.Y., Dong L.-H., Song H.F., Gao X. Harnessing the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated Cas9 system to disrupt the hepatitis B virus. Gene Ther. 2015:1–9. doi: 10.1038/gt.2015.2. [DOI] [PubMed] [Google Scholar]
- 10.Zhen S., Hua L., Takahashi Y., Narita S., Liu Y.-H., Li Y. In vitro andin vivo growth suppression of human papillomavirus16-positive cervical cancer cells by CRISPR/Cas9. Biochem Biophys Res Commun. 2014;450:1422–1426. doi: 10.1016/j.bbrc.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Peters W.A., Liu P.Y., Barrett R.J., II, Stock R.J., Monk B.J., Berek J.S., Souhami L., Grigsby P., Gordon W., Jr., Alberts D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 12.Morris M., Eifel P.J., Lu J., Grigsby P.W., Levenback C., Stevens R.E., Rotman M., Gershenson D.M., Mutch D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 13.Butz K., Geisen C., Ullmann A., Spitkovsky D., Hoppe-Seyler F. Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. Int J Cancer. 1996;68:506–513. doi: 10.1002/(SICI)1097-0215(19961115)68:4<506::AID-IJC17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Wesierska-Gadek J., Schloffer D., Kotala V., Horky M. Escape of p53 protein from E6-mediated degradation in HeLa cells after cisplatin therapy. Int J Cancer. 2002;101:128–136. doi: 10.1002/ijc.10580. [DOI] [PubMed] [Google Scholar]
- 15.Huang H., Huang S.Y., Chen T.T., Chen J.C., Chiou C.L., Huang T.M. Cisplatin restores p53 function and enhances the radiosensitivity in HPV16 E6 containing SiHa cells. J Cell Biochem. 2004;91:756–765. doi: 10.1002/jcb.10769. [DOI] [PubMed] [Google Scholar]
- 16.Decatris M.P., Sundar S., O'Byrne K.J. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat Rev. 2004;30:53–81. doi: 10.1016/S0305-7372(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 17.Siddik Z.H. Biochemical and molecular mechanisms of cisplatin resistance. Cancer Treat Res. 2002;112:263–284. doi: 10.1007/978-1-4615-1173-1_13. [DOI] [PubMed] [Google Scholar]
- 18.Shen B., Zhang J., Wu H., Wang J., Ma K., Li Z., Zhang X., Zhang P., Huang X. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 2013;46:10. doi: 10.1038/cr.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., Dicarlo J.E., Norville J.E., Church G.M. RNA-Guided Human Genome Engineering via Cas9. Science. 2013;10:1126. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz E. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 21.Chang J.T.C., Chan S.H., Lin C.Y., Lin T.Y., Wang H.M., Liao C.T. Differentially expressed genes in radioresistant nasopharyngeal cancer cells: gp96 and GDF15. Mol Cancer Ther. 2007;6:2271–2279. doi: 10.1158/1535-7163.MCT-06-0801. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G., Budker V., Wolff A. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 23.Zur Hausen H., de Villiers E.M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
- 24.Mighty K.K., Laimins L.A. The role of human papillomaviruses in oncogenesis. Recent Results Cancer Res. 2014;193:135–148. doi: 10.1007/978-3-642-38965-8_8. [DOI] [PubMed] [Google Scholar]
- 25.Ebina H., Misawa N., Kanemura Y., Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510. doi: 10.1038/srep02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii W., Kawasaki K., Sugiura K., Naito K. Efficient generation of large-scale genome-modified mice using gRNA and CAS9 endonuclease. Nucleic Acids Res. 2013;41(20):e187. doi: 10.1093/nar/gkt772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Yang L., Hu J., Wang B., Zhao L., Ji K., Guo B. Plasmid-based E6-specific siRNA and co-expression of wild-type p53 suppresses the growth of cervical cancer in vitro and in vivo. Cancer Lett. 2013;10:242–250. doi: 10.1016/j.canlet.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]