Abstract
Introduction: Toxoplasma gondii (T. gondii) is an intracellular protozoan that can infect all mammals, who serve as intermediate host. It causes congenital, neurological, eyes complications and mild or asymptomatic infections in humans. Purpose of this study: To investigate not only the prevalence of T. gondii, but also to find out its genotyping using multiple sequential molecular methods to predict exactly the precise genotyping of T. gondii among Saudi pregnant women. Methods: A cross-sectional study was conducted using multi-stage methods. Initial stage involved enrolment of 250 Saudi pregnant women from multi-centre healthcare and community based settings in the capital of Saudi Arabia Riyadh. The second stage was embracement of the laboratory investigation that included Enzyme immunoassay (ELISA), DNA extraction, PCR, nested-PCR assay, and genotyping of the seropositive cases. Results: 203 women agreed to take part in our study with a response rate of 81.2% (203/250). Using ELISA, we found that the prevalence of Toxoplasma gondii IgG and IgM antibodies was 32.5% and 6.4%, respectively. We found that 29 samples (80.6%) were of genotype II; however 7 samples (19.4%) were of genotype III. Conclusion: Defining the population structure of T. gondii from Saudi Arabia has important implications for transmission, immunogenicity, pathogenesis, and in planning preventive strategies. Relationship between such variation in structure and disease manifestation in pregnant women is still difficult to assess due to the role of host immune status and genetic background on the control of infection, and of other parasitic features such as the infecting dose or parasite stage. Our finding of the genotyping of T. gondii might facilitate and inform future studies on comparative genomics and identification of genes that control important biological phenotypes including pathogenesis and transmission among Saudi women.
Keywords: Toxoplasma, Pregnancy, Women, Genotyping, Prevalence, Saudi
1. Introduction
Toxoplasma gondii (T. gondii) is a single-celled parasitic organism that can infect most animals and birds. Because it reproduces only in cats, wild and domestic felines are the parasite’s ultimate host (Kravetz and Federman, 2005). T. gondii is the third greatest common reason of fatal food-borne infection in United States (Mead et al., 1999). It causes congenital, neurological, eyes complications and minor or asymptomatic infections in humans (Kong et al., 2012). During pregnancy, primary infection, which is transmitted trans-placentally, may cause congenital toxoplasmosis (Many and Koren, 2006). This includes a wide range of manifestations, extending from mild chorioretinitis, which can present many years after birth, to miscarriage, mental retardation, microcephaly, hydrocephalus and seizures (Kravetz and Federman, 2005). Hence, early and accurate diagnosis of toxoplasmosis can be crucial for the prevention and control of the disease, particularly in individual who are at risk, such as pregnant women.
Diagnosing T. gondii is usually achieved by identifying the parasite-specific antibodies in the serum with serological techniques such as Enzyme-linked Immunosorbent Assay (ELISA) and Immuno-fluorescence Antibody Assay (IFA) (Zhang et al., 2009, Lau et al., 2010). However, serology is very difficult to interpret in pregnant patients. Furthermore, Polymerase chain reaction (PCR) methods have substantial advancement for the detection of toxoplasmosis. Among these techniques, nested-PCR has been proved as the most sensitive diagnostic technique for the diagnosis of toxoplasmosis (Kong et al., 2012). There are different strains of T. gondii with variable genetic structure. Three genetically different types (strains) of Toxoplasma gondii are known. These are type-I, type-II and type-III. Due to their genetic differences, the three types also differ in the mode of infection and the severity of symptoms. Therefore, identifying the genetic type helps in better understanding the disease and probably finding the proper treatment (Fuentes et al., 2001). In this context, the identification of atypical strains is of clinical and epidemiological importance because atypical strains are usually associated with severe disease outcomes (Ajzenberg et al., 2010).
Approximately one-third of the global human population is infected with this pathogenic parasite including populations in Europe, South America, Africa and several Asian countries (Fallahi et al., 2014). The prevalence of positive serology for Toxoplasma varies in various regions and cultures. In Saudi Arabia, prevalence studies showed that 29.5–35.6% of pregnant women were found to have T. gondii during pregnancy (Ghazi et al., 2002, Al-Harthi et al., 2006). However, limited studies have been conducted to explore the trend and genotyping of such infection among Saudi pregnant women. Hence, the aim of this study was to investigate not only the prevalence of T. gondii, but also to find out its genotyping using multiple sequential molecular methods to predict exactly the precise genotyping of T. gondii among Saudi pregnant women.
2. Materials and methods
A cross-sectional study was conducted using multi-stage methods over a period of 9 months of the year 2011. Initial stage involved enrolment of 250 Saudi pregnant women from multi-centre healthcare and community based settings in the capital of Saudi Arabia Riyadh. These centres were selected from different regions involving rural and urban areas in order to maximize the variety of our sample. Participants were selected using convenient sampling. They were approached during waiting time for their antenatal care visits and explained the study aim and objectives, and a written consent was obtained accordingly. Inclusion criteria were pregnant women, Saudi, and aged 15–45 years.
The second stage was embracement of the laboratory investigation that included Enzyme immunoassay (ELISA), DNA extraction, PCR, nested-PCR assay, and genotyping of the seropositive cases.
2.1. Enzyme immunoassay
Blood samples were collected from the consented participants (6 mm). Each blood sample was divided into two tubes (3 ml each): one contained the EDTA (EDTA tube) for extracting the DNA and the second one was a plane tube kept to extract serum. The later samples were left for one hour at room temperature until clotting is established. Thereafter, the tubes were centrifuged for 10 min at a speed of 3000 cycles for 15 min. The resulted serum was transferred then into a clean dry tube (Eppendorf tube) and kept in under −20 °C for further analysis.
Specific IgG and IgM antibodies to T. gondii in the serum were measured by the ELISA test following the method of Engvall and his colleagues with the commercial ENZYWELL TOXOPLASMA IgG and IgM KIT, and using the BEP III system (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) according to the manufacturer’s instructions (Engvall and Perlmann, 1971, Engvall and Perlmann, 1972). The extinction value of calibrator identified the upper limit of the reference range of non-infected individual (Cut-off). Therewith, the findings were evaluated quantitatively by calculating a ratio of the extinction value of the control or patient samples over the extinction value of the calibrator. If the absorbance of the sample is higher than that of the Cut-off (>1.3 for IgG and >1.2 for IgM), the sample was considered positive for the presence of specific antibodies and negative if it was <0.7 of IgG and <0.8 for IgM. In case of doubt or borderline test results (0.7–1.3 for IgG and 0.8–1.2 for IgM), the test was repeated.
2.2. DNA extraction
Extraction of DNA was conducted using 3 ml of blood, which was collected in tubes containing EDTA using DNA Isolation Kit (MagNA Pure LC Instrument, Roche Diagnostics, GmbH, Mannheim, Germany) according to the manufacturer’s instructions (Wittwer et al., 1997). This was used to isolate highly purified genomic DNA from the whole blood samples. The isolation procedure was based on magnetic-bead technology. The samples were analysed by incubation with a special buffer containing a chaotropic salt and Proteinase K. Magnetic Glass Particles (MGPs) were added and the DNA is bound to their surfaces. Unbound substances were removed by several washing steps, and then the purified DNA was eluted with a low salt buffer. This was followed by testing the purity of DNA. The purity and concentration of the extracted DNA was determined using spectrophotometer. The purity of a nucleic acid solution can be determined by calculating the A260 nm:A280 nm ratio. Pure DNA has A260 nm/A280 nm ratio of 1.8. The quality of the extracted DNA was assessed by performing gel electrophoresis using Agarose gel (Promega) with a concentration of 0.8% for one hour. The damaged DNA parts were discarded and the rest of the DNA samples were kept under −20 °C for later use.
2.3. PCR
This was done in two stages. The first stage involved applying the PCR in order to identify a certain area of DNA using the amplification of gene B1 for all cases as described by Burg and his colleagues (Burg et al., 1989). PCR master mix was prepared as described in Table 1. To avoid contamination, we used negative control that contained all the matrix mix but not the DNA part. From the seronegative samples, we used one sample as a negative control and one positive control that constituted of DNA as part of T. gondii RH strain. The first cycle took 15 min at 95 °C to activate the enzymes. In this first cycle, pairs of primers (B1R1 and B1F1) were used (Table 2) to apply PCR (Roche Company). It involved 39 repeated cycles with every cycle consisted of 3 steps: denaturation for 30 s at 95 °C, annealing for 30 s at the same temperature (at similar temperature according to the type of the Primer used), and extension for 30 s at 72 °C. At the end of these cycles, a final extension was done for 10 min at 72 °C. These steps were applied in both stages of the PCR and the nested-PCR. The second stage entailed a nested-PCR, which was performed to amplify the products of the first PCR cycle to obtain clear DNA using the method of Lee and his colleagues (Lee et al., 2008). This was done by using 1 μl of the PCR products of the first cycle (diluted at 1:10) with the rest of PCR Master Mix contents plus the primers B1R2 and B1F2 at 60 °C for 30 s (Table 2).
Table 1.
Contents of PCR master mix.
| Contents | 1× | 43× |
|---|---|---|
| d.H2O | 15.8 μl | 679.4 μl |
| 10× Buffer | 2.5 μl | 107.5 μl |
| 5 μM F + R Primers mix | 2 μl | 86 μl |
| DNA 50 ng/μl | 2 μl | — |
| DNA Polymerase 50 units | 0.2 μl | 8.6 μl |
| d.NTPs MIX 0.25 mM | 2.5 μl | 107.5 μl |
| Total volume | 25 μl | 989 μl |
Table 2.
Primers used for amplification for the B1gene.
| Primers used for amplification for B1 gene | |
|---|---|
| B1F1 | 5′-GGAACTGCATCCGTTCATGAG-3′ |
| BIR1 | 5′-TCTTTAAAGCGTTCGTGGTC-3′ |
| B1F2 | 5′-TGCATAGGTTGCAGTCACTG-3′ |
| B1R2 | 5′-GGCGACCAATCTGCGAATACACC-3′ |
2.4. Genotyping
This step was carried out on the positive samples that were obtained using nested PCR for the B1 gene of T. gondii in order to amplify the SAG2 (Surface Antigen of the intracellular T. gondii) gene at both 3′ and 5′ ends separately. We used PCR reaction mix that contained 5 μl of buffer PCR X10 (100 ml Tris HC1 + 15 mmol MgC12 + 500 mmol KC1 from Sigma Company) and added to it 1 mmol MgC12, 500 mmol dNTPs, 10 μmol from each unit of F and R primers of the Taq DNA polymerase enzyme and 100 nanogram of Genomic DNA. Initial stage involved PCR testing for 10 min at temperature 95 °C, followed by 40 cycles (each cycle composed of three steps: denaturation for 60 s at temperature 95 °C, annealing for 60 s at similar temperature according to the type of the Primer used, and extension for 60 s at a temperature of 72 °C). The Primers used to amplify the 5 end of the SAG2 gene were SAG2F4 and SAG2R4. The nested-PCR test was performed by adding 1 μl (diluted 1:10) of the initial PCR product and used as a source for DNA templates for the second cycle (nested-PCR). The same PCR master mix previously described except that the internal Primers were replaced by SAG2F4 and SAG2R2 (TIB-MOLBIOL-Germany) at a temperature of 60 °C for 60 s (Table 3).
Table 3.
Primers used for amplification for the 5′ end of SAG2 gene.
| Primers used for amplification for the 5′ end of SAG2 gene | |
|---|---|
| SAG2F4 | 5′-GACCTCGAACAGGAACAC-3′ |
| SAG2R4 | 5′-GCATCAACAGTCTTCGTTGC-3′ |
| SAG2F | 5′-GAAATGTTTCAGGTTGCTGC-3′ |
| SAG2R2 | 5′-GCAAGAGCGAACTTGAACAC-3′ |
For the amplification of the 3′ end of SAG2, all the previous procedures were applied, but the Primers were replaced with SAG2F3 and SAG2R3 at annealing temperature of 60 °C for 30 s. Additionally, similar PCR cycles and nested-PCR were performed, but the Primers used were replaced by SAG2F2 and SAG2R at annealing temperature of 61 °C for 30 s (Table 4). This was followed by analysing PCR products using Agarose gel (concentration: 2%).
Table 4.
Primers used for amplification for the 3′ end of SAG2 gene.
| Primers used for amplification for the 3′ end of SAG2 gene | |
|---|---|
| SAG2F3 | 5′-TCTGTTCTCCGAAGTGACTCC-3′ |
| SAG2R3 | 5′-TCAAAGCGTGCATTATCGC-3′ |
| SAG2F2 | 5′-ATTCTCATGCCTCCGCTTC-3′ |
| SAG2R | 5′-AACGTTTCACGAAGGCACAC-3′ |
Genotyping was performed using the Restriction Fragment Length Polymorphism (PCR-RFLP) (Fuentes et al., 2001). This involved adding 1.5 μl of Buffer SA (Digestion buffer), 2 μl of Sau3AI enzyme, 10 μl of the PCR products from the 5′ end of SAG2 gene and 11.5 μl distilled water. The digestion was conducted at a temperature of 37 °C for three hours, while the 3′ end was digested using HhaI endonuclease enzyme by adding 1.5 μl of SL Buffer with 2 μl of Hhal enzyme, 10 μl of the PCR product of the 3′ end SAG2, and 11.5 μl of distilled water. The digestion was conducted at a temperature of 37 °C for three hours. The resulted digestives were run on Agarose gel (concentration: 25%) using three types of T. gondii as reference standards and as positive controls. The first type was T. gondii RH strain (type I), the second one was LEG 96-1 (type II), and the third was LEG-NJA (type III). These were used to identify the genotyping of T. gondii of our seropositive cases.
2.5. Data analysis
Data were coded and entered into SPSS (version 15). Data were described using frequencies and percentage. Correlations between variables were done using Chi-square with a significant cut-off p value of <0.05.
2.6. Ethical consideration
The study was approved by the ethics committee of King Saud University. Participants were informed of the study aim and procedures. Consent was obtained and participants were informed that they are free to join or leave the study at any stage without giving any justification.
3. Results
203 women agreed to take part in our study with a response rate of 81.2% (203/250). Using ELISA, we found that the prevalence of Toxoplasma gondii IgG and IgM antibodies was 32.5% and 6.4%, respectively. The mean age was 32 years (SD±). 34.9% of those with positive IgG were in their first trimester, 32.1% were in the second trimester, and 33% were in the last trimester of their pregnancy.
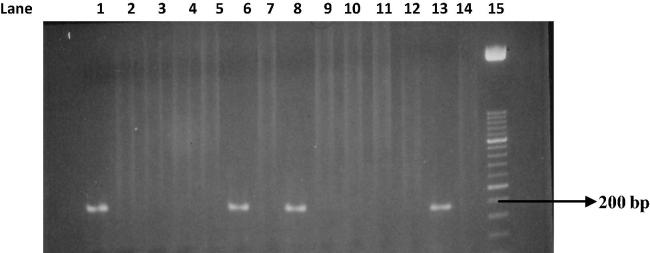
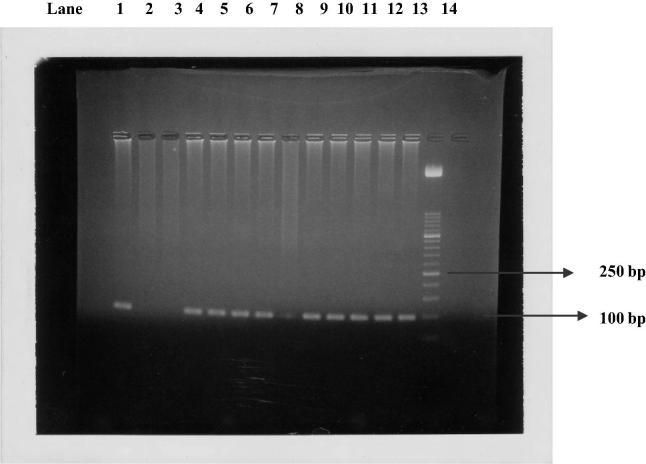
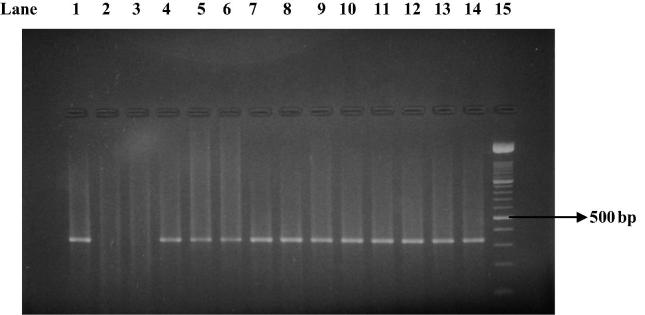
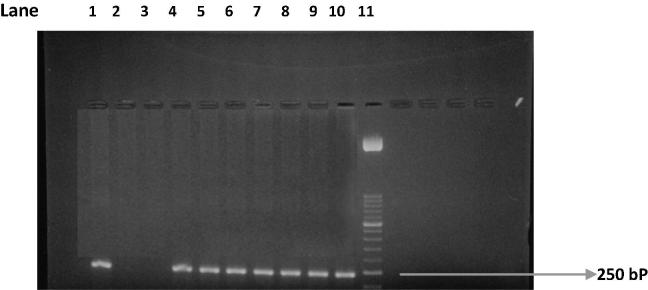
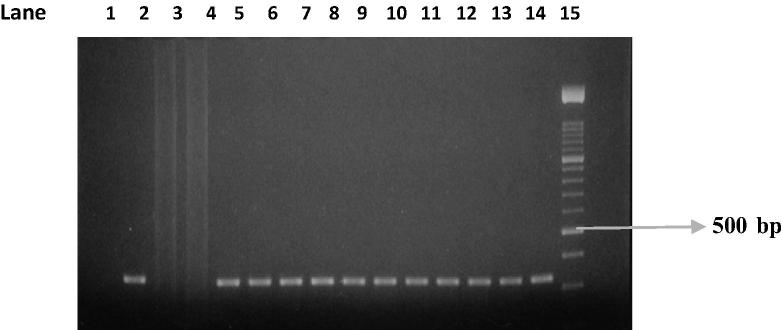
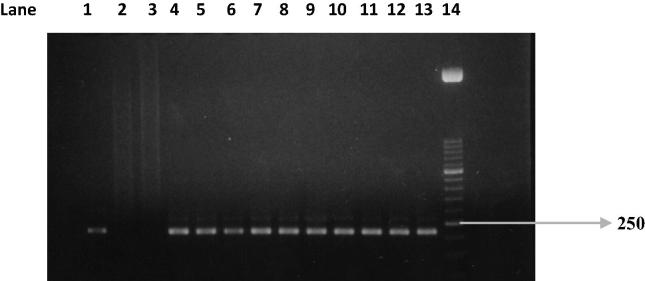
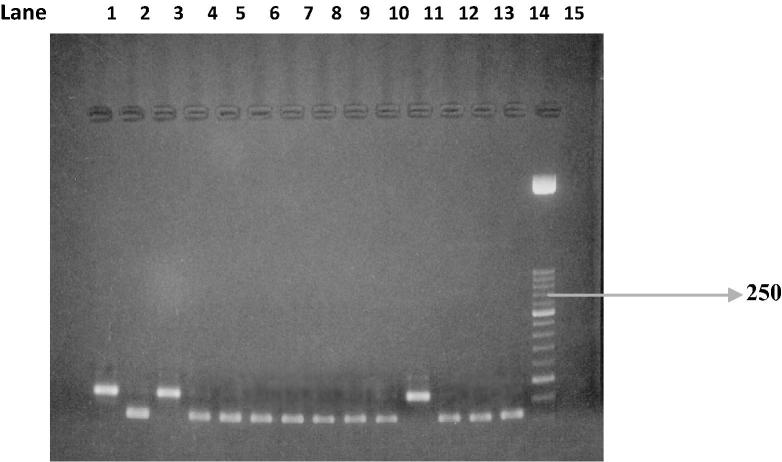
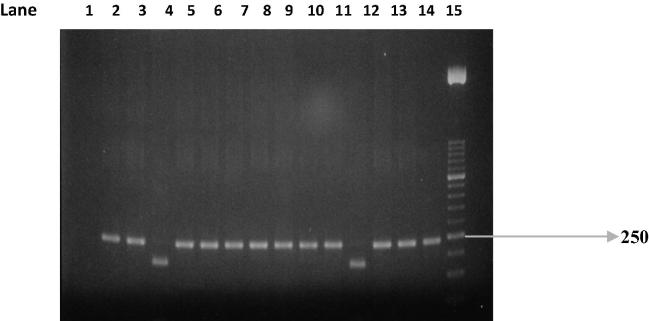
PCR test (first cycle) revealed 22.2% positive samples (45/203) and the amplification of T. gondii gene B1 with a production of a fragment with 196 bp (Fig. 1). The nested-PCR test for positive samples (second cycle) resulted in an expected fragment of 97 bp (Fig. 2). Amplification of the SAG2 locus in both 5′ and 3′ ends of the confirmed cases of T. gondii produced 241 bp and 221 bp fragments respectively (Figure 3, Figure 4, Figure 5, Figure 6).
Figure 1.
Amplification of T. gondii B1 gene. Lane 1: Positive control. Lanes 2 and 3: Negative control. Lanes 6, 8, and 13: Positive samples. Lanes 4, 5, 7, 9, 10, 11, 12, and 14: Negative samples. Lane 15: Molecular weight marker 50 bp.
Figure 2.
Nested PCR on B1 gene. Lane 1: positive control. Lanes 2 and 3: negative control. Lanes 4–13: Positive samples. Lane 14: Molecular weight marker 50 bp.
Figure 3.
First amplification of the 5′ end of SAG2 locus. Lane 1: positive control. Lanes 2 and 3: negative control. Lanes 4–14: Positive samples. Lane 15: Molecular weight marker 100-bp.
Figure 4.
Second amplification of the 5′ end of SAG2 locus. Lane 1: positive control. Lanes 2 and 3: negative control. Lanes 4–10: some positive samples. Lane 11: Molecular weight marker 50-bp.
Figure 5.
First amplification of the 3′ end of SAG2 locus. Lane 1: positive control. Lane 2: negative control. Lane 3: negative control. Lanes 4–14: some positive samples. Lane 15: Molecular weight marker 100-bp.
Figure 6.
Second amplification of the 3′ end of SAG2 locus. Lane 1: positive control. Lane 2: negative control. Lane 3: negative control. Lanes 4–13: some positive samples. Lane 14: Molecular weight marker 50 bp.
We were able to do total genotypic characterization of 80% of the samples (36 out of 45), which were positive by carrying out the nested-PCR technique, with two different genomic targets: B1 and SAG2 genes. From these samples using Agarose gel electrophoresis, we found that 29 samples (80.6%) were of genotype II; however 7 samples (19.4%) were of genotype III. There were 9 samples (only partially characterized) in which we could not completely identify the parasite genotypes. Six of them (the 3′ end) that were digested characterized as type II (Fig. 7). Four were digested using the enzyme HhaI; however, we could not determine that it is type II because the digestion process was not clear enough. The remaining two were not digested and considered as either type I (RH strain) or III (non-type II). In three samples, the 5′ end was only amplified and two of them were Sau3AI digested (Fig. 8) and again, here, it was not possible to characterize them as type III because digestion process was not clear; however, one was not digested and hence was considered as either type I or II (non-type III).
Figure 7.
HhaI Restriction digestion of the 3′ end amplification products. Lane 1: type I strain. Lane 2: Type II strain. Lane 3: Type III strain. Lanes 4–10, 12, 13, and 14: strains from clinical samples of type II strain. Lane 11: sample of nontype II strain. Lane 15: Molecular weight marker 50 bp.
Figure 8.
Sau3AI Restriction digestion of the 5′ end amplification products. Lane 1: type I strain. Lane 2: Type II strain. Lane 3: Type III strain. Lanes: 4–10, 12, 13, and 14: strains from clinical samples of nontype III strains.
4. Discussion
This study showed that T. gondii was prevalent among Saudi pregnant women (32.5% for IgG and 6.4% for IgM). The majority of cases were of genotype II of T. gondii. World-wide, T. gondii prevalence in human varies from region to region with rates range from less than 10% to over 90% (Bouratbine et al., 2001, Torgerson and Mastroiacovo, 2013). For example, a study conducted among ante-natal care patients in Saudi Arabia showed higher rate of IgG (38%) than our finding; however, the researchers did not find IgM among their sample (Almogren, 2011). Another study conducted in Qatar with almost similar results to our findings showed that 35.1% and 5.2% of pregnant women tested positive for IgG and IgM, respectively (Abu-Madi et al., 2010). A literature review showed that high prevalence exists in Latin America, parts of Eastern/Central Europe, the Middle East, parts of south-east Asia and Africa. However, a trend towards lower sero-prevalence is observed in many European countries and the USA. Authors suggested that there is an over-representation of certain countries such as Iran, while information from other, geographically larger, countries may be limited (Pappas et al., 2009).
This variation in the sero-prevalence rate might be explained by several reasons. The presence of specific IgM is generally suggested to be associated with acute infection. However, this may not be the case in toxoplasmosis, as the continuation of IgM for several months in the sera may interfere in calculating the time of exposure (Pelloux et al., 1997). Therefore, different timing of measuring the sero-positivity might give different results, which in turn depends on the stage of the infection of T. gondii whether acute or chronic period of the disease. Moreover, regional variation of prevalence has been attributed to climate, cultural differences in the amount and type of raw meat consumed, and the increased consumption of meat from animal’s farmed indoors and frozen meat. Our present study focuses on the prevalence and genotyping of T. gondii; however, we will report the role of socio-demographic profile and life-style in the occurrence of such infection in another paper.
Another issue in collectively evaluating what sero-prevalence rates provided to us is the difference in diagnostic methods used. Several methods are available which finally allow diagnosing the stage of infection. However, value of these tests in diagnosing the acute T. gondii infections can be questioned particularly from developing countries (Singh and Pandit, 2004). Although the detection of IgM antibodies is the most commonly used method for serological diagnosis of acute infections, yet it has some disadvantages. Moreover, the IgM-ISAGA test which is considered standard test remains positive for up to 12 months after primary infection (bioMérieux, 2000). Therefore, it may be difficult to interpret the single point IgM positive results in women who tested during the different trimesters of their pregnancy. Indeed, it is a measure of the accumulated exposure during a person’s lifetime in a particular social setting.
Another variation exists also in the genotyping of T. gondii. Moreover, assumption about the global evolution of T. gondii has led to a worldwide effort to investigate genetic diversity within this interesting organism. It has been described as a parasite with a low genetic diversity and a clonal population structure (Dardé, 2008). While isolates of T. gondii were historically considered to be highly comparable, molecular analysis revealed that they exhibit very marked genotyping, notably in North America and Europe, where three predominant lineages, known as types I, II, and III, comprise the vast majority of isolates (Sibley and Ajioka, 2008). In our study, type II was the most common type. Most reports propose that the high prevalence of type II strains in humans is caused by the high prevalence of that strain in animals (Fuentes et al., 2001). Genotype analysis may identify the source of an infection. This analysis will also assist in understanding the pathogenesis of T. gondii infection and its epidemiology, and in planning preventive strategies.
Researchers suggested that the majority of human infections that have been studied in North America and Europe are caused by type II strains (Sibley et al., 2009). However, using PCR-RFLP method, recombinant strains representing mixtures of the three clonal types were also occasionally found (approx. 5%). These isolates, presenting different mixtures of classical alleles can be considered as recombinant genotypes: “Atypical”, “unusual”, “non-archetypal” or “exotic” strains (Dardé, 2008).
Most genotypes are locale-specific, but some are found across continents and are closely related to each other, indicating a recent radiation of a pandemic genotype (Lehmann et al., 2006).
5. Conclusion
Defining the population structure of T. gondii from Saudi Arabia has important implications for transmission, immunogenicity, pathogenesis, and in planning preventive strategies. Relationship between such variation in structure and disease manifestation in pregnant women is still difficult to assess due to the role of host immune status and genetic background on the control of infection, and of other parasitic features such as the infecting dose or parasite stage. The situation for the “new” genotypes is yet more complex, due to their diversity and the combination of genes.
Our finding of the genotyping of T. gondii might facilitate and inform future studies on comparative genomics and identification of genes that control important biological phenotypes including pathogenesis and transmission among Saudi women.
Acknowledgment
Many thanks go to King Saudi University for supporting the study. We would like also to thank King Abdulaziz for Science and Technology (KACST) for financial support. Additionally, it was a great support from Professor. Darde M.L, France who supplied the positive controls for free. We thank Dr. Abdularhman Alshwuariq for facilitating the sample collection and their analysis. Furthermore, we appreciate Alymam and King Saudi Medical city Hospitals for their continuing support and approval to conduct the study, especially Dr. Yousef ALomay. Many thanks go to all the team in the PCR unit, particularly Dr. Hassan Hajj, Ms. Rowa, and Mona Alshaafi who worked hard to complete the study. We are grateful to Ms. Jawaher Alzahrani for helping in the supply needed for analyzing the samples.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Jawahir Alghamdi, Email: Joooj-2013@hotmail.com.
Maha Hussein Elamin, Email: malamyn@ksu.edu.sa.
Samia Alhabib, Email: smalhabib@yahoo.com.
References
- Abu-Madi M.A., Behnke J.M. Toxoplasma gondii seropositivity and co-infection with TORCH pathogens in high-risk patients from Qatar. Am. J. Trop. Med. Hygiene. 2010;82(4):626–633. doi: 10.4269/ajtmh.2010.09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajzenberg D., Collinet F. Genotyping of Toxoplasma gondii isolates with 15 microsatellite markers in a single multiplex PCR assay. J. Clin. Microbiol. 2010;48(12):4641–4645. doi: 10.1128/JCM.01152-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harthi S.A., Jamjoom M.B. Seroprevalence of Toxoplasma gondii among pregnant women in Makkah, Saudi Arabia. Umm Al-Qura Univ. J. Sci. Med. Eng. 2006;18:217–227. [Google Scholar]
- Almogren A. Antenatal screening for Toxoplasma gondii infection at a tertiary care hospital in Riyadh, Saudi Arabia. Ann. Saudi Med. 2011;31(6):569. doi: 10.4103/0256-4947.87090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- bioMérieux, M.l.E., 2000. Toxo-immunosorbent agglutination assay for the detection of Toxoplasma IgM antibodies, France.
- Bouratbine, A., Siala, E., et al., 2001. [Sero-epidemiologic profile of toxoplasmosis in northern Tunisia]. Parasite (Paris, France) 8(1), 61–66. [DOI] [PubMed]
- Burg J.L., Grover C.M. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 1989;27(8):1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardé M.L. Toxoplasma gondii, “new” genotypes and virulence. Parasite. 2008;15(3):366–371. doi: 10.1051/parasite/2008153366. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry. 1971;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa: III. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J. Immunol. 1972;109(1):129–135. [PubMed] [Google Scholar]
- Fallahi S., Kazemi B. Comparison of the RE and B1 gene for detection of Toxoplasma gondii infection in children with cancer. Parasitol. Int. 2014;63(1):37–41. doi: 10.1016/j.parint.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Fuentes I., Rubio J.M. Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J. Clin. Microbiol. 2001;39(4):1566–1570. doi: 10.1128/JCM.39.4.1566-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi H.O., Telmesani A.M. TORCH agents in pregnant Saudi women. Med. Princip. Pract. 2002;11(4):180–182. doi: 10.1159/000065813. [DOI] [PubMed] [Google Scholar]
- Kong Q.M., Lu S.H. Loop-mediated isothermal amplification (LAMP): early detection of Toxoplasma gondii infection in mice. Parasit Vect. 2012;3(5):2–7. doi: 10.1186/1756-3305-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravetz J.D., Federman D.G. Toxoplasmosis in pregnancy. Am. J. Med. 2005;118(3):212–216. doi: 10.1016/j.amjmed.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Lau Y.L., Meganathan P. Specific, sensitive, and rapid diagnosis of active toxoplasmosis by a loop-mediated isothermal amplification method using blood samples from patients. J. Clin. Microbiol. 2010;48(10):3698–3702. doi: 10.1128/JCM.00462-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Lee S. Nested PCR-based detection of Toxoplasma gondii in German shepherd dogs and stray cats in South Korea. Res. Vet. Sci. 2008;85(1):125–127. doi: 10.1016/j.rvsc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Lehmann T., Marcet P.L. Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. 2006;103(30):11423–11428. doi: 10.1073/pnas.0601438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Many A., Koren G. Toxoplasmosis during pregnancy. Can. Fam. Physician. 2006;52(1):29–30. [PMC free article] [PubMed] [Google Scholar]
- Mead P.S., Slutsker L. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5(5):607. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G., Roussos N. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009;39(12):1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Pelloux H., Fricker-Hidalgo H. Detection of anti-Toxoplasma immunoglobulin M in pregnant women. J. Clin. Microbiol. 1997;35(8) doi: 10.1128/jcm.35.8.2187-2187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L.D., Ajioka J.W. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu. Rev. Microbiol. 2008;62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- Sibley L.D., Khan A. Genetic diversity of Toxoplasma gondii in animals and humans. Philosop. Trans. R. Soc. B: Biolog. Sci. 2009;364(1530):2749–2761. doi: 10.1098/rstb.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Pandit A.J. Incidence and prevalence of toxoplasmosis in Indian pregnant women: a prospective study. Am. J. Reprod. Immunol. 2004;52(4):276–283. doi: 10.1111/j.1600-0897.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull. World Health Organ. 2013;91(7):501–508. doi: 10.2471/BLT.12.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer C.T., Ririe K.M. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. Biotechniques. 1997;22(1):176–181. doi: 10.2144/97221pf02. [DOI] [PubMed] [Google Scholar]
- Zhang H., Thekisoe O.M.M. Toxoplasma gondii: sensitive and rapid detection of infection by loop-mediated isothermal amplification (LAMP) method. Exp. Parasitol. 2009;122(1):47–50. doi: 10.1016/j.exppara.2009.01.012. [DOI] [PubMed] [Google Scholar]