Abstract
Aim
The treatment of large (>5 cm) hepatocellular carcinoma (HCC) remains controversial. The aim of this study was to report short and long term outcomes and analyze the factors associated with long term survival for patients who underwent hepatic resection for large HCC.
Methods
All patients who underwent hepatic resection for large HCC at the department of Hepato-Pancreato-Biliary Surgery of the First Affiliated Hospital of Anhui Medical University between August 2005 and December 2011 were identified and included for analysis. Demographic and operative data, pathological findings and post-operative outcomes were entered into a computer database. Prognostic factors were analyzed by univariate and multivariate analysis.
Results
Ninety-nine patients were included for analysis. Two patients died within 30 days of surgery secondary to hepatic failure. The 1-, 3-, 5-year disease-free survival and overall survival rates following hepatic resection were 67%, 49%, 37% and 77%, 56%, 43%, respectively. Poor histological grade was the only independent predictor of a reduced 5-year disease-free survival. Spontaneous tumor rupture and tumor recurrence were independent predictors of a reduced 5-year overall survival.
Conclusions
For selected patients with large HCC, hepatic resection can be performed safely and effectively with moderate expectation of long term survival. True cure however remains rare.
Introduction
Hepatocellular carcinoma (HCC) is usually clinically asymptomatic and screening for HCC has not been widely applied in China, Therefore many patients present with large tumors (>5 cm) at diagnosis. The optimal treatment options for large HCC remain limited and controversial. Because of the increased risk of local or vascular invasion, spontaneous tumor rupture and occult metastatic disease, large HCC is generally excluded from liver transplantation (LT). According to the Barcelona Clinic Liver Cancer (BCLC) staging classification and treatment schedule, patients with large HCC are not candidates for hepatic resection, and are recommended to receive locoregional therapies such as TACE.1 Non-surgical options for large HCC remain limited with poor long term outcomes. Poon et al.2 reported that the overall 5-year survival rates of patients with HCC (>10 cm) were less than 10% after transcatheter arterial chemoembolization (TACE). Resection options are often limited by a reduced hepatic reserve. With recent advances of perioperative management and surgical techniques, hepatic resection has been performed safely for selected patients with large HCC.3, 4 For those patients with large HCC who can be resected, the overall survival has been shown to be better than the nonsurgical treatment.5
Various studies have demonstrated that the long-term disease-free survival and overall survival rates of patients who undergo resection for HCC >10 cm are significantly worse than those with smaller tumors.5, 6, 7 However, other studies have shown that equivalent 5-year overall survival rates irrespective of size (>10 cm vs. <10 cm).8, 9 A retrospective study (n = 300) from five major hepatobiliary centers reported that clinical factors including tumor size do not reliably predict patient outcomes for HCC patients (>10 cm) undergoing hepatic resection.10
The aim of this study was to report the short and long term outcomes and prognostic factors for patients undergoing hepatic resection for large HCC.
Patients and methods
All patients who underwent hepatic resection for large HCC between August 2005 and December 2011 at the Organ Transplantation Center of the First Affiliated Hospital of Anhui Medical University were identified and included for further analysis. Ethical approval for this was obtained from the local institute review board. Demographic data, operative data, pathological findings and post-operative outcomes and follow up were recorded for all patients.
Pre-operative assessment
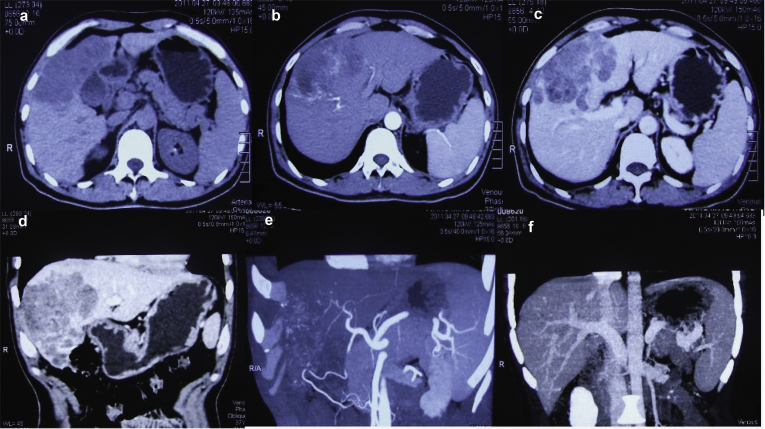
The diagnosis of HCC was based on radiological findings (including abdominal ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI), or positron emission tomography-computed tomography (PET/CT), or hepatic angiography) and/or serum alpha fetoprotein (AFP) level >400 ng/ml (Fig. 1). Patients' hepatic function was classified using the Child–Pugh classification. For patients to be considered for resection, the following criteria were required: in good general condition and with preserved hepatic function (Child–Pugh A, and no evidence of portal hypertension), no distant metastases, no evidence of macroscopic vascular invasion, and technically resectable. Volumetric liver measurements using CT were used to predict the remnant liver volume before deciding the extent of hepatic resection for each patient. The minimum acceptable ratio of remnant liver volume to functional total liver volume was approximately 40% in patients with cirrhosis.11 The hepatic resection was defined according to Couinaud's segmentation of liver anatomy. Hepatic resection of 3 or more segments was defined as major resection, and resection of less than 3 segments was minor resection. Post-operative complications were classified according to Clavien–Dindo classification of surgical complications.12
Figure 1.
Preoperative computed tomography scans and hepatic angiography showing a hepatocellular carcinoma (>10 cm) without intrahepatic metastasis and portal vein invasion
Operative details
Mesohepatectomy was used for centrally located large HCC patients, especially for patients with chronic hepatitis or cirrhosis.13 The anterior approach had been adopted for major hepatic resection for HCC >10 cm located in the right lobe of the liver when the conventional approach for complete mobilization of the liver was difficult.14 Intraoperative ultrasonography was performed routinely to exclude occult intrahepatic metastases, tumor margin, the line of parenchymal transection and the relationship of the tumor with the hepatic pedicles. The parenchymal transection was performed by combining Kelly clamps crushing and Biclamp forceps (ERBE Elektromedizin, Tübingen, Germany). During the parenchymal transection, the central venous pressure (CVP) was maintained below 5 cm H2O, and intermittent Pringle's maneuver was prepared routinely to occlude the hepatic pedicle. Selective sectoral clamping was mainly used for right hepatic resection. For tumors (>10 cm) located in the central parts or adjacent to the major intrahepatic vein or infrahepatic inferior vena cava (IVC), total vascular exclusion (TVE) was prepared routinely. The resection margin of specimens was pathologically examined for a curative resection (R0).
Follow-up
Telephone interviews and a thorough review of the outpatient clinic records were used to investigate the long-term survival. Final follow up date for this analysis was July of 2016. Liver functions tests, AFP, hepatitis B virus-DNA (HBV-DNA), chest radiography, and ultrasonography were performed every 3 months for the first year after surgery and then half a year thereafter. When patients were suspected with HCC local recurrence or distant metastasis, CT, or MRI, PET/CT, or hepatic angiography was performed. For patients with intrahepatic recurrence, treatment strategy was made based on their general status, remnant liver function, the number, size and site of recurrent tumors. Repeated hepatic resection, or salvage liver transplantation, or radiofrequency ablation or percutaneous transcatheter arterial chemoembolization was all considered as potential options. Postoperative mortality was defined as death within 30 days after hepatic resection. Disease-free survival (DFS) and overall survival (OS) were defined as the time from the date of hepatic resection to the date of diagnosis of HCC recurrence or the date of patient death.
Statistical analysis
The statistical analysis was performed with SPSS 16.0 software. The Kaplan–Meier method was used to analyze the DFS and OS curve. The clinicopathological variables that might be associated with DFS and OS were analyzed by using the log-rank test for categorical variables and the Wald test from Cox regression for continuous variables. All the factors that were significantly associated with survival from univariate analysis were subjected to a stepwise multivariate analysis using a Cox proportional hazard model. P value < 0.05 (two-tailed) was considered to be statistically significant.
Results
During the study period 137 consecutive patients with suspected large HCC underwent hepatic resection. Of these 137 patients, 99 (72%) patients were pathologically diagnosed to be primary hepatocellular carcinoma and included in the final analysis while 38 (28%) patients were excluded as the final pathology confirmed 26 (19%) patients with intrahepatic cholangiocarcinoma and 12 (9%) patients with combined cholangio-hepatocellular carcinoma. The median (range) age was 52 (20–84) years and 88 (89%) were male. The median (range) tumor size was 8.7 (5.5–20) cm. Thirty-seven (37%) patients had tumors (≥10 cm). Eighty (81%) patients were positive for hepatitis B surface antigen and one (1%) patient was positive for hepatitis C antibody.
Spontaneous tumor rupture was identified in 13 (13%) patients preoperatively. In 29 (29%) patients, the tumors were centrally located and underwent mesohepatectomy. A total of 33 (33%) patients underwent major resection. During transaction, 82 (82%) patients required continuous or intermittent Pringle's maneuver, 4 (4%) patients required portal vein clamping, and another 13 patients had no inflow clamping. The median (range) clamping time was 26 (12–70) min. The median (range) intra-operative blood loss was 290 (50–1500) ml. Blood transfusion was required in 19 (19%) patients. The median (range) duration of surgery was 180 (135–360) min. The median (range) hospital stay was 21 (9–32) days. Postoperative complications occurred in 21 (21%) patients, and twelve (12%) patients suffered morbidity classified as grade III or greater. Liver-related complications included hepatic failure (n = 4, 4%), transient hepatic dysfunction (n = 9, 9%), bile leakage (n = 7, 7%), ascites (n = 21, 21%). Other common complications included pleural effusion (N = 18, 18%), pulmonary infection (N = 11, 11%), pulmonary atelectasis (N = 6, 6%), incision infections (N = 4, 4%). The postoperative 30-day mortality of the entire cohort was 2 (2%), and the cause of death was hepatic failure.
Liver cirrhosis was diagnosed histologically in 79 patients (80%). Microvascular invasion was present in 25 (25%) patients. There were 79 (79%) patients with a solitary tumor, 43 (54%) of whom had an intact capsule or pseudocapsule. Histological differentiation was graded as: well (n = 13, 13%), moderate (n = 39, 39%), poor (n = 43, 43%).
Long-term outcomes
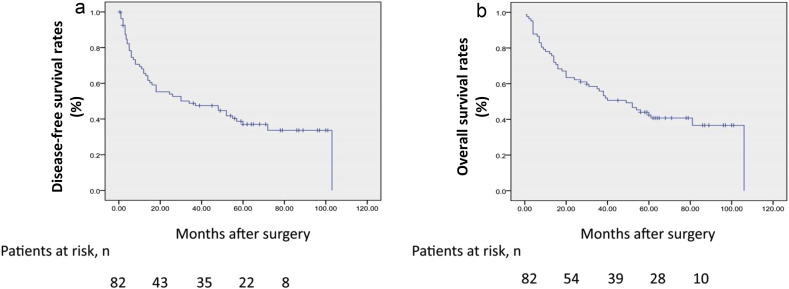
The median (range) follow-up of this cohort of patients was 39 (1–106) months, 15 (15%) patients were lost to follow up. 25 (30%) patients remained disease-free, 49 (60%) patients had died, and 8 (10%) patients were alive with tumor recurrence. The major causes of death were recorded as local recurrence in the remnant liver (45 patients, 55%) and tumor dissemination in the lung or bone (7 patients, 9%). The median DFS and OS after hepatic resection were 26 and 38 months, respectively. The 1-, 3-, 5-year DFS and OS rates after hepatic resection were 67%, 49%, 37%, respectively and 77%, 56%, 43%, respectively (Fig. 2). The 1-, 3-, 5-year DFS and OS rates for patients who underwent mesohepatectomy were 68%, 46%, 38%, respectively and 86%, 60%, 43%, respectively. The 1-, 3-, 5-year DFS and OS rates after hepatic resection in patients with solitary tumors were 72%, 55%, 40%, respectively and 75%, 59%, 44%, respectively.
Figure 2.
Disease-free survival and overall survival rates of 82 patients with large hepatocellular carcinoma (>5 cm) who underwent hepatic resection
Prognostic factors influencing DFS and OS
Uni- and subsequent multi-variable analysis of significant variables for DFS are shown in Table 1, Table 2, respectively. Uni- and subsequent multi-variable analysis of significant variables for OS are shown in Table 3, Table 4, respectively. Survival curves for the whole cohort are shown in Fig. 2.
Table 1.
Univariate analysis of prognostic factors associated with 5-year disease free survival of 82 patients with large hepatocellular carcinoma who underwent hepatic resection
| Variables | No of patients | Median DFS (months) | No of recurrence | P value |
|---|---|---|---|---|
| Gender | 0.923 | |||
| Male/female | 73/9 | 35/37 | 44/6 | |
| Age (years) | 0.641 | |||
| ≥60/<60 | 22/60 | 33/37 | 13/37 | |
| Tumor size (cm) | 0.037 | |||
| >10/≤10 | 19/63 | 22/40 | 11/39 | |
| Hepatitis status | 0.870 | |||
| HBsAg (+)/(−) | 66/15 | 36/37 | 40/9 | |
| Liver cirrhosis | 0.862 | |||
| Present/absent | 65/17 | 35/34 | 38/12 | |
| AFP (ug/L) | 0.024 | |||
| ≥400/<400 | 35/47 | 27/42 | 27/23 | |
| HR type | 0.735 | |||
| Major/minor | 26/56 | 34/36 | 16/34 | |
| Vascular clamping | 0.832 | |||
| Yes/no | 71/11 | 36/34 | 45/5 | |
| Perioperative transfusions | 0.857 | |||
| Yes/no | 46/36 | 36/35 | 25/25 | |
| Number of tumor | 0.074 | |||
| Solitary/multiple | 68/14 | 38/22 | 38/12 | |
| Tumor capsule | 0.006 | |||
| Present/absent | 48/34 | 44/24 | 24/26 | |
| Microvascular invasion | 0.047 | |||
| Present/absent | 19/63 | 23/39 | 12/38 | |
| Grade of differentiation | 0.003 | |||
| Poor or moderate | 70 | 31 | 47 | |
| Well | 12 | 60 | 3 | |
| Spontaneous tumor rupture | 0.029 | |||
| Present/absent | 12/70 | 17/39 | 9/41 | |
| Complications | 0.677 | |||
| Present/absent | 38/44 | 34/37 | 23/27 |
HBsAg, hepatitis B virus surface antigen; AFP, α-fetoprotein; HR, hepatic resection.
Table 2.
Multivariate analysis of prognostic factors associated with 5-year disease free survival of 82 patients with large hepatocellular carcinoma who underwent hepatic resection
| Variables | RR | 95% CI | P value |
|---|---|---|---|
| Tumor size | 2.090 | 1.002–4.361 | 0.050 |
| AFP | 1.505 | 0.832–2.722 | 0177 |
| Tumor capsule | 0.634 | 0.324–1.241 | 0.184 |
| Microvascular invasion | 1.044 | 0.520–2.097 | 0.904 |
| Grade of differentiation | 4.122 | 1.167–14.564 | 0.028 |
| Spontaneous tumor rupture | 1.555 | 0.680–3.558 | 0.296 |
RR, risk ratio; CI, confidence interval; AFP, α-fetoprotein.
Table 3.
Univariable analysis of prognostic factors associated with 5-year overall survival of 82 patients with large hepatocellular carcinoma who underwent hepatic resection
| Variable | No of survival | No of death | P value | |
|---|---|---|---|---|
| Gender | Male/female | 29/4 | 44/5 | 0.713 |
| Age (years) | ≥60/<60 | 7/26 | 15/34 | 0.403 |
| Tumor size (cm) | >10/≤10 | 5/28 | 14/35 | 0.057 |
| Hepatitis status | HBsAg (+)/(−) | 26/7 | 40/8 | 0.850 |
| Liver cirrhosis | Present/absent | 26/7 | 39/10 | 0.985 |
| AFP (ug/L) | ≥400/<400 | 7/26 | 28/21 | 0.018 |
| HR type | Major/minor | 7/26 | 19/30 | 0.486 |
| Vascular clamping | Yes/no | 29/4 | 42/7 | 0.591 |
| Perioperative transfusions | Yes/no | 18/15 | 28/21 | 0.975 |
| Number of tumor | Solitary/multiple | 29/4 | 39/10 | 0.286 |
| Tumor capsule | Present/absent | 23/10 | 25/24 | 0.032 |
| Microvascular invasion | Present/absent | 6/27 | 13/36 | 0.051 |
| Grade of differentiation | Poor and moderate/well | 24/9 | 46/3 | 0.001 |
| Spontaneous tumor rupture | Present/absent | 3/30 | 9/40 | 0.029 |
| Complications | Present/absent | 11/22 | 27/22 | 0.419 |
| Tumor recurrence | Yes/no | 7/26 | 43/6 | 0.000 |
HBsAg, hepatitis B virus surface antigen; AFP, α-fetoprotein; HR, hepatic resection.
Table 4.
Multivariate analysis of prognostic factors associated with 5-year overall survival of 82 patients with large hepatocellular carcinoma who underwent hepatic resection
| Variables | RR | 95% CI | P value |
|---|---|---|---|
| AFP | 1.302 | 0.708–2.397 | 0.396 |
| Tumor capsule | 1.154 | 0.591–2.254 | 0.675 |
| Grade of differentiation | 2.515 | 0.716–8.832 | 0.150 |
| Spontaneous tumor rupture | 2.659 | 1.118–6.325 | 0.027 |
| Tumor recurrence | 7.540 | 3.054–18.617 | 0.000 |
RR, risk ratio; CI, confidence interval; AFP, α-fetoprotein.
Discussion
This study describes the short- and long-term outcomes for patients who underwent resection for large HCC. Although some progress has been made in the treatment of large HCC, hepatic resection remains the only potential curative treatment option for patients with large HCC. Several studies have shown that hepatic resection can be successfully performed in well selected patients with satisfactory long-term outcomes. Yang et al.15 reported that the median DFS of patients with solitary large HCC (37 months) after hepatic resection was significantly better than that of patients with nodular (node number ≥2) HCC (25 months), but similar with that of small HCC (46 months). The 1-, 3-, 5-year DFS and OS rates of solitary large HCC patients after LR were 82%, 51%, 35% and 87%, 56%, 38%, respectively.15 The author proposed that hepatic resection should be the treatment choice patients with preserved hepatic function and solitary large HCC. In a study of 66 patients with large HCC (>5 cm) who underwent hepatic resection, no peri-operative death occurred, and the 5-year DFS rate and OS rate were 29% and 32%.16 In the current cohort of patients, the surgical mortality rate was 2%, the 5-year DFS and OS rate after hepatic resection were 37% and 43%. Therefore, there is now increasing evidence to support hepatic resection in well selected patients with large tumors.
Two patients died of hepatic failure within 30 days of hepatic resection. Hepatic failure is a known major cause of postoperative death after hepatic resection, especially for patients who require major resection on a background of cirrhosis. Preoperative assessment of hepatic reserve function is extremely important for avoiding postoperative hepatic failure. All cirrhotic patients of this study were classified into Child A. Indocyanine green clearance and three-dimensional CT volumetry was introduced to evaluate hepatic reserve function and future liver remnant (FLR) of patients with large HCC during the study period. Preoperative portal vein embolization (PVE) before hepatice resection has been widely applied to induce the hypertrophy of FLR for patients undergoing a major resection.2 Sequential preoperative TACE and PVE before major hepatic resection have been shown to strengthen the effect of PVE and simultaneously prevent tumor progression during the waiting time.3 In recent years, the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) has been proposed as a novel approach to induce the rapid liver regeneration, resulting in increasing the chance of resectability.17 These may be appropriate in patients who are identified as marginal candidates for resection. In addition, mesohepatectomy has been used for centrally located large HCC, thus preserving more FLR compared with extended hepatectomy.13
Several prognostic factors that affect tumor recurrence and patient long-term survival of patients with large HCC after hepatic resection have been identified, which is critical to patient selection for receiving hepatic resection. Previous studies have demonstrated that high AFP levels (>400 ng/ml or ≥1000 ng/ml) were correlated with poor DFS and OS for patients with HCC (>10 cm).7, 10 High levels of AFP are thought to suppress the ability of immune system to target against tumor cells.7 Compared with non-encapsulated tumors, capsulated tumors showed a much lower incidence of direct liver invasion, tumor microsatellites, and venous permeation.18 Moreover, the DFS and OS of capsulated HCC patients were significantly better than non-capsulated HCC patients. In a retrospective study of 1240 HCC patients underwent hepatic resection, tumor encapsulation was associated with lower incidence of vascular invasion, and was a prognostic factor when the tumor size was larger than 5 cm.19 Vascular invasion has also been shown to predict early recurrence and poor OS.20 Yang et al.15 has reported that only large amount of intraoperative blood transfusion and presence of vein invasion are independently poor factors of OS in 481 HCC patients including 260 patients with large HCC after hepatic resection. As hepatic resection is not always be the best option for each patient with large HCC, it is of great significance to select potential candidates for the surgery in clinic practice. Tumor size has long been considered an independent risk factor for tumor recurrence and survival of patients with large HCC. In the current cohort, tumor size was statistically associated with tumor recurrence on univariate analysis, but was not a significant predictor of patient survival on multivariate analysis. Delis et al.16 showed that tumor size and grade of differentiation were two independent predictors for tumor recurrence and adverse long-term survival in patients with large HCC. Spontaneous tumor rupture, a life-threatening situation for patients with large HCC, is also a known adverse prognostic factor after hepatic resection.7 Spontaneous tumor rupture might be caused by the rapid expansion of the tumor, but the molecular mechanism of spontaneous tumor rupture is still not fully understood.21 Transhepatic arterial embolization followed by hepatic resection has been proposed to be a rational treatment for the majority HCC patients with tumor rupture if the tumor could be resected.22
Conclusions
Patients with large HCC should not be excluded from hepatic resection simply because of tumor size. Selected patients with large HCC can benefit from hepatic resection, and achieve long-term survival after surgery. However, it is suggested that large HCC patients with poor histological grade and spontaneous tumor rupture might be less favorable candidates for hepatic resection.
Conflicts of interest
The authors declare no competing interests.
Acknowledgements
The authors would sincerely thank the reviewers and editors for critically reviewing and editing this manuscript and for the constructive and thoughtful comments and suggestions. This work was supported by grants from the Medical Scientific Research Foundation of Anhui Province, China (No.: 2010A009). The authors acknowledge Dr. Lin Lin and Ms. Dai-Zhen Xing from Department of Medical Records Management and Dr. Qiang Wei from Department of Neurology for very kind help with data collection and analysis.
Footnotes
Grant Sponsor: Medical Scientific Research Foundation of Anhui Province, China; Grant number: 2010A009.
This manuscript is being submitted as a original article. This manuscript has not been presented on a society or meeting before.
References
- 1.Llovet J.M., Fuster J., Bruix J., Barcelona-Clínic Liver Cancer Group The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 2.Poon R.T., Fan S.T., Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592–602. doi: 10.1016/s1072-7515(02)01163-8. [DOI] [PubMed] [Google Scholar]
- 3.Meier R.P., Toso C., Terraz S., Breguet R., Berney T., Andres A. Improved liver function after portal vein embolization and an elective right hepatectomy. HPB. 2015;17:1009–1018. doi: 10.1111/hpb.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronot M., Cauchy F., Gregoli B., Breguet R., Allaham W., Paradis V. Sequential transarterial chemoembolization and portal vein embolization before resection is a valid oncological strategy for unilobar hepatocellular carcinoma regardless of the tumor burden. HPB. 2016;18:684–690. doi: 10.1016/j.hpb.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita Y., Taketomi A., Shirabe K., Aishima S., Tsuijita E., Morita K. Outcomes of hepatic resection for huge hepatocellular carcinoma (≥10 cm in diameter) J Surg Oncol. 2011;104:292–298. doi: 10.1002/jso.21931. [DOI] [PubMed] [Google Scholar]
- 6.Taniai N., Yoshida H., Tajiri T. Adaptation of hepatectomy for huge hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:10–416. doi: 10.1007/s00534-007-1317-3. [DOI] [PubMed] [Google Scholar]
- 7.Yeh C.N., Lee W.C., Chen M.F. Hepatic resection and prognosis for patients with hepatocellular carcinoma larger than 10 cm: two decades of experience at Chang Gung memorial hospital. Ann Surg Oncol. 2003;10:1070–1076. doi: 10.1245/aso.2003.03.072. [DOI] [PubMed] [Google Scholar]
- 8.Shah S.A., Wei A.C., Cleary S.P., Yang I., McGilvray I.D., Gallinger S. Prognosis and results after resection of very large (>or=10 cm) hepatocellular carcinoma. J Gastrointest Surg. 2007;11:589–595. doi: 10.1007/s11605-007-0154-7. [DOI] [PubMed] [Google Scholar]
- 9.Thng Y., Tan J.K., Shridhar I.G., Chang S.K., Madhavan K., Kow A.W. Outcomes of resection of giant hepatocellular carcinoma in a tertiary institution: does size matter? HPB. 2015;17:988–993. doi: 10.1111/hpb.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlik T.M., Poon R.T., Abdalla E.K., Zorzi D., Ikai I., Curley S.A. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140:450–457. doi: 10.1001/archsurg.140.5.450. [DOI] [PubMed] [Google Scholar]
- 11.Kubota K., Makuuchi M., Kusaka K., Kobayashi T., Miki K., Hasegawa K. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 12.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 13.Yang L.Y., Chang R.M., Lau W.Y., Ou D.P., Wu W., Zeng Z.J. Mesohepatectomy for centrally located large hepatocellular carcinoma: indications, techniques, and outcomes. Surgery. 2014;156:1177–1187. doi: 10.1016/j.surg.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Liu C.L., Fan S.T., Lo C.M., Tung-Ping Poon R., Wong J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25–31. doi: 10.1097/00000658-200007000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L.Y., Fang F., Ou D.P., Wu W., Zeng Z.J., Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249:118–123. doi: 10.1097/SLA.0b013e3181904988. [DOI] [PubMed] [Google Scholar]
- 16.Delis S.G., Bakoyiannis A., Tassopoulos N., Athanassiou K., Kelekis D., Madariaga J. Hepatic resection for hepatocellular carcinoma exceeding Milan criteria. Surg Oncol. 2010;19:200–207. doi: 10.1016/j.suronc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Schnitzbauer A.A., Lang S.A., Goessmann H., Nadalin S., Baumgart J., Farkas S.A. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 18.Ng I.O., Lai E.C., Ng M.M., Fan S.T. Tumor encapsulation in hepatocellular carcinoma: a pathologic study of 189 cases. Cancer. 1992;70:45–49. doi: 10.1002/1097-0142(19920701)70:1<45::aid-cncr2820700108>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Wu T.H., Yu M.C., Chen T.C., Lee C.F., Chan K.M., Wu T.J. Encapsulation is a significant prognostic factor for better outcome in large hepatocellular carcinoma. J Surg Oncol. 2012;105:85–90. doi: 10.1002/jso.22060. [DOI] [PubMed] [Google Scholar]
- 20.Schiffman S.C., Woodall C.E., Kooby D.A., Martin R.C., Staley C.A., Egnatashvili V. Factors associated with recurrence and survival following hepatectomy for large hepatocellular carcinoma: a multicenter analysis. J Surg Oncol. 2010;101:105–110. doi: 10.1002/jso.21461. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L.X., Geng X.P., Fan S.T. Spontaneous rupture of hepatocellular carcinoma and vascular injury. Arch Surg. 2001;136:682–687. doi: 10.1001/archsurg.136.6.682. [DOI] [PubMed] [Google Scholar]
- 22.Zhu L.X., Wang G.S., Fan S.T. Spontaneous rupture of hepatocellular carcinoma. Br J Surg. 1996;83:602–607. doi: 10.1002/bjs.1800830507. [DOI] [PubMed] [Google Scholar]