Abstract
Background
Exposure to chemotherapeutic agents such as acetaminophen may lead to serious liver injury. Calcium deregulation, angiotensin II production and xanthine oxidase activity are suggested to play mechanistic roles in such injury.
Objective
This study evaluates the possible protective effects of the calcium channel blocker amlodipine, the angiotensin converting enzyme inhibitor lisinopril, and the xanthine oxidase inhibitor allopurinol against experimental acetaminophen-induced hepatotoxicity, aiming to understand its underlying hepatotoxic mechanisms.
Material and methods
Animals were allocated into a normal control group, a acetaminophen hepatotoxicity control group (receiving a single oral dose of acetaminophen; 750 mg/kg/day), and four treatment groups receive N-acetylcysteine (300 mg/kg/day; a reference standard), amlodipine (10 mg/kg/day), lisinopril (20 mg/kg/day) and allopurinol (50 mg/kg/day) orally for 14 consecutive days prior to acetaminophen administration. Evaluation of hepatotoxicity was performed by the assessment of hepatocyte integrity markers (serum transaminases), oxidative stress markers (hepatic malondialdehyde, glutathione and catalase), and inflammatory markers (hepatic myeloperoxidase and nitrate/nitrite), in addition to a histopathological study.
Results
Rats pre-treated with amlodipine, lisinopril or allopurinol showed significantly lower serum transaminases, significantly lower hepatic malondialdehyde, myeloperoxidase and nitrate/nitrite, as well as significantly higher hepatic glutathione and catalase levels, compared with acetaminophen control rats. Serum transaminases were normalized in the lisinopril treatment group, while hepatic myeloperoxidase was normalized in the all treatment groups. Histopathological evaluation strongly supported the results of biochemical estimations.
Conclusion
Amlodipine, lisinopril or allopurinol can protect against acetaminophen-induced hepatotoxicity, showing mechanistic roles of calcium channels, angiotensin converting enzyme and xanthine oxidase enzyme in the pathogenesis of hepatotoxicity induced by acetaminophen.
Keywords: Acetaminophen, Allopurinol, Amlodipine, Hepatotoxicity, Lisinopril, Rat
1. Introduction
Liver is the main detoxifying organ in the body. However, continuous exposure to certain chemotherapeutic agents, drugs, environmental toxins, viral infections or bacterial invasion can trigger liver injury and eventually lead to various liver diseases (Stephens et al., 2014). Susceptibility of the liver to injury by such agents is much higher than any other organ because of its central role in metabolism as well as its ability to concentrate and biotransform xenobiotics (Kumar et al., 2015).
Acetaminophen is an over-the-counter drug commonly used for its analgesic and antipyretic properties. Although considered a safe drug, it is the most frequent cause of severe liver failure in the world, having a mortality rate of about 90% (Zyoud et al., 2010). In therapeutic levels, most of the administered dose is normally metabolized via phase II reactions and excreted as glucuronide and sulfate conjugates, while only a small portion is metabolized via phase I pathway to yield the highly toxic intermediate N-acetyl-p-benzoquinoneimine (NAPQI), which is normally detoxified by interaction with cellular glutathione (GSH). When GSH becomes depleted by the overproduction of NAPQI caused by saturation of the conjugation pathways at high doses, NAPQI binds to cellular macromolecules, leading to oxidative stress, cellular necrosis and finally cell death (Coen, 2014).
N-acetylcysteine (NAC) is a thiol containing antioxidant which acts as a direct scavenger of free radicals and a precursor for GSH biosynthesis (Tobwala et al., 2015). It can inhibit the induction of pro-inflammatory cytokines and can also block the tumor necrosis factor-α (TNF-α)-induced apoptotic cell death (Sen et al., 2014). It is old known as the standard antidote of acetaminophen poisoning (Bateman et al., 2014).
Amlodipine is a third generation dihydropyridine-type calcium channel blocker commonly used for the treatment of hypertension. Calcium channel blockers were reported to possess hepatoprotective activities in previous studies (Kamal, 2013) based on the mechanistic role of calcium deregulation in the progression of hepatotoxicity (Kheradpezhouh et al., 2014). In addition, the antioxidant activity of amlodipine was reported. This was attributed to its physicochemical properties where its high lipophilicity and chemical structure could facilitate proton-donating and resonance-stabilization mechanisms that quench free radicals (Mason et al., 2014). The anti-inflammatory potential of amlodipine was also reported (Zhang et al., 2012).
Lisinopril is a lipophilic non-sulfhydryl angiotensin converting enzyme (ACE) inhibitor reported to have the ability to enhance endogenous antioxidant enzyme activities (Velayutham et al., 2013). Inhibition of the rennin-angiotensin-aldosterone system may have beneficial effects on oxidative injury as angiotensin II was recently reported to cause mitochondrial oxidative injury (Li et al., 2014b). Additionally, lisinopril was reported to have anti-inflammatory effects via suppression of the pro-inflammatory cytokines such as TNF-α production (Morsy, 2011).
The prototypical xanthine oxidase inhibitor allopurinol has been applied in different models of tissue injury, based on its reported ability to inhibit the production of reactive oxygen species (ROS), and the release of inflammatory mediators such as TNF-α (Rachmat et al., 2013, Prieto-Moure et al., 2014).
Based on the aforementioned background, the present study aims to investigate the possible protective effects of three agents acting through different mechanisms, namely amlodipine as a calcium channel blocker, lisinopril as an ACE inhibitor, and allopurinol as a xanthine oxidase inhibitor, on acetaminophen-induced hepatotoxicity in experimental rats, using NAC as a reference standard agent, aiming also to declare the role of calcium channels, ACE enzyme and xanthine oxidase enzyme in the pathogenesis of acetaminophen-induced hepatotoxicity.
2. Materials and methods
2.1. Chemicals and diagnostic kits
N-acetylcysteine powder was obtained as a kind gift from SEDICO Pharmaceutical Company, Egypt. Amlodipine was obtained as a kind gift from Pfizer Pharmaceutical Company, Egypt. Lisinopril was obtained as a kind gift from AstraZeneca Pharmaceutical Company, Egypt. Allopurinol was obtained as a kind gift from GlaxoSmithKline pharmaceutical company, Egypt. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) reagent kits were purchased from Spinreact (Spain). 1,1-3,3-tetramethoxypropane, 5,5′-Dithiobis-2-nitrobenzoicacid (DTNB), GSH powder, Horseradish peroxidase, N-(1-Naphthyl) ethylenediamine dihydrochloride, o-dianisidine hydrochloride, thiobarbituric acid (TBA), malondialdehyde (MDA), Tris-hydroxymethyl-amino methane, hexadecyltrimethylammonium bromide (HTAB) and sulfanilamide were purchased from Sigma–Aldrich (USA). Vanadium chloride was obtained from Acros (Belgium). All other chemicals used were of analytical grade.
2.2. Animals
Adult male Wistar rats, purchased from the Modern Veterinary Office for Laboratory Animals, Cairo, Egypt, were fed with standard laboratory rat diet (Modern Veterinary Office) and water ad libitum until reaching weights of 180–200 g. Animals were housed in a room kept at 22–25 °C with 12-h light/12-h dark cycles, in individual stainless steel wire-bottomed cages having an upper water supply to avoid coprophagy. All animal housing and handling were conducted in compliance with the Beni-Sueif University guidelines and in accordance with the research protocols established by the Animal Care Committee of the National Research Center (Cairo, Egypt) which followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
2.3. Experimental design
Rats were distributed into 6 groups, each of 6–8 rats, namely a normal control group, a acetaminophen hepatotoxicity control group, a standard treatment group receiving NAC, and three treatment groups receiving amlodipine, lisinopril and allopurinol, orally on a daily basis for 14 consecutive days prior to acetaminophen dose. All test agents were prepared in 1% tween 80 solution in normal saline. Drug doses were guided by the literature and adjusted through pilot trials. NAC was given in a dose of 300 mg/kg/day (Abla et al., 2005, Hsieh et al., 2014), amlodipine was given in a dose of 10 mg/kg/day (Begum and Akhter, 2007), lisinopril was given in a dose of 20 mg/kg/day (Albarwani et al., 2015), and allopurinol was given in a dose 50 mg/kg/day (Aldaba-Muruato et al., 2013).
2.4. Induction of hepatotoxicity
After drug or vehicle treatment for 14 consecutive days, animals were fasted for 18 h and then received a single oral dose of acetaminophen (750 mg/kg) guided by the method described previously (Olaleye et al., 2014, Omidi et al., 2014) with dose adjusted through pilot trials. Animals were anaesthetized with a single dose of thiopental sodium (75 mg/kg, i.p.) 24 h after acetaminophen administration, and blood samples were collected from retro-orbital plexus using heparinized micro-capillary tubes. Rats were sacrificed thereafter by cervical dislocation to separate liver samples (Kiran et al., 2012).
2.5. Sample preparation
2.5.1. Serum preparation
After collecting blood samples in centrifuge tubes, the tubes were allowed to coagulate at room temperature and then placed in a water bath at 37 °C for 10 min. Centrifugation at 1000g for 20 min was performed. The clear serum was separated and used for analysis of biochemical parameters (ALT and AST).
2.5.2. Liver tissue preparation
After animals were sacrificed, the abdominal cavities were opened with livers carefully separated and washed with ice-cooled saline. Hepatic lobes were used for the preparation of liver homogenates and histopathology sections.
To prepare a 20% liver homogenate, a portion of the liver was homogenized with 5 volumes of isotonic ice-cooled normal saline using a homogenizer (Ultra-Turrax T25, made in Germany) for the estimation of hepatic thiobarbituric acid-reactive substances (TBARS), GSH, catalase (CAT) and total nitrate/nitrite (NOx; nitric oxide end products).
Another portion of the liver was homogenized with 60 volumes of ice-cooled HTAB (1%) solution in normal saline and centrifuged at 4000g for 15 min at 4 °C in a cooling centrifuge (model 2-16KL, Germany). The supernatant was used for the estimation of hepatic myeloperoxidase (MPO) activity.
2.6. Histopathological study
A portion of the isolated livers was immediately fixed in 10% buffered formalin solution in normal saline, dehydrated in graded concentrations of ethanol (50–100%), cleared in xylene and embedded in paraffin. Liver sections (4–5 μm) were prepared and stained with hematoxylin-eosin (H&E) dye using standard techniques for photomicroscopic observations (Banchroft and Steven, 1983). Sections of samples were viewed and evaluated by two experienced pathologists blinded to the experiment.
2.7. Assessment of biochemical parameters
Serum ALT and AST levels were assayed colorimetrically using a UV–visible double-beam spectrophotometer (model T80, made in England) according to the method described by Young (1995). Lipid peroxidation was determined in liver homogenate as TBARS, measured as MDA according to the method of Uchiyama and Mihara (1978). Hepatic GSH was measured in the liver homogenate according to the method described by Sedlak and Lindsay (1968). Hepatic CAT activity was measured according to the method described by Claiborne (1985). Hepatic NOx production was assayed according to the method described by Miranda et al. (2001). Liver MPO activity was measured in liver homogenate as previously described by Harada et al. (1999).
2.8. Statistical analysis
Results were expressed as means of 6–8 rats ± standard error of the mean (SEM). All statistical analyses were performed using one way analysis of variance (ANOVA) test followed by Student–Newman–Keuls post hoc test using the statistical package for social sciences (SPSS; version 19.0) computer software program (SPSS Inc., Chicago, IL, USA) with the value of p < 0.05 considered statistically significant.
3. Results
3.1. Hepatocyte integrity markers
Single dose acetaminophen administration significantly increased serum ALT and AST levels by about 2.6 and 3.2 folds, respectively, as compared to normal control animals. Daily oral administration of NAC, amlodipine, lisinopril or allopurinol significantly decreased serum ALT levels to about 56%, 71%, 52% and 65%, and serum AST levels to about 43%, 60%, 45% and 61%, respectively, as compared to acetaminophen control toxicity group. With NAC or lisinopril treatments, serum transaminases were restored back to normal levels (Table 1). Administration of NAC, amlodipine, lisinopril or allopurinol to normal rats did not significantly affect any of the hepatocyte integrity markers (data not included).
Table 1.
Effect of NAC, amlodipine, lisinopril or allopurinol administration on markers of liver injury.
| Parameters | Control groupA | Acetaminophen groupB | NAC treatment groupC | Amlodipine treatment groupD | Lisinopril treatment groupE | Allopurinol treatment groupF |
|---|---|---|---|---|---|---|
| Serum ALT (U/L) | 45.3 ± 2.68 | 117.8 ± 18.53a | 65.7 ± 5.08b | 84.1 ± 4.39ab | 61.8 ± 9.23b | 76.8 ± 5.09b |
| Serum AST (U/L) | 155.3 ± 9.98 | 503.0 ± 76.78a | 218.0 ± 3.69b | 303.0 ± 7.63ab | 225.5 ± 21.45b | 307.8 ± 10.79ab |
Data are expressed as mean of 6–8 rats ± SEM. Multiple comparisons were done using one-way ANOVA test followed by Student–Newman–Keuls as post hoc test.
ALT: Alanine transaminase, ANOVA: Analysis of variance, AST: Aspartate transaminase, NAC: N-acetylcysteine, SEM: Standard error of the mean.
Control group receiving only vehicles.
Acetaminophen group, subjected to a single oral dose of acetaminophen (750 mg/kg).
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where NAC was given in a dose of 300 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where amlodipine was given in a dose of 10 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where lisinopril was given in a dose of 20 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where allopurinol was given in a dose of 50 mg/kg/day.
Significantly different from control group.
Significantly different from acetaminophen group at p < 0.05.
3.2. Oxidative stress markers
Administration of acetaminophen to rats significantly increased liver TBARS content to about 575%, significantly decreased liver GSH content to about 34%, and significantly decreased CAT activity to about 4% as compared to normal control rats. Pre-treatment of rats with NAC, amlodipine, lisinopril or allopurinol prior to acetaminophen administration significantly decreased hepatic TBARS content to about 51%, 48%, 45% and 46%, significantly increased hepatic GSH content to about 179%, 145%, 160% and 178%, and significantly increased hepatic CAT activity to about 1150%, 1025%, 1100% and 950%, respectively, as compared to acetaminophen hepatotoxicity control rats (Table 2).
Table 2.
Effect of NAC, amlodipine, lisinopril or allopurinol administration on markers of oxidative stress.
| Parameters | Control groupA | Acetaminophen groupB | NAC treatment groupC | Amlodipine treatment groupD | Lisinopril treatment groupE | Allopurinol treatment groupF |
|---|---|---|---|---|---|---|
| Hepatic TBARS (nmol/g) | 24.2 ± 3.20 | 139.1 ± 7.21a | 70.6 ± 5.91ab | 67.3 ± 6.85ab | 61.9 ± 4.15ab | 63.5 ± 5.68ab |
| Hepatic GSH (μmol/g) | 394.6 ± 24.47 | 135.2 ± 1.98a | 242.0 ± 9.54ab | 195.5 ± 5.58abc | 215.8 ± 2.83ab | 240.5 ± 7.43ab |
| Hepatic CAT (U/mg protein) | 0.091 ± 0.0078 | 0.004 ± 0.0017a | 0.046 ± 0.0177ab | 0.041 ± 0.0057ab | 0.044 ± 0.0076ab | 0.038 ± 0.0036ab |
Data are expressed as mean of 6–8 rats ± SEM. Multiple comparisons were done using one-way ANOVA test followed by Student–Newman–Keuls as post hoc test.
ANOVA: Analysis of variance, CAT: Catalase, GSH: Glutathione reduced, NAC: N-acetylcysteine, SEM: Standard error of the mean, TBARS: Thiobarbituric acid reactive substances.
Control group receiving only vehicles.
Acetaminophen group, subjected to a single oral dose of acetaminophen (750 mg/kg).
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where NAC was given in a dose of 300 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where amlodipine was given in a dose of 10 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where lisinopril was given in a dose of 20 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where allopurinol was given in a dose of 50 mg/kg/day.
Significantly different from control group.
Significantly different from acetaminophen group.
Significantly different from standard treatment (NAC) group at p < 0.05.
3.3. Inflammatory markers
Acetaminophen administration significantly increased hepatic NOx content and MPO activity to about 176% and 1005%, respectively, as compared to normal rats. Alternatively, pre-treatment of rats with NAC, amlodipine, lisinopril or allopurinol prior to acetaminophen administration significantly decreased hepatic NOx content to about 71%, 80%, 81% and 78%, and hepatic MPO activity to about 15%, 21%, 21% and 14%, respectively, as compared to acetaminophen hepatotoxicity control rats. Hepatic MPO activity was restored back to normal levels in all the treatment groups (Table 3).
Table 3.
Effect of NAC, amlodipine, lisinopril or allopurinol administration on markers of inflammation.
| Parameters | Control groupA | Acetaminophen groupB | NAC treatment groupC | Amlodipine treatment groupD | Lisinopril treatment groupE | Allopurinol treatment groupF |
|---|---|---|---|---|---|---|
| Hepatic NOx (nmol/g) | 123.5 ± 5.99 | 216.7 ± 7.05a | 153.7 ± 9.43ab | 174.4 ± 2.51ab | 175.9 ± 3.63ab | 168.8 ± 3.25ab |
| Hepatic MPO (U/g) | 0.380 ± 0.0574 | 3.820 ± 0.3739a | 0.574 ± 0.0779b | 0.819 ± 0.1429b | 0.790 ± 0.0929b | 0.547 ± 0.1240b |
Data are expressed as mean of 6–8 rats ± SEM. Multiple comparisons were done using one-way ANOVA test followed by Student–Newman-Keuls as post hoc test.
ANOVA: Analysis of variance, MPO: Myeloperoxidase, NAC: N-acetylcysteine, NOx: Total nitrate/nitrite, SEM: Standard error of the mean.
Control group receiving only vehicles.
Acetaminophen group, subjected to a single oral dose of acetaminophen (750 mg/kg).
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where NAC was given in a dose of 300 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where amlodipine was given in a dose of 10 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where lisinopril was given in a dose of 20 mg/kg/day.
Test agents were given orally on a daily basis for 14 consecutive days prior to acetaminophen dose, where allopurinol was given in a dose of 50 mg/kg/day.
Significantly different from control group.
Significantly different from acetaminophen group at p < 0.05.
3.4. Histopathological study
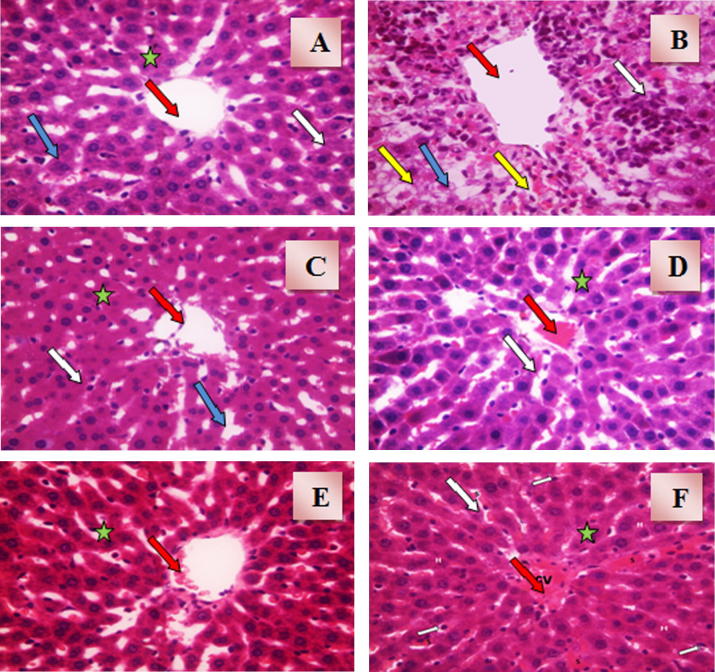
Histopathological examination of liver sections obtained from normal control group showed normal hepatic architecture with normal central vein and radiating cords of hepatocytes. Cords of hepatocytes were separated by blood sinusoids lined with Kupffer cells as shown in Fig. 1(A). Liver sections obtained from acetaminophen hepatotoxicity control group showed irregularly dilated central vein with massive inflammatory reactions and activated Kupffer cells. Hepatocytes showed cellular degeneration and centrilobular necrosis, and were separated with dilated congested sinusoids as shown in Fig. 1(B). Animal pre-treated with NAC prior to acetaminophen dose showed that all hepatocytes were normal with slightly congested central vein. Hepatocytes were separated with slightly congested blood sinusoids with some activated Kupffer cells as shown in Fig. 1(C). Treatment of rats with amlodipine showed that all hepatocytes were normal with dilated congested central vein and activated Kupffer cells as shown in Fig. 1(D). Liver sections obtained from animals treated with lisinopril showed nearly normal architecture and normal hepatocytes with minimal congested central vein and some activated Kupffer cells as shown in Fig. 1(E). Histopathological examination of liver sections obtained from animals treated with allopurinol showed nearly normal architecture. It was observed that hepatocytes were normal with irregularly dilated congested central vein and some activated Kupffer cells as shown in Fig. 1(F).
Figure 1.
(A) A photomicrograph of liver section obtained from control rats (H&E × 40). The section shows normal hepatic architecture with central vein (red arrow) and radiating cords of hepatocytes (star). Cords of hepatocytes are separated by blood sinusoids (blue arrow) lined with Kupffer cells (white arrow). (B) A photomicrograph of liver section obtained from acetaminophen hepatotoxicity control rats (H&E × 40). The section shows irregularly dilated central vein (red arrow) with massive inflammatory reactions and activated Kupffer cells (white arrow). Hepatocytes are showing cellular degeneration and centrilobular necrosis (yellow arrow). Hepatocytes are separated with dilated congested sinusoids (blue arrow). (C) A photomicrograph of liver section obtained from rats intoxicated with acetaminophen and pre-treated with N-acetylcysteine (H&E × 40). The section shows that all hepatocytes are normal with slightly congested central vein (red arrow). Hepatocytes are separated with slightly congested blood sinusoids (blue arrow) with some activated Kupffer cells (white arrow). (D) A photomicrograph of liver section obtained from rats intoxicated with acetaminophen and pre-treated with amlodipine (H&E × 40). The section shows that all hepatocytes (star) are normal with dilated congested central vein (red arrow) and activated Kupffer cells (white arrow). (E) A photomicrograph of liver section obtained from rats intoxicated with acetaminophen and pre-treated with lisinopril (H&E × 40). The section shows nearly normal architecture and normal hepatocytes (star) with minimal congested central vein (red arrow) and some activated Kupffer cells (white arrow). (F) A photomicrograph of liver section obtained from rats intoxicated with acetaminophen and pre-treated with allopurinol (H&E × 40). The section shows normal architecture and normal hepatocytes (star) with irregularly dilated congested central vein (red arrow) and some activated Kupffer cells (white arrow).
4. Discussion
The present investigation aims to evaluate the possible hepatoprotective effects of the calcium channel blocker amlodipine, the ACE inhibitor lisinopril, and the xanthine oxidase inhibitor allopurinol, as compared to the standard treatment NAC, on acute liver injury induced experimentally in adult male albino rats with acetaminophen.
The current investigation showed that a single oral dose administration of acetaminophen caused acute liver damage to rats as evidenced by significant increases in serum activities of ALT and AST (Table 1), which are among the most sensitive indicators of hepatocyte integrity loss (Amacher et al., 2013). Liver damage was coupled with oxidative stress evidenced by significant elevation of tissue TBARS associated with significant reductions in tissue GSH and CAT levels (Table 2). Inflammatory progression was also evident, reported as significant elevations of tissue NOx production and MPO activity (Table 3). Biochemical findings were strongly supported by the results of histopathological examination (Fig. 1(A and B)). In agreement, previous investigations showed similar elevations of serum transaminases with comparable doses of acetaminophen in rats (Alipour et al., 2013). In addition, a similar increase in hepatic MDA content was observed by Lahouel et al. (2004) and Chandrasekaran et al. (2009) in the same model. On the other hand, the decreases in hepatic GSH content and CAT activity are in harmony with the results reported by Yousef et al., 2010, Gupta et al., 2014. Additionally, the elevations in the inflammatory biomarkers MPO and NOx are in agreement with the work of Gardner et al. (2002).
Acute liver injury caused by acetaminophen is a severe condition in which metabolic homeostasis is affected. The toxicity of acetaminophen develops when its dose exceeds safe hepatic detoxification pathways such as glucuronidation and sulfation (Eesha et al., 2011) where the very reactive metabolite N-acetyl-p-benzoquinoneimine (NAPQI) is formed in a rate that depletes cellular GSH faster than its re-synthesis. Semiquinone radicals, obtained by one electron reduction of NAPQI, can then covalently bind to the macromolecules of cellular membrane and increase the lipid peroxidation and MDA production resulting in massive tissue damage. The damaged hepatocytes trigger a cascade of inflammatory responses leading to various degrees of liver damage which is further propagated by the migration of different extrahepatic inflammatory cells to the area of injury (Chan et al., 2007, Krenkel et al., 2014).
Results of the present investigation showed that the calcium channel blocker amlodipine protected the liver against acetaminophen-induced hepatotoxicity evidenced by significant decreases in serum ALT and AST levels (Table 1; Fig. 1(D)), coupled with anti-oxidant and anti-inflammatory potentials (Table 2, Table 3). Although we have no reported experimental trials on amlodipine as a hepatoprotective agent against acetaminophen toxicity, amlodipine was reported to have hepatoprotective potential in other animal models of hepatotoxicity like CCl4-induced hepatic damage (Abdel Salam et al., 2007).
Calcium channel blockers in general were reported to possess hepatoprotective activities in several in vivo and in vitro studies (Rajaraman et al., 2007, Kamal, 2013). Hepatocytes were old known to contain several types of calcium channels (Barritt et al., 2008, Liu et al., 2013). Calcium channel blockers are of particular protective effect when hepatotoxicity is mediated by disturbed calcium homeostasis as in the case of acetaminophen-induced injury where calcium influx plays a mechanistic role in the progression of hepatotoxicity (Corcoran et al., 1987, Kheradpezhouh et al., 2014).
The antioxidant activity of amlodipine could be related to the intrinsic structural characteristics of the dihydropyridine ring which belongs to the chain breaking group of antioxidants (Berkels et al., 2005). The dihydropyridine compounds have a reducing nature or hydrogen donor properties making them having the ability to donate protons and electrons to the lipid peroxide molecules to reduce it into a non-reactive form thereby blocking the peroxidation process (Vitolina et al., 2012). Regarding the anti-inflammatory effect of amlodipine, Salman et al. (2011) reported similar decreases in MPO activity during studying the effects of chronic administration of some antihypertensive drugs on enzymatic and non-enzymatic oxidant/antioxidant parameters in rat ovarian tissue. Side by side, Yasu et al. (2013) concluded that amlodipine could inhibit leucocyte adhesion and MPO release.
According to our study, pre-treatment with the ACE inhibitor lisinopril showed a hepatoprotective potential evidenced from decreased serum ALT and AST levels in acetaminophen-treated rats, supported by histopathological results (Table 1; Fig.1(E)). No reported trials were evident regarding the hepatoprotective effect of lisinopril on acetaminophen-induced injury. However, Ohishi et al. (2001) reported anti-fibrogenic effect of lisinopril on chronic CCl4-induced hepatic fibrosis in rats, while Morsy (2011) reported hepatoprotective potential of lisinopril on ischemia–reperfusion injury in rats. In addition, these results are in agreement with Yirmibeşoğlu et al. (2011) who reported an inhibitory effect of lisinopril on endothelin-1 elevation in a partial hepatectomy model. On the other hand these results are in disagreement with Gokcimen et al. (2007) who observed that lisinopril increased ALT level during studying the effect of lisinopril on rat liver tissues in L-NAME induced hypertension model.
The anti-oxidant activity of lisinopril, evidenced by significant corrections of oxidative stress biomarkers in the present study, came in harmony with the result of Yilmaz et al. (2011) who reported a decreased level of MDA by lisinopril in the hippocampus of rats with L-NAME-induced hypertension. More recently, Li et al. (2014a) reported antioxidant potential for lisinopril represented as attenuation of oxidative stress in rostral ventrolateral medulla in hypertensive rats. The anti-oxidant potential of lisinopril may be related to its ability to stimulate the antioxidant defense components like CAT activity and GSH stores evident in this study. In agreement, Öktem et al. (2011) reported that lisinopril increased CAT activity and attenuated renal oxidative injury in L-NAME-induced hypertension in rats, while Mohanty et al. (2013) demonstrated that lisinopril increased GSH level in ischemic cardiac toxicity. It should be mentioned that lisinopril is a non-thiol-containing ACE inhibitor, which indicates that lisinopril antioxidant effect, unlike thiol-containing ACE inhibitors such as captopril, is independent to thiol moiety (Thakur et al., 2014).
In the present investigation, lisinopril also showed a potent anti-inflammatory effect evidenced by decreased hepatic NOx production and MPO activity. In agreement, Yirmibeşoğlu et al. (2011) observed significant decreases in NO and ONOO− levels in the liver tissue by lisinopril in a partial hepatectomy model. In addition, Shaker and Sourour (2010) reported that lisinopril significantly decreased cardiac inducible nitric oxide synthase (iNOS) mRNA expression. Lisinopril was reported to have immune-modulatory functions as it could suppress IL-12 which is a cytokine produced primarily by monocytes and macrophages with an essential role in cell-mediated immunity (Agha and Mansour, 2000).
Results of the present investigation also revealed that allopurinol could protect rat liver against acetaminophen-induced injury, evidenced by decreased serum ALT and AST levels, supported by histopathological improvements (Table 1; Fig. 1(F)). In agreement, Demirel et al. (2012) showed similar results in thioacetamide-induced acute liver failure. Moreover, Kataoka et al. (2015) reported a similar hepatoprotective potential for another xanthine oxidase inhibitor, febuxostat, against acetaminophen and uric acid-induced hepatitis.
The current investigation demonstrated that allopurinol has a potent antioxidant activity which was evidenced by modulation of oxidative stress biomarkers. It was found that allopurinol significantly increased hepatic GSH content and CAT activity. These findings confirmed the result of Al Maruf et al. (2014) who reported a similar increase in GSH in a model of azathioprine-induced cytotoxicity, Rodrigues et al. (2014) who studied a protective effect for allopurinol on hypoxanthine-induced oxidative stress in rat kidney, and Akbulut et al. (2015) who studied the beneficial effects of allopurinol against cyclosporine-induced hepatotoxicity.
In addition, the present work showed that allopurinol had anti-inflammatory activity proved by significant decreases in hepatic MPO activity and NOx production. These results came in agreement with the work of Ansari et al. (2013) who reported that allopurinol decreased MPO activity and exerted a neuroprotective effect against cerebral ischemia reperfusion injury in diabetic rats. In addition, Margaritis et al. (2011) found that allopurinol decreased MPO activity in intestinal ischemic injury in rats. A further support for this idea was provided by Makay et al. (2009) who reported a similar decrease in NOX production during studying the role of allopurinol on oxidative stress in experimental hyperthyroidism.
Allopurinol competitively inhibits the action of xanthine oxidoreductase enzyme responsible for the generation of massive amounts of reactive oxygen intermediates. Allopurinol could therefore prevent liver injury by the inhibition of free radical formation (Marotto et al., 1988, Aldaba-Muruato et al., 2013). Another possible explanation for the beneficial effects of allopurinol is the preservation of hypoxanthine through the blockade of xanthine oxidase, which can act as a substrate to form ATP (McCord, 1985, Hirsch et al., 2012). Recently, Williams et al. (2014) reported that the beneficial effect of allopurinol on acetaminophen-induced liver injury may be attributed to the effect of the former on aldehyde oxidase-mediated liver preconditioning.
Allopurinol was also reported to have immunomodulatory properties evidenced as suppression of nuclear expression of nuclear factor kappa B (NF-κB) as well as attenuation of the expression of inflammatory adhesion molecules in murine models (Muir et al., 2008, Aldaba-Muruato et al., 2013).
Results of the present investigation suggest three good hepatoprotective strategies, namely calcium channel blockade, ACE inhibition and xanthine oxidase inhibition, through amlodipine, lisinopril and allopurinol, respectively. Hepatoprotective potentials are mostly through anti-oxidant, anti-inflammatory, immunomodulatory and calcium regulatory activities. This study may give a good guide to acetaminophen hepatotoxic mechanisms, which may give a helpful key for further trials on other drugs with similar mechanisms against such injury.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nesreen E.M. Mohammed, Email: nesr_pharma@yahoo.com.
Basim A.S. Messiha, Email: drbasimanwar2006@yahoo.com.
Ali A. Abo-Saif, Email: aliabosaif@hotmail.com.
References
- Abdel Salam O.M.E., Hassan N.S., Baiuomy A.R., Karam S.H. The effect of amlodipine, diltiazem and enalapril on hepatic injury caused in rats by the administration of CCl4. J. Pharmacol. Toxicol. 2007;2:610–620. [Google Scholar]
- Abla L.E., Gomes H.C., Percario S., Ferreira L.M. Acetylcysteine in random skin flap in rats. Acta Cir. Bras. 2005;20:121–123. doi: 10.1590/s0102-86502005000200004. [DOI] [PubMed] [Google Scholar]
- Agha A.M., Mansour M. Effects of captopril on interleukin-6, leukotriene b and oxidative stress markers in serum and inflammatory exudate of arthritic rats: evidence of anti-inflammatory activity. Toxicol. Appl. Pharmacol. 2000;168:123–130. doi: 10.1006/taap.2000.8985. [DOI] [PubMed] [Google Scholar]
- Akbulut S., Elbe H., Eris C., Dogan Z., Toprak G., Yalcin E., Turkoz Y. Effects of antioxidant agents against CsA-induced hepatotoxicity. J. Surg. Res. 2015;193:658–666. doi: 10.1016/j.jss.2014.08.042. [DOI] [PubMed] [Google Scholar]
- Al Maruf A., Wan L., O’Brien P.J. Evaluation of azathioprine-induced cytotoxicity in an in vitro rat hepatocyte system. Biomed. Res. Int. 2014;2014:379748. doi: 10.1155/2014/379748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarwani S., Al-Siyabi S., Al-Husseini I., Al-Ismail A., Al-Lawati I., Al-Bahrani I., Tanira M.O. Lisinopril alters contribution of nitric oxide and K(Ca) channels to vasodilatation in small mesenteric arteries of spontaneously hypertensive rats. Physiol. Res. 2015;64(1):39–49. doi: 10.33549/physiolres.932780. [DOI] [PubMed] [Google Scholar]
- Aldaba-Muruato L.R., Moreno M.G., Shibayama M., Tsutsumi V., Muriel P. Allopurinol reverses liver damage induced by chronic carbon tetrachloride treatment by decreasing oxidative stress, TGF-β production and NF-κB nuclear translocation. Pharmacology. 2013;92:138–149. doi: 10.1159/000339078. [DOI] [PubMed] [Google Scholar]
- Alipour M., Buonocore C., Omri A., Szabo M., Pucaj K., Suntres Z.E. Therapeutic effect of liposomal-N-acetylcysteine against acetaminophen-induced hepatotoxicity. J. Drug Target. 2013;21:466–473. doi: 10.3109/1061186X.2013.765443. [DOI] [PubMed] [Google Scholar]
- Amacher D.E., Schomaker S.J., Aubrecht J. Development of blood biomarkers for drug-induced liver injury: an evaluation of their potential for risk assessment and diagnostics. Mol. Diagn. Ther. 2013;17:343–354. doi: 10.1007/s40291-013-0049-0. [DOI] [PubMed] [Google Scholar]
- Ansari M.A., Hussain S.K., Mudagal M.P., Goli D. Neuroprotective effect of allopurinol and nimesulide against cerebral ischemic reperfusion injury in diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2013;17:170–178. [PubMed] [Google Scholar]
- Banchroft G.D., Steven A. fourth ed. Churchil Livingstone Publications; London: 1983. Theory and practice of histological technique. [Google Scholar]
- Barritt G.L., Chen J., Rychkov G.Y. Ca2+-permeable channels in the hepatocyte plasma membrane and their roles in hepatocyte physiology. Biochim. Biophys. Acta. 2008;1783:651–672. doi: 10.1016/j.bbamcr.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Bateman D.N., Dear J.W., Thanacoody H.K., Thomas S.H., Eddleston M., Sandilands E.A., Coyle J., Cooper J.G., Rodriguez A., Butcher I., Lewis S.C., Vliegenthart A.D., Veiraiah A., Webb D.J., Gray A. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet. 2014;383:697–704. doi: 10.1016/S0140-6736(13)62062-0. [DOI] [PubMed] [Google Scholar]
- Begum S., Akhter N. Cardioprotective effect of amlodipine in oxidative stress induced by experimental myocardial infarction in rats. Bangladesh J. Pharmacol. 2007;2:55–60. [Google Scholar]
- Berkels R., Breitenbach T., Bartels H., Taubert D., Rosenkranz A., Klaus W., Roesen R. Different antioxidative potencies of dihydropyridine calcium channel modulators in various models. Vascul. Pharmacol. 2005;42:145–152. doi: 10.1016/j.vph.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Chan H., Leung P.S., Tam M.S. Effect of angiotensin AT1 receptor antagonist on D-galactosamine-induced acute liver injury. Clin. Exp. Pharmacol. Physiol. 2007;34:985–991. doi: 10.1111/j.1440-1681.2007.04669.x. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran V.R., Hsu D.Z., Liu M.Y. The protective effect of sesamol against mitochondrial oxidative stress and hepatic injury in acetaminophen-overdosed rats. Shock. 2009;32:89–93. doi: 10.1097/SHK.0b013e31818ede6f. [DOI] [PubMed] [Google Scholar]
- Claiborne A. Catalase activity. In: Greenwald R.A., editor. Handbook of Methods for Oxygen Radical Research. CRC Press; Boca Raton: 1985. pp. 283–284. [Google Scholar]
- Coen M. Metabolic phenotyping applied to pre-clinical and clinical studies of acetaminophen metabolism and hepatotoxicity. Drug Metab. Rev. 2014;23:1–16. doi: 10.3109/03602532.2014.982865. [DOI] [PubMed] [Google Scholar]
- Corcoran G.B., Wong B.K., Neese B.L. Early sustained rise in total liver calcium during acetaminophen hepatotoxicity in mice. Res. Commun. Chem. Pathol. Pharmacol. 1987;58:291–305. [PubMed] [Google Scholar]
- Demirel U., Yalniz M., Aygün C., Orhan C., Tuzcu M., Sahin K., Ozercan I.H., Bahçecioğlu I.H. Allopurinol ameliorates thioacetamide-induced acute liver failure by regulating cellular redox-sensitive transcription factors in rats. Inflammation. 2012;35:1549–1557. doi: 10.1007/s10753-012-9470-5. [DOI] [PubMed] [Google Scholar]
- Eesha B.R., Mohanbabu Amberkar V., Meena Kumari K., Sarath B., Vijay M., Lalit M., Rajput R. Hepatoprotective activity of Terminalia paniculata against paracetamol induced hepatocellular damage in Wistar albino rats. Asian Pac. J. Trop. Med. 2011;4:466–469. doi: 10.1016/S1995-7645(11)60127-2. [DOI] [PubMed] [Google Scholar]
- Gardner C.R., Laskin J.D., Dambach D.M., Sacco M., Durham S.K., Bruno M.K., Cohen S.D., Gordon M.K., Gerecke D.R., Zhou P., Laskin D.L. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol. Appl. Pharmacol. 2002;184:27–36. [PubMed] [Google Scholar]
- Gokcimen A., Kocak A., Kilbas S., Bayram D., Kilbas A., Cim A., Kockar C., Kutluhan S. Effect of lisinopril on rat liver tissues in L-NAME induced hypertension model. Mol. Cell. Biochem. 2007;296:159–164. doi: 10.1007/s11010-006-9310-8. [DOI] [PubMed] [Google Scholar]
- Gupta G., Krishna G., Chellappan D.K., Gubbiyappa K.S., Candasamy M., Dua K. Protective effect of pioglitazone, a PPARγ agonist against acetaminophen-induced hepatotoxicity in rats. Mol. Cell. Biochem. 2014;393:223–228. doi: 10.1007/s11010-014-2064-9. [DOI] [PubMed] [Google Scholar]
- Harada N., Okajima K., Kushimoto S., Isobe H., Tanaka K. Antithrombin reduces ischemia/reperfusion injury of rat liver by increasing the hepatic level of prostacyclin. Blood. 1999;93:157–164. [PubMed] [Google Scholar]
- Hirsch G.A., Bottomley P.A., Gerstenblith G., Weiss R.G. Allopurinol acutely increases adenosine triphospate energy delivery in failing human hearts. J. Am. Coll. Cardiol. 2012;59:802–808. doi: 10.1016/j.jacc.2011.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.C., Hsieh S.C., Chiu J.H., Wu Y.L. Protective effects of N-acetylcysteine and a prostaglandin E1 analog, alprostadil, against hepatic ischemia: reperfusion injury in rats. J. Tradit. Complement. Med. 2014;4:64–71. doi: 10.4103/2225-4110.124351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal S.M. Possible hepatoprotective effects of lacidipine in irradiated DOCA-salt hypertensive albino rats. Pak. J. Biol. Sci. 2013;16:1353–1357. doi: 10.3923/pjbs.2013.1353.1357. [DOI] [PubMed] [Google Scholar]
- Kataoka H., Yang K., Rock K.L. The xanthine oxidase inhibitor Febuxostat reduces tissue uric acid content and inhibits injury-induced inflammation in the liver and lung. Eur. J. Pharmacol. 2015;746:174–179. doi: 10.1016/j.ejphar.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradpezhouh E., Ma L., Morphett A., Barritt G.J., Rychkov G.Y. TRPM2 channels mediate acetaminophen-induced liver damage. Proc. Natl. Acad. Sci. USA. 2014;111:3176–3181. doi: 10.1073/pnas.1322657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran P.M., Raju A.V., Rao B.G. Investigation of hepatoprotective activity of Cyathea gigantea (Wall. ex. Hook.) leaves against paracetamol-induced hepatotoxicity in rats. Asian Pac. J. Trop. Biomed. 2012;2:352–356. doi: 10.1016/S2221-1691(12)60055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenkel O., Mossanen J.C., Tacke F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg. Nutr. 2014;3:331–343. doi: 10.3978/j.issn.2304-3881.2014.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Kalita J., Misra U.K., Bora H.K. A study of dose response and organ susceptibility of copper toxicity in a rat model. J. Trace Elem. Med Biol. 2015;29:269–274. doi: 10.1016/j.jtemb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Lahouel M., Boulkour S., Segueni N., Fillastre J.P. The flavonoids effect against vinblastine, cyclophosphamide and paracetamol toxicity by inhibition of lipid-peroxydation and increasing liver glutathione concentration. Pathol. Biol. 2004;52:314–322. doi: 10.1016/j.patbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Li H.B., Qin D.N., Ma L., Miao Y.W., Zhang D.M., Lu Y., Song X.A., Zhu G.Q., Kang Y.M. Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicol. Appl. Pharmacol. 2014;279:141–149. doi: 10.1016/j.taap.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Li Y., Shen G., Yu C., Li G., Shen J., Gong J., Xu Y. Angiotensin II induces mitochondrial oxidative stress and mtDNA damage in osteoblasts by inhibiting SIRT1–FoxO3a–MnSOD pathway. Biochem. Biophys. Res. Commun. 2014;455:113–118. doi: 10.1016/j.bbrc.2014.10.123. [DOI] [PubMed] [Google Scholar]
- Liu H.M., Yan L.H., Luo Z., Sun X.M., Cui R.B., Li X.H., Yan M. Role of store-operated Ca2+ channels in ethanol-induced intracellular Ca2+ increase in HepG2 cells. Zhonghua Gan. Zang. Bing. Za Zhi. 2013;21 doi: 10.3760/cma.j.issn.1007-3418.2013.12.015. 949-254. [DOI] [PubMed] [Google Scholar]
- Makay O., Yenisey C., Icoz G., Genc Simsek N., Ozgen G., Akyildiz M., Yetkin E. The role of allopurinol on oxidative stress in experimental hyperthyroidism. J. Endocrinol. Invest. 2009;32:641–646. doi: 10.1007/BF03345734. [DOI] [PubMed] [Google Scholar]
- Margaritis E.V., Yanni A.E., Agrogiannis G., Liarakos N., Pantopoulou A., Vlachos I., Papachristodoulou A., Korkolopoulou P., Patsouris E., Kostakis M., Perrea D.N., Kostakis A. Effects of oral administration of (L)-arginine, (L)-NAME and allopurinol on intestinal ischemia/reperfusion injury in rats. Life Sci. 2011;88:1070–1076. doi: 10.1016/j.lfs.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Marotto M.E., Thurman R.G., Lemasters J.J. Early midzonal cell death during low-flow hypoxia in the isolated, perfused rat liver: protection by allopurinol. Hepatology. 1988;8:585–590. doi: 10.1002/hep.1840080325. [DOI] [PubMed] [Google Scholar]
- Mason R.P., Jacob R.F., Corbalan J.J., Kaliszan R., Malinski T. Amlodipine increased endothelial nitric oxide and decreased nitroxidative stress disproportionately to blood pressure changes. Am. J. Hypertens. 2014;27:482–488. doi: 10.1093/ajh/hpt202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J.M. Oxygen-derived free radicals in postischemic tissue injury. New Engl. J. Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Miranda K.M., Espey M.G., Wink D.A. A rapid, simple spectro-photometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- Mohanty I.R., Arya D.S., Dinda A., Gupta S.K. Comparative cardioprotective effects and mechanisms of vitamin E and lisinopril against ischemic reperfusion induced cardiac toxicity. Environ. Toxicol. Pharmacol. 2013;35:207–217. doi: 10.1016/j.etap.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Morsy M.A. Protective effect of lisinopril on hepatic ischemia/reperfusion injury in rats. Indian. J. Pharmacol. 2011;43:652–655. doi: 10.4103/0253-7613.89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir S.W., Harrow C., Dawson J., Lees K.R., Weir C.J., Sattar N., Walters M.R. Allopurinol use yields potentially beneficial effects on inflammatory indices in those with recent ischemic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2008;39:3303–3307. doi: 10.1161/STROKEAHA.108.519793. [DOI] [PubMed] [Google Scholar]
- Ohishi T., Saito H., Tsusaka K., Toda K., Inagaki H., Hamada Y., Kumagai N., Atsukawa K., Ishii H. Anti-fibrogenic effect of an angiotensin converting enzyme inhibitor on chronic carbon tetrachloride-induced hepatic fibrosis in rats. Hepatol. Res. 2001;21:147–158. doi: 10.1016/s1386-6346(01)00102-4. [DOI] [PubMed] [Google Scholar]
- Öktem F., Kirbas A., Armagan A., Kuybulu A.E., Yilmaz H.R., Ozguner F., Uz E. Lisinopril attenuates renal oxidative injury in L-NAME-induced hypertensive rats. Mol. Cell. Biochem. 2011;352:247–253. doi: 10.1007/s11010-011-0760-2. [DOI] [PubMed] [Google Scholar]
- Olaleye M.T., Amobonye A.E., Komolafe K., Akinmoladun A.C. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi J. Biol. Sci. 2014;21:486–492. doi: 10.1016/j.sjbs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidi A., Riahinia N., Montazer Torbati M.B., Behdani M.A. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J. Phytomed. 2014;4:330–336. [PMC free article] [PubMed] [Google Scholar]
- Prieto-Moure B., Carabén-Redaño A., Aliena-Valero A., Cejalvo D., Toledo A.H., Flores-Bellver M., Martínez-Gil N., Toledo-Pereyra L.H., Lloris Carsí J.M. Allopurinol in renal ischemia. J. Invest. Surg. 2014;27:304–316. doi: 10.3109/08941939.2014.911395. [DOI] [PubMed] [Google Scholar]
- Rachmat F.D., Rachmat J., Sastroasmoro S., Wanandi S.I. Effect of allopurinol on oxidative stress and hypoxic adaptation response during surgical correction of tetralogy of fallot. Acta Med. Indones. 2013;45:94–100. [PubMed] [Google Scholar]
- Rajaraman G., Wang G., Smith H.J., Gong Y., Burczynski F.J. Effect of diltiazem isomers and thiamine on piglet liver microsomal peroxidation using dichlorofluorescein. J. Pharm. Pharm. Sci. 2007;10:380–387. [PubMed] [Google Scholar]
- Rodrigues A.F., Roecker R., Junges G.M., de Lima D.D., da Cruz J.G., Wyse A.T., Dal Magro D.D. Hypoxanthine induces oxidative stress in kidney of rats: protective effect of vitamins E plus C and allopurinol. Cell Biochem. Funct. 2014;32:387–394. doi: 10.1002/cbf.3029. [DOI] [PubMed] [Google Scholar]
- Salman S., Kumbasar S., Yilmaz M., Kumtepe Y., Borekci B., Bakan E., Suleyman H. Investigation of the effects of the chronic administration of some antihypertensive drugs on enzymatic and non-enzymatic oxidant/antioxidant parameters in rat ovarian tissue. Gynecol. Endocrinol. 2011;27:895–899. doi: 10.3109/09513590.2010.551564. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Sen H., Deniz S., Yedekci A.E., Inangil G., Muftuoglu T., Haholu A., Ozkan S. Effects of dexpanthenol and N-acetylcysteine pretreatment in rats before renal ischemia/reperfusion injury. Ren. Fail. 2014;36:1570–1574. doi: 10.3109/0886022X.2014.949768. [DOI] [PubMed] [Google Scholar]
- Shaker O., Sourour D.A. How to protect doxorubicin-induced cardiomyopathy in male albino rats? J. Cardiovasc. Pharmacol. 2010;55:262–268. doi: 10.1097/FJC.0b013e3181cf91ac. [DOI] [PubMed] [Google Scholar]
- Stephens C., Andrade R.J., Lucena M.I. Mechanisms of drug-induced liver injury. Curr. Opin. Allergy Clin. Immunol. 2014;14:286–292. doi: 10.1097/ACI.0000000000000070. [DOI] [PubMed] [Google Scholar]
- Thakur, K.S., Prakash, A., Bisht, R., Bansal, P.K., 2014. Beneficial effect of candesartan and lisinopril against haloperidol-induced tardive dyskinesia in rat. J. Renin Angiotensin Aldosterone Syst. (in press). [DOI] [PubMed]
- Tobwala S., Khayyat A., Fan W., Ercal N. Comparative evaluation of N-acetylcysteine and N-acetylcysteineamide in acetaminophen-induced hepatotoxicity in human hepatoma HepaRG cells. Exp. Biol. Med. 2015;240:261–272. doi: 10.1177/1535370214549520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Velayutham P.K., Adhikary S.D., Babu S.K., Vedantam R., Korula G., Ramachandran A. Oxidative stress-associated hypertension in surgically induced brain injury patients: effects of β-blocker and angiotensin-converting enzyme inhibitor. J. Surg. Res. 2013;179:125–131. doi: 10.1016/j.jss.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Vitolina R., Krauze A., Duburs G., Velena A. Aspects of the amlodipine pleiotropy in biochemistry, pharmacology and clinics. Int. J. Pharm. Sci. Res. 2012;3:1215–1232. [Google Scholar]
- Williams C.D., McGill M.R., Lebofsky M., Bajt M.L., Jaeschke H. Protection against acetaminophen-induced liver injury by allopurinol is dependent on aldehyde oxidase-mediated liver preconditioning. Toxicol. Appl. Pharmacol. 2014;274:417–424. doi: 10.1016/j.taap.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasu T., Kobayashi M., Mutoh A., Yamakawa K., Momomura S., Ueda S. Dihydropyridine calcium channel blockers inhibit non-esterified-fatty-acid-induced endothelial and rheological dysfunction. Clin. Sci. 2013;125:247–255. doi: 10.1042/CS20120311. [DOI] [PubMed] [Google Scholar]
- Yilmaz N., Vural H., Yilmaz M., Sutcu R., Sirmali R., Hicyilmaz H., Delibas N. Calorie restriction modulates hippocampal NMDA receptors in diet-induced obese rats. J. Recept. Signal. Transduct. Res. 2011;31:214–219. doi: 10.3109/10799893.2011.569724. [DOI] [PubMed] [Google Scholar]
- Yirmibeşoğlu O.A., Büyükgebiz O., Ars D., Unay O., Cevik D. Lisinopril inhibits endothelin-1 in the early period of hepatic reperfusion injury in a partial hepatectomy model. Transplant. Proc. 2011;43:2524–2530. doi: 10.1016/j.transproceed.2011.06.043. [DOI] [PubMed] [Google Scholar]
- Young D.S. fourth ed. AACC Press; Washington: 1995. Effects of Drugs on Clinical Laboratory Tests. [Google Scholar]
- Yousef M.I., Omar S.A., El-Guendi M.I., Abdelmegid L.A. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem. Toxicol. 2010;48:3246–3261. doi: 10.1016/j.fct.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Zhang X., Tian F., Kawai H., Kurata T., Deguchi S., Deguchi K., Shang J., Liu N., Liu W., Ikeda Y., Matsuura T., Kamiya T., Abe K. Anti-inflammatory effect of amlodipine plus atorvastatin treatment on carotid atherosclerosis in zucker metabolic syndrome rats. Transl. Stroke Res. 2012;3:435–441. doi: 10.1007/s12975-012-0198-1. [DOI] [PubMed] [Google Scholar]
- Zyoud S.H., Awang R., Sulaiman S.A., Al-Jabi S.W. Effects of delay in infusion of N-acetylcysteine on appearance of adverse drug reactions after acetaminophen overdose: a retrospective study. Pharmacoepidemiol. Drug Saf. 2010;19:1064–1070. doi: 10.1002/pds.1955. [DOI] [PubMed] [Google Scholar]