Abstract
Background
Laparoscopic cholecystectomy is commonly performed, and several factors increase the risk of open conversion, prolonging operating time and hospital stay. Preoperative stratification would improve consent, scheduling and identify appropriate training cases. The aim of this study was to develop a validated risk score for conversion for use in clinical practice.
Patients and methods
Preoperative patient and disease-related variables were identified from a prospective cholecystectomy database (CholeS) of 8820 patients, divided into main and validation sets. Preoperative predictors of conversion were identified by multivariable binary logistic regression. A risk score was developed and validated using a forward stepwise approach.
Results
Some 297 procedures (3.4%) were converted. The risk score was derived from six significant predictors: age (p = 0.005), sex (p < 0.001), indication for surgery (p < 0.001), ASA (p < 0.001), thick-walled gallbladder (p = 0.040) and CBD diameter (p = 0.004). Testing the score on the validation set yielded an AUROC = 0.766 (p < 0.001), and a score >6 identified patients at high risk of conversion (7.1% vs. 1.2%).
Conclusion
This validated risk score allows preoperative identification of patients at six-fold increased risk of conversion to open cholecystectomy.
Introduction
Laparoscopic cholecystectomy is the gold standard treatment for symptomatic gallstones. Conversion to an open procedure is necessary in 5–10% of patients, and is associated with increased morbidity, prolonged hospitalization and longer recovery compared to a laparoscopic approach.1, 2, 3, 4 Common indications for conversion include failure to demonstrate the ‘critical view of safety’,1, 2, 5, 6 or the presence of an intraoperative complication, such as intestinal perforation, haemorrhage or bile duct injury. Several factors increase the risk of conversion to open, including age,4 male sex,3, 7 obesity,1, 2 cholecystitis2, 3, 7 and previous ERCP.8 Conversion to open surgery usually indicates a difficult procedure, and rather than being considered a complication, the decision to convert should be regarded as a sign of good judgement in the presence of adverse conditions.
Preoperative prediction of patients at increased risk of conversion to open cholecystectomy has several potential advantages. Low risk patients could be identified and appropriately scheduled in an ambulatory care facility, and selected as training cases for surgical trainees,9 whilst high risk patients should be appropriately counselled and operated by experienced surgeons. The majority of studies that have evaluated risk factors for conversion to open cholecystectomy are small retrospective series or population-based databases1, 2, 3, 4, 6, 7, 10, 11, 12, 13, 14 (Table 1). Several studies have developed risk scores, but their clinical utility is limited by retrospective data, small sample sizes and/or lack of validation. The aim of this study was to develop and validate a preoperative risk score to predict conversion from laparoscopic to open cholecystectomy, using data derived from a large, prospective cholecystectomy database of 8820 patients.15
Table 1.
Preoperative risk factors for conversion from laparoscopic to open cholecystectomy
| Reference | N | Conversions (%) | Design | Risk score | Patient-related | Disease-related |
|---|---|---|---|---|---|---|
| aSippey (2015)10 | 7242 | 6.0 | Retrospective | No | Age, sex, BMI Comorbidity |
|
| Goonawardena (2015)1 | 732 | 6.4 | Retrospective | Yes | BMI Previous surgery |
CBD stone, GB wall thickness |
| Vivek (2014)11 | 323 | 7.5 | Prospective | No | Age, sex, BMI Previous surgery |
ERCP |
| Stanisic (2013)12 | 369 | 2.7 | Prospective | No | BMI | Cholecystitis, GB wall thickness, WCC |
| Kaafarani (2010)13 | 9530 | 9.0 | Retrospective | No | Age, sex Previous surgery |
|
| Randhawa (2009)14 | 228 | 1.3 | Retrospective | Yes | BMI | Cholecystitis, GB wall thickness |
| Ballal (2009)4 | 43,821 | 5.2 | Retrospective | No | Age, sex | |
| Lipman (2007)3 | 1377 | 8.1 | Retrospective | Yes | Age, sex, diabetes | Cholecystitis |
| Ishizaki (2006)6 | 1179 | 7.5 | Retrospective | No | Sex | Cholecystitis, CBD stone, GB wall thickness, ERCP |
| Rosen (2002)2 | 1347 | 5.3 | Retrospective | No | Age, BMI | Cholecystitis, GB wall thickness |
| Kama (2001)7 | 1000 | 4.8 | Retrospective | Yes | Age, sex Previous surgery |
GB wall thickness Abdominal tenderness |
GB = gallbladder; CBD = common bile duct; BMI = body mass index; ERCP = endoscopic retrograde cholangiopancreatography; WCC = white cell count.
Included patients with cholecystitis only.
Patients and methods
Data for this study were derived from the CholeS study, a multicentre, prospective population-based cohort study of variation of cholecystectomy.15 Data was collected from 8820 patients who underwent laparoscopic cholecystectomy in 166 hospitals across the UK, during a two-month period from March to April 2014, and has been found to be 99.2% accurate by independent data validation.16 Data was collected prospectively by surgical trainees, who formed a network of surgical research collaborative groups across the UK. Data regarding postoperative follow-up was obtained by review of medical records, including outpatient attendances or hospital readmissions up to 30 days postoperatively. Preoperative variables included patient demographics, indications for surgery, ASA grade, admission type, ultrasound findings and preoperative endoscopic retrograde cholangiopancreatography (ERCP). Operative data were also gathered prospectively, and surgeons were asked to grade the difficulty of the procedure using the Nassar scale (grades 1–4).17 Duration of surgery was calculated from time (minutes) of skin incision to end of skin closure. 30-day follow-up was obtained for all patients and included rates of morbidity and mortality. All cause 30-day morbidity included bile leak, bile duct injury, wound infection, intra-abdominal collection, pancreatitis, bile duct stones, as well as non-surgical complications such as cardiac, respiratory, urinary and other complications. Bile duct injury was defined as any injury to the main biliary tree and was classified using the Stewart-Way classification.18 Bile leak was defined using a standardized definition from the International Study Group of Liver Surgery.19
Perioperative outcomes were compared between those patients who underwent laparoscopic surgery, and those that required conversion to open surgery. Continuous variables were found to be skewed, and so were reported as medians and interquartile ranges, with Mann–Whitney tests used to compare the two groups. Nominal variables were compared between the groups using Fisher's exact test, where this was calculable, or with Chi2 where this was not possible, whilst Kendall's tau was used to compare ordinal variables.
The data were then randomly divided 3:1 into a main and validation set. Within the main set, univariable analyses were used to compare the conversion rates across the preoperative variables being considered, using Chi2 tests or Kendall's tau for nominal and ordinal factors, respectively. A multivariate binary logistic regression model was then produced using a forward stepwise approach. The coefficients of the model were multiplied by two, and rounded to the nearest integer, in order to produce a simplified risk score. Receiver operating characteristic (ROC) curves were then produced for this score in both sets of data to determine accuracy. All analyses were performed using IBM SPSS Statistics 22 (IBM Corp. Armonk, NY). Missing data were excluded on a per-analysis basis, and p < 0.05 was considered to be statistically significant.
Results
Overall, 297 out of 8820 (3.4%) laparoscopic cholecystectomies were converted to open. Mean patient age was 51 ± 17 years and 73.9% were female. Procedural difficulty was graded 3–4 in 94% of converted procedures compared to only 27% of laparoscopic procedures (p < 0.001; Fisher's exact test). Compared to laparoscopic procedures, converted procedures took significantly longer, and were associated with longer hospital stay, as well as increased morbidity and mortality (Table 2). Although the specific indications for conversion were not collected prospectively in the CholeS study protocol, bile duct injury (N = 7), bowel injury (N = 12) and bleeding (N = 64) were observed in 83 out of 297 (28%) converted patients. Of the remainder, operative difficulty was graded 3 or 4 in 170 (57%), and 35 patients (12%) underwent bile duct exploration. The reason for conversion was unclear in 9 patients (3%).
Table 2.
Perioperative outcomes after laparoscopic cholecystectomy
| Laparoscopic (n = 8523) | Converted (n = 297) | p-Value | |
|---|---|---|---|
| Procedural difficultya | <0.001∗ | ||
| 1 | 3535 (42%) | 7 (2%) | |
| 2 | 2618 (31%) | 12 (4%) | |
| 3 | 1724 (20%) | 65 (22%) | |
| 4 | 572 (7%) | 212 (71%) | |
| Median operating time (min) | 60 (45–88) | 120 (90–160) | <0.001∗ |
| Median time to conversion (min) | – | 34 (20–60) | – |
| Median hospital stay (days) | 1 (0–2) | 6 (4–10) | <0.001∗ |
| Morbidity (30 day) | |||
| All | 840 (10%) | 99 (33%) | <0.001∗ |
| Bile leak | 92 (1%) | 25 (8%) | <0.001∗ |
| Bile duct injury | 23 (0.3%) | 6 (2%) | <0.001∗ |
| Mortality (30 day) | 8 (0.1%) | 2 (0.7%) | 0.043∗ |
Data reported as medians and interquartile ranges, with p-values from Mann–Whitney tests, or numbers and column percentages, with p-values from Fisher's exact tests, as applicable.
∗Significant at p < 0.05.
Nassar scale (Ref. 17), with p-value from Kendall's tau.
Of 8523 patients who successfully underwent laparoscopic cholecystectomy without conversion, the operating time was longer than 2 h in 544 patients (6.4%). However, a prolonged laparoscopic procedure (>2 h) was associated with increased overall complications (17.5% vs. 9.3%; p < 0.001), bile leak (3.7% vs. 0.8%; p < 0.001), bile duct injury (1.8% vs. 0.13%; p < 0.001) and longer median hospital stay (2 vs. 1 days; p < 0.001) compared to shorter laparoscopic procedures (less than 2 h). Thirty three percentage of patients who underwent a prolonged laparoscopic cholecystectomy had a postoperative length of stay ≥5 days. The morbidity (18% vs. 33%; p < 0.001) and median length of hospital stay (2 vs. 6 days; p < 0.001) after a prolonged laparoscopic cholecystectomy were significantly less than after converted cholecystectomy.
The data were then divided into two random groups, a main set (N = 6615; 3.3% converted to open), which could be used to produce a risk score, and a validation set (N = 2205; 3.4% converted to open) to validate the resulting score. Within the main set, the association between preoperative variables and conversion was evaluated by univariable analysis of a range of patient and surgical factors (Table 3). Several patient-related (age, gender, ASA) and disease-related factors (gallbladder wall thickness, bile duct diameter, indication for surgery, previous ERCP) were found to be significantly associated with conversion to open surgery. Body mass index was not found to be a risk factor for conversion in this analysis. On multivariable analysis, six factors (age, gender, ASA, indication, gallbladder wall thickness and bile duct diameter) were identified as significant independent predictors of conversion, whilst type of admission (p = 0.225) and previous ERCP (p = 0.141) were no longer significant (Table 4).
Table 3.
Univariable analysis of preoperative variables (main dataset)
| N (n = 6615) | Converted (n = 221) | p-Value | |
|---|---|---|---|
| Age# | <0.001#* | ||
| <30 | 803 | 3 (0.4%) | |
| 30–39 | 983 | 11 (1.1%) | |
| 40–49 | 1255 | 28 (2.2%) | |
| 50–59 | 1365 | 57 (4.2%) | |
| 60–69 | 1234 | 54 (4.4%) | |
| 70+ | 970 | 68 (7.0%) | |
| Gender | <0.001* | ||
| Female | 4889 | 111 (2.3%) | |
| Male | 1726 | 110 (6.4%) | |
| BMI# | 0.667# | ||
| <25 | 1318 | 42 (3.2%) | |
| 25–30 | 2261 | 76 (3.4%) | |
| 31–35 | 1536 | 50 (3.3%) | |
| >35 | 1198 | 43 (3.6%) | |
| ASA# | <0.001#* | ||
| 1 | 2505 | 28 (1.1%) | |
| 2 | 3340 | 131 (3.9%) | |
| 3+ | 712 | 57 (8.0%) | |
| Primary indication | <0.001* | ||
| Colic | 3647 | 45 (1.2%) | |
| CBD stone | 429 | 39 (9.1%) | |
| Cholecystitis | 1912 | 124 (6.5%) | |
| Pancreatitis | 623 | 13 (2.1%) | |
| Gallbladder wall | <0.001* | ||
| Normal | 4363 | 90 (2.1%) | |
| Thick walled | 2094 | 122 (5.8%) | |
| CBD diameter | <0.001* | ||
| Normal | 5449 | 145 (2.7%) | |
| Dilated | 1011 | 66 (6.5%) | |
| Admission type | <0.001* | ||
| Elective | 3125 | 52 (1.7%) | |
| Delayed | 2450 | 111 (4.5%) | |
| Acute | 1040 | 58 (5.6%) | |
| Preoperative ERCP | <0.001* | ||
| No | 5848 | 160 (2.7%) | |
| Yes | 692 | 58 (8.4%) | |
ASA – American Society of Anesthesiologists physical status classification system.
BMI – body mass index; CBD – common bile duct.
ERCP – Endoscopic Retrograde Cholangio-Pancreatography.
p-Values from Chi2 test, unless stated otherwise.
#p-Value from Kendall's tau.
*Significant at p < 0.05.
Table 4.
Multivariable binary logistic regression model (main dataset)
| Coefficient# | Odds ratio (95% CI) | p-Value | |
|---|---|---|---|
| Age | 0.005* | ||
| <30 | – | – | – |
| 30–39 | 0.88 | 2.41 (0.66–8.72) | 0.181 |
| 40–49 | 1.26 | 3.53 (1.05–11.94) | 0.042* |
| 59–59 | 1.74 | 5.72 (1.75–18.69) | 0.004* |
| 60–69 | 1.48 | 4.41 (1.33–14.59) | 0.015* |
| 70+ | 1.77 | 5.88 (1.78–19.40) | 0.004* |
| Gender | <0.001* | ||
| Female | – | – | – |
| Male | 0.57 | 1.76 (1.30–2.39) | <0.001* |
| Indication | <0.001* | ||
| Biliary colic | – | – | – |
| CBD stone | 1.26 | 3.54 (2.06–6.08) | <0.001* |
| Cholecystitis | 1.12 | 3.05 (2.01–4.65) | <0.001* |
| Pancreatitis | −0.24 | 0.79 (0.38–1.62) | 0.517 |
| ASA | <0.001* | ||
| 1 | – | – | – |
| 2 | 0.81 | 2.26 (1.44–3.53) | <0.001* |
| 3+ | 1.38 | 3.97 (2.37–6.67) | <0.001* |
| Gallbladder wall | 0.040* | ||
| Normal | – | – | – |
| Thick walled | 0.36 | 1.43 (1.02–2.01) | 0.040* |
| CBD diameter | 0.004* | ||
| Normal | – | – | – |
| Dilated | 0.53 | 1.70 (1.18–2.45) | 0.004* |
ASA – American Society of Anesthesiologists physical status classification system.
CBD – common bile duct.
Factors not in the final model: BMI (p = 0.466), Admission Type (p = 0.225).
Preoperative ERCP (p = 0.141).
#Loge-odds.
*Significant at p < 0.05.
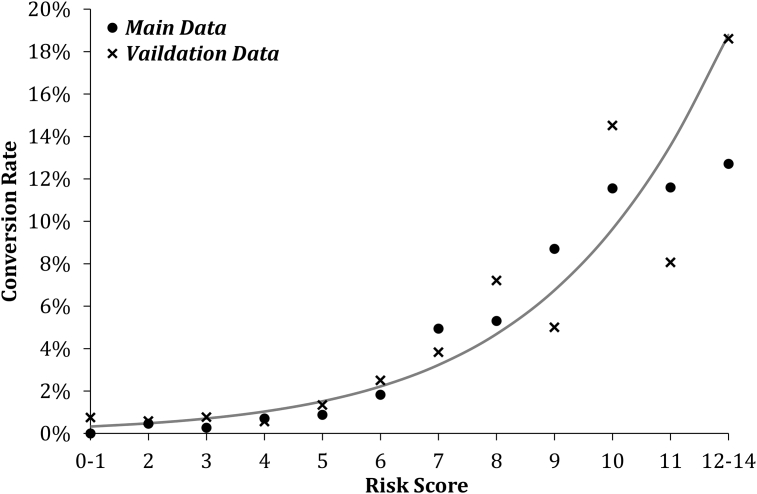
The model's accuracy was tested using ROC analysis, and returned an area under the curve (AUROC) of 0.811 (SE = 0.013; p < 0.001). The model was then transformed into a Conversion from Laparoscopic to Open Cholecystectomy risk score (CLOC score), by rounding the coefficients to the nearest integer, after multiplying by two to minimize the effect of rounding errors. The resulting ‘points’ scores for each of the factors in the model are reported in Table 5. In order to generate a CLOC score for a patient, the number of points for each of the factors is looked up in the table, and added together, giving a score in the range of 0–14. The simplification of the logistic regression model into the CLOC score had minimal impact on its accuracy (AUROC 0.802; SE = 0.013; p < 0.001). The score was then applied to the validation set of patients, resulting in an AUROC of 0.766 (SE = 0.027; p < 0.001). The performance of the risk score for both sets of data is shown graphically in Fig. 1. The ROC curve from the validation set was then used to identify the best cut off from the risk score (Fig. 1). Based on the Youden's J statistic, the optimal grouping was to classify patients with scores >6 as high risk, which yielded a sensitivity of 77.1% and a specificity of 65.4%. In the validation set, the risk of conversion to open for low (CLOC ≤ 6) and high risk (CLOC > 6) patients was 1.2% and 7.1%, respectively. Hence, patients identified as high risk have a near six-fold higher rate of conversion than low risk patients.
Table 5.
Conversion from laparoscopic to open cholecystectomy (CLOC) risk score
| Points | |
|---|---|
| Age | |
| <30 | 0 |
| 30–39 | 2 |
| 40–69 | 3 |
| 70+ | 5 |
| Gender | |
| Female | 0 |
| Male | 1 |
| Indication | |
| Colic/Pancreatitis | 0 |
| Cholecystitis | 2 |
| CBD Stone | 3 |
| ASA | |
| 1 | 0 |
| 2 | 2 |
| 3+ | 3 |
| Gallbladder wall | |
| Normal | 0 |
| Thick walled | 1 |
| CBD diameter | |
| Normal | 0 |
| Dilated | 1 |
ASA – American Society of Anesthesiologists physical status classification system.
CBD – common bile duct.
Figure 1.
Relationship between conversion risk score and conversion rates. Reference line is based on a binary logistic model using the combination of the two datasets, with the risk score as a covariate
The CLOC score was found to be significantly associated with the intraoperative assessment of operative difficulty (Spearman's rho = 0.386; p < 0.001), and also correlated with bile duct injury, whether diagnosed intra- (p = 0.032) or postoperatively (p = 0.035) (see Table 6).
Table 6.
Correlation between conversion risk score and operative difficulty (entire cohort)
| N | Risk score | p-Value | |
|---|---|---|---|
| Operative difficulty | <0.001#∗ | ||
| 1 | 3404 | 4.5 ± 2.6 | |
| 2 | 2547 | 5.6 ± 2.6 | |
| 3 | 1726 | 6.7 ± 2.6 | |
| 4 | 742 | 8.2 ± 2.4 | |
| Bile duct injury (intraoperative diagnosis) | 0.032∗ | ||
| No | 8333 | 5.6 ± 2.8 | |
| Yes | 23 | 6.9 ± 2.8 | |
| Bile duct injury (delayed diagnosis) | |||
| No | 8481 | 5.6 ± 2.8 | 0.035∗ |
| Yes | 7 | 7.9 ± 3.0 | |
Data reported as mean ± SD, with p-values from t-tests, unless stated otherwise.
#p-Value from Spearman's rho – correlation coefficient = 0.386.
*Significant at p < 0.05.
Discussion
In this study, a risk score has been developed and validated using a large prospective cholecystectomy database, and accurately predicts the risk of conversion from laparoscopic to open cholecystectomy (“CLOC score”). The CLOC score correlated with indicators of operative difficulty, such as the Nassar scale and bile duct injury, and could therefore potentially be used preoperatively to predict difficult cases. The score has several potential applications, including consent, training and resource utilization. Low risk patients (CLOC score ≤ 6) may be selected for ambulatory care facilities, and are suitable training cases for surgical registrars in the early phase of training. High risk patients (CLOC score > 6) should be operated by experienced surgeons in an inpatient facility. Depending on the availability of local expertise and resources, consideration should be given to referring very high risk patients to a specialist unit. Several risk scores have been published previously,1, 3, 8, 20, 21, 22 but all have failed to be incorporated into routine clinical practice. Early scores have been derived from small, retrospective series using subjective variables and included data from the learning curve of the laparoscopic era.7, 20, 21, 22 Recently, Goonawardena et al. developed a predictive nomogram from a retrospective series of 732 patients.1 Similar variables were found to be significant predictors of conversion in this and the present report.1
Laparoscopic cholecystectomy has been the standard approach for symptomatic gallstones for more than two decades, and is associated with improved recovery and lower morbidity.23 Conversion to open surgery may be necessary to prevent injury (e.g. bile duct injury), treat an intraoperative complication (e.g. bleeding, bowel injury, bile duct injury) or due to failure to progress.1, 2, 6 In this analysis, the precise indication for conversion was not collected prospectively, since it was not part of the CholeS dataset. It was assumed that the presence of a bile duct injury, bowel injury or bleeding was the reason for conversion. Although it is feasible that an intraoperative complication occurred after conversion to open in some or all patients, this was considered unlikely. Nonetheless this is a potential weakness of the present study. Of the converted patients without intraoperative complications (N = 214), conversion was due to procedural difficulty (Nassar grade 3–4 or bile duct exploration) in the majority of cases (96%). In patients with significant inflammation and/or fibrosis in the region of Calot's triangle, accurate identification of anatomical landmarks may prove difficult or impossible using a laparoscopic approach. In this scenario, conversion to open surgery permits assessment by palpation and is an essential step if the critical view of safety has not been achieved. In patients in whom the critical view of safety cannot be obtained at open surgery, dissection in this region should be avoided and a subtotal cholecystectomy should be performed. Importantly, there were no cases of delayed diagnosis of bile duct injury in converted patients in this series. A third of converted patients in this study developed a postoperative complication, including bile leak in 8% and mortality (Table 2). This is in part due to selection bias, and these findings are compatible with previously published reports.1, 2, 3, 4
The threshold for conversion is likely to vary between surgeons, and may relate to several factors, such as experience, procedural difficulty (e.g. bile duct exploration) and possibly logistic issues (e.g. time pressures and inpatient bed availability). Such variation in threshold may explain the range of times to conversion observed in this study (up to 4 h). The CLOC score may help to identify high risk patients, in whom an early decision to convert may avoid a lengthy laparoscopic procedure.
Conversion from laparoscopic to open cholecystectomy is a strategy to prevent and/or treat bile duct injuries. In this study, the incidence of bile duct injury in the entire cohort was 0.33% (29 patients). 23 out of 29 (79%) bile duct injuries were diagnosed intraoperatively, of which 16 were managed laparoscopically and 7 were converted to open. The remaining six bile duct injuries were diagnosed postoperatively. It is important to note that patients who were diagnosed with iatrogenic bile duct injury after thirty days (e.g. ischaemic biliary stricture) would not be included in this analysis, and this may underestimate the true incidence of iatrogenic bile duct injury after cholecystectomy. 18 out of 29 (62%) bile duct injuries were in high risk patients based on a CLOC score > 6, including 4 of 6 (67%) bile duct injuries that were diagnosed postoperatively. Although a high CLOC score may alert the surgeon to a potentially difficult cholecystectomy, a significant proportion of bile duct injuries developed in ‘low risk’ cases in this cohort, and a high degree of suspicion is needed both intra- and post-operatively. The critical view of safety (CVS) was described more than twenty years ago to prevent bile duct injury during laparoscopic cholecystectomy.5 However, the incidence of bile duct injuries has failed to reduce in the intervening period.3 The reasons for this are unclear, although it is possible that the CVS is either not being routinely used or is being applied incorrectly. The ‘infundibular technique’, which is flawed and may predispose to bile duct injury, cannot be recommended.24 In a recent single centre series of over 1000 patients, the CVS was associated with a zero incidence of bile duct injury.25 Information regarding the use of CVS in our cohort was not collected, and it is therefore not possible to comment on the aetiology of bile duct injury in this series, but it is hypothesised that bile duct injuries in low risk patients (CLOC score ≤ 6) occurred due to improper or failure to use CVS. Other strategies designed to reduce bile duct injuries, including the use of the anatomical landmark Rouvière's sulcus or a cholecystectomy checklist, have not been rigorously tested.26, 27
This study has some limitations. The primary aim of the CholeS study was to assess the variation in practice of cholecystectomy in the UK and was not designed to develop a risk score to predict conversion. Consequently, several variables potentially of interest were not included in the original dataset, such as history of previous surgery, comorbidity, reason for conversion and use of the critical view of safety. It is unclear whether history of previous surgery independently predicts conversion, since previous reports are conflicting.1, 2, 3 Although correlation between the risk score and operative difficulty was demonstrated in this study, the threshold for conversion to open is variable as outlined above, and the optimal time to convert cannot be deduced from this study. Interestingly, body mass index did not independently predict conversion in this study, in contrast to data from other series.1, 2, 10, 11, 12 The reasons for this discrepancy are unclear, but given the large sample size and number of variables included in this multivariable analysis, it may indicate that important confounding factors could have been omitted from other studies.
In summary, this is the first validated risk score to predict conversion from laparoscopic to open cholecystectomy, and is based on a large prospective, contemporary database. Stratifying patients according to the risk of conversion has several potential clinical applications.
Conflict of interest
None declared.
Acknowledgements
We would like to acknowledge the help of Amanda Kirkham, Statistician, Birmingham Clinical Trials Unit for help with setting up the CholeS database and Paul Marriott, West Midlands Research Collaborative for organising the data validation.
Contributor Information
Robert P. Sutcliffe, Email: robert.sutcliffe@uhb.nhs.uk.
the CholeS study group, West Midlands Research Collaborative:
Stephen Fenwick, Mohamed Elmasry, Quentin Nunes, David Kennedy, Raja B. Khan, Muhammad A.S. Khan, Conor J. Magee, Steven M. Jones, Denise Mason, Ciny P. Parappally, Pawan Mathur, Michael Saunders, Sara Jamel, Samer U.l. Haque, Sara Zafar, Muhammad H. Shiwani, Nehemiah Samuel, Farooq Dar, Andrew Jackson, Bryony Lovett, Shiva Dindyal, Hannah Winter, Saquib Rahman, Kevin Wheatley, Tom Nieto, Soofiyah Ayaani, Haney Youssef, Rajwinder S. Nijjar, Helen Watkin, David Naumann, Sophie Emeshi, Piyush B. Sarmah, Kathryn Lee, Nikita Joji, Jonathan Heath, Rebecca L. Teasdale, Chamindri Weerasinghe, Paul J. Needham, Hannah Welbourn, Luke Forster, David Finch, Jane M. Blazeby, William Robb, Angus G.K. McNair, Alex Hrycaiczuk, Alexandros Charalabopoulos, Sritharan Kadirkamanathan, Cheuk-Bong Tang, Naga V.G. Jayanthi, Nigel Noor, Brian Dobbins, Andrew J. Cockbain, April Nilsen-Nunn, Jonathan de Siqueira, Mike Pellen, Jonathan B. Cowley, Wei-Min Ho, Victor Miu, Timothy J. White, Kathryn A. Hodgkins, Alison Kinghorn, Matthew G. Tutton, Yahya A. Al-Abed, Donald Menzies, Anwar Ahmad, Joanna Reed, Shabuddin Khan, David Monk, Louis J. Vitone, Ghulam Murtaza, Abraham Joel, Stephen Brennan, David Shier, Catherine Zhang, Thusidaran Yoganathan, Steven J. Robinson, Iain J.D. McCallum, Michael J. Jones, Mohammed Elsayed, Liz Tuck, John Wayman, Kate Carney, Somaiah Aroori, Kenneth B. Hosie, Adam Kimble, David M. Bunting, Kenneth B. Hosie, Adeshina S. Fawole, Mohammed Basheer, Rajiv V. Dave, Janahan Sarveswaran, Elinor Jones, Chris Kendal, Michael P. Tilston, Martin Gough, Tom Wallace, Shailendra Singh, Justine Downing, Katherine A. Mockford, Eyad Issa, Nayab Shah, Neal Chauhan, Timothy R. Wilson, Amir Forouzanfar, Jonathan R.L. Wild, Emma Nofal, Catherine Bunnell, Khaliel Madbak, Sudhindra T.V. Rao, Laurence Devoto, Najaf Siddiqi, Zechan Khawaja, James C. Hewes, Laura Gould, Alice Chambers, Daniel U. Rodriguez, Gourab Sen, Stuart Robinson, Kate Carney, Francis Bartlett, David M. Rae, Thomas E.J. Stevenson, Kas Sarvananthan, Simon J. Dwerryhouse, Simon M. Higgs, Oliver J. Old, Thomas J. Hardy, Reena Shah, Steve T. Hornby, Ken Keogh, Lucinda Frank, Musallam Al-Akash, Emma A. Upchurch, Richard J. Frame, Michael Hughes, Clare Jelley, Simon Weaver, Sudipta Roy, Toritseju O. Sillo, Giorgios Galanopoulos, Tamzin Cuming, Pedro Cunha, Salim Tayeh, Sarantos Kaptanis, Mohamed Heshaishi, Abdalla Eisawi, Michael Abayomi, Wee S. Ngu, Katie Fleming, Dalvir S. Bajwa, Vivek Chitre, Kamal Aryal, Paul Ferris, Michael Silva, Simon Lammy, Sarah Mohamed, Amir Khawaja, Adnan Hussain, Mudassar A. Ghazanfar, Maria I. Bellini, Hamdi Ebdewi, Mohamed Elshaer, Gianpiero Gravante, Benjamin Drake, Arikoge Ogedegbe, Dipankar Mukherjee, Chanpreet Arhi, Lola Giwa, Nusrat Iqbal, Nicholas F. Watson, Smeer K. Aggarwal, Philippa Orchard, Eduardo Villatoro, Peter D. Willson, Kam W.J. Mok, Thomas Woodman, Jean Deguara, Giuseppe Garcea, Benoy I. Babu, Alistair R. Dennison, Deep Malde, David Lloyd, John P. Slavin, Robert P. Jones, Laura Ballance, Stratos Gerakopoulos, Periyathambi Jambulingam, Sami Mansour, Naomi Sakai, Vikas Acharya, Mohammed M. Sadat, Lawen Karim, David Larkin, Khalid Amin, Amarah Khan, Jennifer Law, Saurabh Jamdar, Stella R. Smith, Keerthika Sampat, Kathryn M. O'shea, Mangta Manu, Fotini M. Asprou, Nabeela S. Malik, Jessica Chang, Marianne Johnstone, Michael Lewis, Geoffrey P. Roberts, Babu Karavadra, Evangelos Photi, James Hewes, Laura Gould, Alice Chambers, Dan Rodriguez, Derek A. O'Reilly, Anthony J. Rate, Hema Sekhar, Lucy T. Henderson, Benjamin Z. Starmer, Peter O. Coe, Sotonye Tolofari, Jenifer Barrie, Gareth Bashir, Jake Sloane, Suroosh Madanipour, Constantine Halkias, Alexander E.J. Trevatt, David W. Borowski, Jane Hornsby, Michael J. Courtney, Suvi Virupaksha, Keith Seymour, Sarah Robinson, Helen Hawkins, Sadiq Bawa, Paul V. Gallagher, Alistair Reid, Peter Wood, Jonathan G. Finch, J.Guy Finch, Jitesh Parmar, Euan Stirland, James Gardner-Thorpe, Ahmed Al-Muhktar, Mark Peterson, Ali Majeed, Farrukh M. Bajwa, Jack Martin, Alfred Choy, Andrew Tsang, Naresh Pore, David R. Andrew, Waleed Al-Khyatt, Christopher Taylor Santosh Bhandari, Adam Chambers, Dhivya Subramanium, Simon K.C. Toh, Nicholas C. Carter, Stuart J. Mercer, Benjamin Knight, Vardhini Vijay, Swethan Alagaratnam, Sidhartha Sinha, Shahab Khan, Shamsi S. El-Hasani, Abdulzahra A. Hussain, Vish Bhattacharya, Nisheeth Kansal, Tani Fasih, Claire Jackson, Midhat N. Siddiqui, Imran A. Chishti, Imogen J. Fordham, Zohaib Siddiqui, Harald Bausbacher, Ileana Geogloma, Kabita Gurung, George Tsavellas, Pradeep Basynat, Ashish K. Shrestha, Sanjoy Basu, Alok Chhabra, Mohan Harilingam, Mohamed Rabie, Mansoor Akhtar, Pradeep Kumar, Sadaf F. Jafferbhoy, Najam Hussain, Soulat Raza, Manzarul Haque, Imran Alam, Rabiya Aseem, Shakira Patel, Mehek Asad, Michael I. Booth, William R. Ball, Christopher P.J. Wood, Ana C. Pinho-Gomes, Ambareen Kausar, Mohammed Obeidallah, Joseph Varghase, Joshil Lodhia, Donal Bradley, Carla Rengifo, David Lindsay, Sivakumar Gopalswamy, Ian Finlay, Stacy Wardle, Naomi Bullen, Syed Y. Iftikhar, Altaf Awan, Javed Ahmed, Paul Leeder, Guiseppe Fusai, Giles Bond-Smith, Alicja Psica, Yogesh Puri, David Hou, Fergus Noble, Karoly Szentpali, Jack Broadhurst, Ravindra Date, Martin R. Hossack, Yan L. Goh, Paul Turner, Vinutha Shetty, Manel Riera, Christina A.W. Macano, Anisha Sukha, Shaun R. Preston, Jennifer R. Hoban, Daniel J. Puntis, Sophie V. Williams, Richard Krysztopik, James Kynaston, Jeremy Batt, Matthew Doe, Andrzej Goscimski, Gareth H. Jones, Stella R. Smith, Claire Hall, Nick Carty, Jamil Ahmed, Sofoklis Panteleimonitis, Rohan T. Gunasekera, Andrea R.G. Sheel, Hannah Lennon, Caroline Hindley, Marcus Reddy, Ross Kenny, Natalie Elkheir, Emma R. McGlone, Rajasundaram Rajaganeshan, Kate Hancorn, Anita Hargreaves, Raj Prasad, David A. Longbotham, Dhakshinamoorthy Vijayanand, Imeshi Wijetunga, Paul Ziprin, Christopher R. Nicolay, Geoffrey Yeldham, Edward Read, James A. Gossage, Rachel C. Rolph, Husam Ebied, Manraj Phull, Mohammad A. Khan, Matthew Popplewell, Dimitrios Kyriakidis, Anwar Hussain, Natasha Henley, Jessica R. Packer, Laura Derbyshire, Jonathan Porter, Shaun Appleton, Marwan Farouk, Melvinder Basra, Neil A. Jennings, Shahda Ali, Venkatesh Kanakala, Haythem Ali, Risha Lane, Richard Dickson-Lowe, Prizzi Zarsadias, Darius Mirza, Sonia Puig, Khalid Al Amari, Deepak Vijayan, Robert Sutcliffe, Ravi Marudanayagam, Zayed Hamady, Abheesh R. Prasad, Abhilasha Patel, Damien Durkin, Parminder Kaur, Laura Bowen, James P. Byrne, Katherine L. Pearson, Theo G. Delisle, James Davies, Mark A. Tomlinson, Michelle A. Johnpulle, Corinna Slawinski, Andrew Macdonald, James Nicholson, Katy Newton, James Mbuvi, Ansar Farooq, Bhavani S. Mothe, Zakhi Zafrani, Daniel Brett, James Francombe, Philip Spreadborough, James Barnes, Melanie Cheung, Ahmed Z. Al-Bahrani, Giuseppe Preziosi, Tomas Urbonas, Justin Alberts, Mekhlola Mallik, Krashna Patel, Ashvina Segaran, Triantafyllos Doulias, Pratik A. Sufi, Caroline Yao, Sarah Pollock, Antonio Manzelli, Saj Wajed, Michail Kourkulos, Roberto Pezzuto, Martin Wadley, Emma Hamilton, Shameen Jaunoo, Robert Padwick, Mazin Sayegh, Richard C. Newton, Madhusoodhana Hebbar, Sameh F. Farag, Madhu Hebbar, John Spearman, Mohammed F. Hamdan, Conrad D'Costa, Christine Blane, Mathew Giles, Mark B. Peter, Natalie A. Hirst, Tanvir Hossain, Arslan Pannu, Yesar El-Dhuwaib, Tamsin E.M. Morrison, Greg W. Taylor, Ronald L.E. Thompson, Ken McCune, Paula Loughlin, Roger Lawther, Colman K. Byrnes, Duncan J. Simpson, Abi Mawhinney, Conor Warren, Damian McKay, Colin McIlmunn, Serena Martin, Matthew MacArtney, Tom Diamond, Phil Davey, Claire Jones, Joshua M. Clements, Ruairi Digney, Wei M. Chan, Stephen McCain, Sadaf Gull, Adam Janeczko, Emmet Dorrian, Andrew Harris, Suzanne Dawson, Dorothy Johnston, Barry McAree, Essam Ghareeb, George Thomas, Martin Connelly, Stephen McKenzie, Krzysztos Cieplucha, Gary Spence, William Campbell, Gareth Hooks, Neil Bradley, Arnold D.K. Hill, John T. Cassidy, Michael Boland, Paul Burke, Deirdre M. Nally, Arnold D.K. Hill, Elmoataz Khogali, Wael Shabo, Edrin Iskandar, Gerry P. McEntee, Maeve A. O'Neill, Colin Peirce, Emma M. Lyons, Adrian W. O'Sullivan, Rohan Thakkar, Paul Carroll, Ivan Ivanovski, Paul Balfe, Matthew Lee, Des C. Winter, Michael E. Kelly, Emir Hoti, Donal Maguire, Priyadarssini Karunakaran, Justin G. Geoghegan, Sean T. Martin, Keith S. Cross, Fiachra Cooke, Saquib Zeeshan, James O. Murphy, Ken Mealy, Helen M. Mohan, Yuwaraja Nedujchelyn, Muhammad F. Ullah, Irfan Ahmed, Francesco Giovinazzo, James Milburn, Sarah Prince, Eleanor Brooke, Joanna Buchan, Ahmed M. Khalil, Elizabeth M. Vaughan, Michael I. Ramage, Roland C. Aldridge, Simon Gibson, Gary A. Nicholson, David G. Vass, Alan J. Grant, David J. Holroyd, Angharad Jones, Cherith M.L.R. Sutton, Patrick O'Dwyer, Frida Nilsson, Beatrix Weber, Tracey K. Williamson, Kushik Lalla, Alice Bryant, Ross Carter, Craig R. Forrest, David I. Hunter, Ahmad H. Nassar, Mavis N. Orizu, Katrina Knight, Haitham Qandeel, Stuart Suttie, Rowena Belding, Andrew McClarey, Alan T. Boyd, Graeme J.K. Guthrie, Pei J. Lim, Andreas Luhmann, Angus J.M. Watson, Colin H. Richards, Laura Nicol, Marta Madurska, Ewen Harrison, Kathryn M. Boyce, Amanda Roebuck, Graeme Ferguson, Pradeep Pati, Michael S.J. Wilson, Faith Dalgaty, Laura Fothergill, Peter J. Driscoll, Kirsty L. Mozolowski, Victoria Banwell, Stephen P. Bennett, Paul N. Rogers, Brendan L. Skelly, Claire L. Rutherford, Ahmed K. Mirza, Taha Lazim, Henry C.C. Lim, Diana Duke, Talat Ahmed, William D. Beasley, Marc D. Wilkinson, Geta Maharaj, Cathy Malcolm, Timothy H. Brown, Guy M. Shingler, Nicholas Mowbray, Rami Radwan, Paul Morcous, Simon Wood, Abbas Kadhim, Duncan J. Stewart, Andrew L. Baker, Nicola Tanner, and Hrishikesh Shenoy
References
- 1.Goonawardena J., Gunnarsson R., de Costa A. Predicting conversion from laparoscopic to open cholecystectomy presented as a probability nomogram based on preoperative patient risk factors. Am J Surg. 2015;210:492–500. doi: 10.1016/j.amjsurg.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Rosen M., Brody F., Ponsky J. Predictive factors for conversion of laparoscopic cholecystectomy. Am J Surg. 2002;184:254–258. doi: 10.1016/s0002-9610(02)00934-0. [DOI] [PubMed] [Google Scholar]
- 3.Lipman J.M., Claridge J.A., Haridas M., Martin M.D., Yao D.C., Grimes K.L. Preoperative findings predict conversion from laparoscopic to open cholecystectomy. Surgery. 2007;142:556–563. doi: 10.1016/j.surg.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Ballal M., David G., Willmott S., Corless D.J., Deakin M., Slavin J.P. Conversion after laparoscopic cholecystectomy in England. Surg Endosc. 2009;23:2338–2344. doi: 10.1007/s00464-009-0338-1. [DOI] [PubMed] [Google Scholar]
- 5.Strasberg S.M., Hertl M., Soper N.J. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101–125. [PubMed] [Google Scholar]
- 6.Ishizaki Y., Miwa K., Yoshimoto J., Sugo H., Kawasaki S. Conversion of elective laparoscopic to open cholecystectomy between 1993 and 2004. Br J Surg. 2006;93:987–991. doi: 10.1002/bjs.5406. [DOI] [PubMed] [Google Scholar]
- 7.Kama N.A., Kologlu M., Doganay M., Reis E., Atli M., Dolapci M. A risk score for conversion from laparoscopic to open cholecystectomy. Am J Surg. 2001;181:520–525. doi: 10.1016/s0002-9610(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 8.Reinders J.S., Gouma D.J., Heisterkamp J., Tromp E., van Ramshorst B., Boerma D. Laparoscopic cholecystectomy is more difficult after a previous endoscopic retrograde cholangiography. HPB. 2013;15:230–234. doi: 10.1111/j.1477-2574.2012.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kologlu M., Tutuncu T., Yuksek Y.N., Gozalan U., Daglar G., Kama N.A. Using a risk score for conversion from laparoscopic to open cholecystectomy in resident training. Surgery. 2004;135:282–287. doi: 10.1016/s0039-6060(03)00395-7. [DOI] [PubMed] [Google Scholar]
- 10.Sippey M., Grzybowski M., Manwaring M.L., Kasten K.R., Chapman W.H., Pofahl W.E. Acute cholecystitis: risk factors for conversion to an open procedure. J Surg Res. 2015;199:357–361. doi: 10.1016/j.jss.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Vivek M.A., Augustine A.J., Rao R. A comprehensive predictive scoring method for difficult laparoscopic cholecystectomy. J Minim Access Surg. 2014;10:62–67. doi: 10.4103/0972-9941.129947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanisic V., Andjelkovic I., Vlaovic D., Babic I., Kocev N., Nikolic B. Feasibility of applying data mining techniques for predicting technical difficulties during laparoscopic cholecystectomy based on routine patient work-up in a small community hospital. Hepatogastroenterology. 2013;60:1561–1568. doi: 10.5754/hge13213. [DOI] [PubMed] [Google Scholar]
- 13.Kaafarani H.M., Smith T.S., Neumayer L., Berger D.H., Depalma R.G., Itani K.M. Trends, outcomes, and predictors of open and conversion to open cholecystectomy in Veterans Health Administration hospitals. Am J Surg. 2010;200:32–40. doi: 10.1016/j.amjsurg.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Randhawa J.S., Pujahari A.K. Preoperative prediction of difficult lap chole: a scoring method. Indian J Surg. 2009;71:198–201. doi: 10.1007/s12262-009-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vohra R.S., Spreadborough P., Johnstone M., Marriott P., Bhangu A., Alderson D., on behalf of the West Midlands Research Collaborative Protocol for a multicenter, prospective, population-based cohort study of variation in practice of cholecystectomy and surgical outcomes (The CholeS study) BMJ Open. 2015;5:e006399. doi: 10.1136/bmjopen-2014-006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CholeS Study Group Independent predictors of all cause 30-day readmissions following cholecystectomy: a contemporary, multi-centre, prospective, population-based cohort study. Br J Surg. 2015;102:68. [Google Scholar]
- 17.Nassar A.H., Ashkar K.A., Mohamed A.Y., Hafiz A.A. Is laparoscopic cholecystectomy possible without video technology? Minim Invasive Ther Allied Technol. 1995;4:63–65. [Google Scholar]
- 18.Way L.W., Stewart L., Gantert W., Liu K., Lee C.M., Whang K. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237:460–469. doi: 10.1097/01.SLA.0000060680.92690.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch M., Garden O.J., Padbury R., Rahbari N.N., Adam R., Capussotti L. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Livingston E.H., Rege R.V. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205–211. doi: 10.1016/j.amjsurg.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Fried G.M., Barkun J.S., Sigman H.H., Joseph L., Clas D., Garzon J. Factors determining conversion to laparotomy in patients undergoing laparoscopic cholecystectomy. Am J Surg. 1994;167:35–39. doi: 10.1016/0002-9610(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 22.Alponat A., Kum C.K., Koh B.C., Rajnakova A., Goh P.M. Predictive factors for conversion of laparoscopic cholecystectomy. World J Surg. 1997;21:629–633. doi: 10.1007/pl00012288. [DOI] [PubMed] [Google Scholar]
- 23.Keus F., de Jong J.A., Gooszen H.G., van Laarhoven C.J. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;4:CD006231. doi: 10.1002/14651858.CD006231. [DOI] [PubMed] [Google Scholar]
- 24.Strasberg S.M. Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2002;9:543–547. doi: 10.1007/s005340200071. [DOI] [PubMed] [Google Scholar]
- 25.Avgerinos C., Kelgiorgi D., Touloumis Z., Baltatzi L., Dervenis C. One thousand laparoscopic cholecystectomies in a single surgical unit using the “critical view of safety” technique. J Gastrointest Surg. 2009;13:498–503. doi: 10.1007/s11605-008-0748-8. [DOI] [PubMed] [Google Scholar]
- 26.Connor S.J., Perry W., Nathanson L., Hugh T.B., Hugh T.J. Using a standardized method for laparoscopic cholecystectomy to create a concept operation-specific checklist. HPB. 2014;16:422–429. doi: 10.1111/hpb.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hugh T.B. New strategies to prevent laparoscopic bile duct injury–surgeons can learn from pilots. Surgery. 2002;132:826–835. doi: 10.1067/msy.2002.127681. [DOI] [PubMed] [Google Scholar]