Abstract
Background
Although pancreaticoduodenectomy (PD) outcomes have improved, complications including surgical site infection (SSI) remain common. We present a stratification tool to predict risk for SSI after PD.
Methods
Data was retrospectively reviewed on all patients undergoing PD at a tertiary hospital (9/2011-8/2014). Potential SSI risk factors identified by univariate analysis were incorporated into a multivariate logistic regression model. The resulting odds ratios were converted into a point system to create an SSI risk score with internal validation.
Results
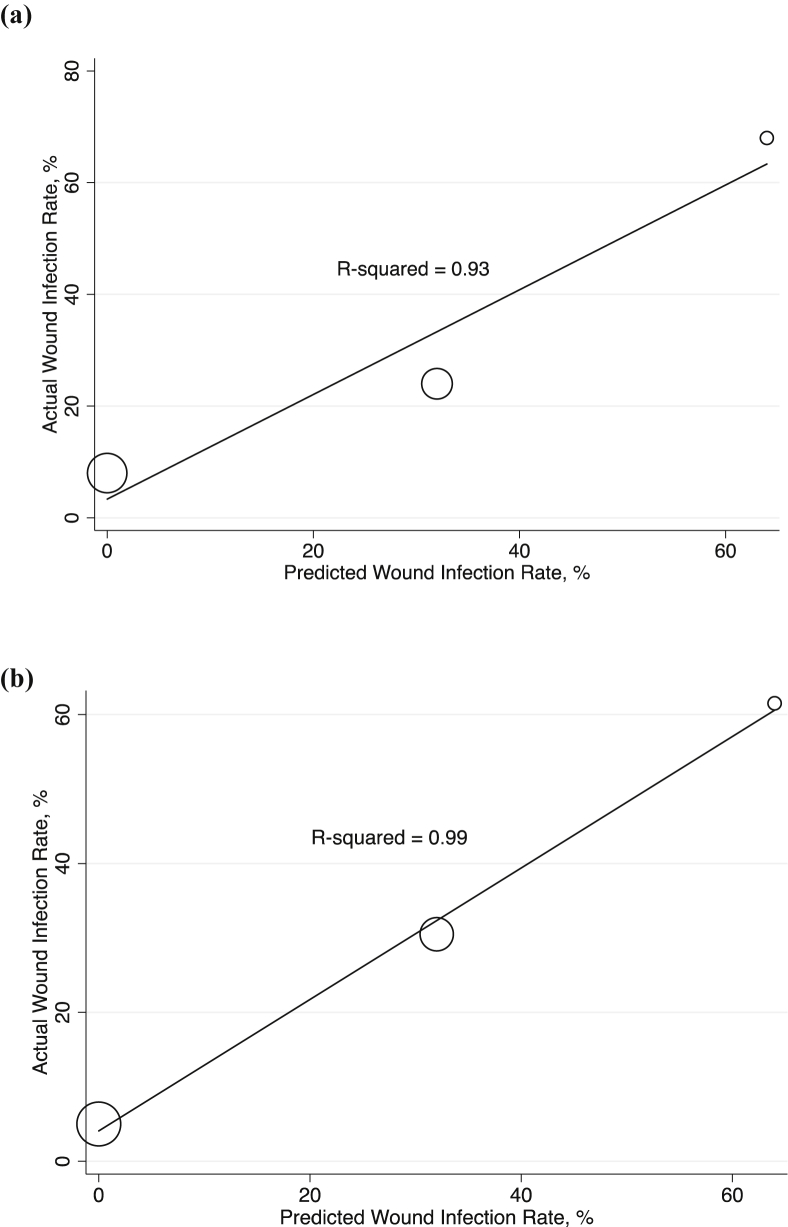
Six hundred seventy nine patients underwent PD and were chronologically split into derivation (443 patients) and validation (236 patients) groups. There was no difference in demographics or perioperative outcomes between groups. Overall thirty-day SSI was observed in 17.2% (n = 117). Neoadjuvant chemotherapy and/or radiation, intraoperative red blood cell transfusion, operative time greater than 7 h, preoperative bile stent/drain, and vascular resection were associated with SSI in univariate analysis (all p < 0.05). On multivariate analysis, preoperative bile stent/drain and neoadjuvant chemotherapy were independent predictors of SSI, each assigned 1 point (both p < 0.001). Patients with 0, 1, and 2 points, respectively, had 0%, 32%, and 64% predicted risk of SSI (AUC = 0.73, R2 = 0.93). The model performed equivalently in the validation group (AUC = 0.77, R2 = 0.99).
Conclusion
This novel, validated risk score accurately predicts SSI risk after pancreaticoduodenectomy. Identifying the highest risk patients can help target interventions to reduce SSI.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer deaths in the United States with an estimated 40,560 deaths in 2015.1 Surgical resection remains the best opportunity for cure, and for tumors in the head and neck of the pancreas, this involves performing a pancreaticoduodenectomy (PD).2, 3 Although mortality has improved significantly for patients undergoing pancreatic resection, postoperative morbidity remains high after PD. Surgical site infection (SSI) is one of the most common complications after PD and can lead to delayed time to adjuvant therapy, decreased disease-free survival and decreased overall survival.2, 4, 5, 6
Surgical site infection (SSI) is defined by the Centers for Disease Control (CDC) as an infection involving only the skin or subcutaneous tissue of a surgical incision within 30 days after an operation.7 It is characterized by erythema, pain, heat, or swelling and may require that the wound be opened.7 The development of an SSI after abdominal surgery leads to a prolonged hospital length of stay in the majority of patients, contributing to dramatically increased post-operative healthcare expenditures.8, 9, 10 Because of the impact SSIs can have on perioperative morbidity and hospital cost as well as disease-free and overall survival, it is important to have a clear understanding of the factors that may contribute to their occurrence. There is limited data on risk factors for SSI in patients who have undergone PD, although previously identified factors include obesity, prolonged operative time, and pancreatic duct diameter.11 In addition, neoadjuvant chemotherapy has been shown to be a risk factor for SSI in surgical treatment of rectal and breast cancer, but has not previously been definitively associated with SSI after PD.12, 13 Finally, preoperative placement of a biliary stent or drain may increase the risk for an SSI after pancreatic resection. Early studies showed decreased complication rates with preoperative drainage and stenting,14, 15, 16 but more recent studies have yielded equivocal results17, 18 or concluded that biliary stents are associated with increased surgical complications, including SSI.19, 20, 21, 22, 23 Other perioperative measures can be employed to decrease SSI rates including using a laparoscopic approach and prophylactically applying a wound vacuum closure device instead of standard closure.24
There is clearly a need for improved SSI risk stratification among patients undergoing PD to allow for preoperative identification of the highest-risk patients and implementation of preventative strategies. This study aimed to identify risk factors associated with an increased risk of SSI after PD and use them to create a risk stratification score.
Methods
Patient selection
All patients who underwent PD at the Johns Hopkins Hospital between September 2011 and August 2014 were included in this study. Prospectively obtained data was analyzed and included patient demographics (age, sex, medical co-morbidities), operative factors, history of chemotherapy or radiation for another malignancy, neoadjuvant or adjuvant therapy for treatment of PDAC, perioperative complications, long-term complications, and tumor histopathology. SSI was identified by retrospective chart review using criteria defined by the Center for Disease Control (CDC).7 Patients were followed with routine postoperative visits, including a visit approximately two weeks and one month after hospital discharge. This study was carried out with the approval of the Institutional Review Board at the Johns Hopkins Hospital.
Vacuum dressing placement
In a minority of patients, a superficial wound vacuum (VAC) dressing was applied to the wound at the time of surgical closure in a manner previously described.25 In short, VAC closure involved closure of the dermis using interrupted 3-0 vicryl sutures. Rectangular strips of reticulated open-cell polyurethane white foam (KCI) were cut to size and inserted at 6- to 8-cm intervals through the dermal layer into the subcutaneous space. Exposed areas of skin between the white foam wicks were covered with silver-impregnanted nonadherent dressing (Restore Silver Contact Layer). A negative pressure VAC therapy was applied over the Restore layer, at a continuous pressure of −125 mmHg. The dressing was replaced every 2–3 days until the white foam strips were dry, at which point an SVAC remained in place for two additional days before removal. None of these patients had any evidence of infection at the time of surgery. The VAC dressing was applied at the discretion of the individual surgeon in patients felt to be at higher risk for SSI. This was based on data demonstrating a reduced incidence of SSI and surgical site occurrence (SSO) with prophylactic VAC dressing closure after open ventral hernia repair.24, 25
Statistical analysis and risk score creation
Summary statistics for patients are presented as percentages for categorical variables and mean values for ranges for continuous variables. Differences between patients with and without SSI were assessed using Fisher's exact test for categorical values and Student's t-test for continuous variables. Univariate logistic regression modeling was used to identify individual factors associated with SSI. Covariates with a p-value < 0.20 on univariate analysis met criteria for further analysis and were entered into a multivariable logistic regression model. The Akaike Information Criterion (AIC), Hosmer–Lemeshow goodness-of-fits test and likelihood ratio were used to assess model strength at each step, leading to the derivation of the most parsimonious model. Receiver operator curves were calculated to ensure appropriate sensitivity and specificity of the risk score. Variables significantly associated with SSI by multivariate analysis were assigned point values based on the rounded-off ratios of their relative odds ratios. Weighted linear regression was used to compare observed to predicted rates of SSI based upon the risk score calculated for all patients. Patients were split into derivation and validation cohorts based upon the time period of surgical intervention. The derivation cohort consisted of all patients between September 2012 and August 2014, while the validation cohort consisted all patients between September 2011 and August 2012. Statistical analysis was performed using STATA Version 13.0 (StataCorp, College Station, TX). Significance for all analyses performed was defined as a p-value < 0.05.
Results
Patient characteristics
During the study period, 679 patients underwent pancreaticoduodenectomy and were included for analysis. The average age was 63.7 (range, 19–92 years) and 56% were male. One hundred twenty one patients (18%) were diabetic preoperatively, and 178 patients (26%) presented with weight loss. The average body mass index (BMI) was 26.4 kg/m2 (range, 15.6–48.4 kg/m2) and 374 patients (55.1%) were considered overweight with a BMI > 25 kg/m2. Obstructive jaundice was a presenting symptom in 262 patients (38.6%) and 256 patients (38%) underwent placement of an endoscopic or percutaneous biliary stent/drain. In 43 patients (17%), the biliary stent or drain was placed to alleviate obstructive jaundice while receiving neoadjuvant chemotherapy, as the patient was not an immediate surgical candidate. In the remaining patients, stent placement occurred prior to the patient seeing a surgeon and was most often at the discretion of the gastroenterologist during endoscopy for workup of the biliary obstruction. Seventy-eight patients (12%) underwent neoadjuvant chemotherapy while 41 patients (6%) received concomitant neoadjuvant chemoradiation therapy.
The majority of patients (n = 631, 93%) underwent open pancreaticoduodenectomy while the remaining patients underwent either laparoscopic or robotic resection. All surgeries were elective, and no patient had signs of clinical infection at the time of surgery. All patients received appropriate perioperative antibiotics 1 h prior to surgical incision and for 24 h after the operation, based upon SCIP (Surgical Care Improvement Project) guidelines by the Joint Commission. Patients who underwent prolonged surgical operations were re-dosed with the same antibiotic during the case. The individual antibiotic administered depended on the patient's individual medication allergies, but usually involved a dose of cefotetan or cefoxitin for pancreaticoduodenectomy. Patients with an allergy to beta-lactam antibiotics were instead given clindamycin and a secondary antibiotic such as a fluoroquinilone based on SCIP guidelines. Malignant disease was found in 541 patients (80%). Postoperatively, a superficial wound vacuum (VAC) dressing was applied to the incisions of 50 patients (7%), at the discretion of the surgeon.
All patients were seen in the surgical clinic for follow-up at 2 weeks and 30 days post-operative, at which time the wound was assessed for any signs of infection. The rate of SSI within 30 days of PD was 17.2% (n = 117). Median time to clinical diagnosis of SSI was 10 days (IQR 6 days–16 days). Treatment of the SSI involved opening of the wound in 104 patients (89%) with or without the administration of antibiotics in 54 patients (46%). The most common antibiotics prescribed for therapy included sulfamethoxazole/trimethoprim (Bactrim), amoxicillin/clavulanate (Augmentin) and cephalexin (Keflex). Cultures of the wound were not routinely obtained in this patient cohort and were not available at the time of this analysis.
Creation of risk score for SSI after pancreaticoduodenectomy
For the risk score analysis, the derivation cohort consisted of 443 patients who underwent PD between September 2012 and August 2014 and the validation cohort consisted of 236 patients who underwent PD between September 2011 and August 2012. Baseline characteristics were similar between the derivation and validation cohorts, including the incidence of post-operative SSI (Table 1).
Table 1.
Comparison of patient variables between the derivation and validation cohorts
| Characteristic | Derivation cohort (n = 443) | Validation cohort (n = 236) | p-value |
|---|---|---|---|
| Mean age, years, (range) | 63.5 (19–92) | 63.9 (21–91) | 0.69 |
| Male gender | 248 (56%) | 130 (55%) | 0.87 |
| Diabetes | 80 (18%) | 41 (17%) | 0.92 |
| Current or prior smoker | 137 (45%) | 74 (51%) | 0.19 |
| Weight loss | 118 (27%) | 60 (25%) | 0.78 |
| Mean BMI, kg/m2, (range) | 26.4 (15.6–48.4) | 26.6 (17–44.3) | 0.69 |
| BMI > 30 | 101 (23%) | 56 (24%) | 0.78 |
| Obstructive jaundice | 176 (40%) | 86 (36%) | 0.41 |
| Preoperative biliary stent/drain | 169 (38%) | 87 (37%) | 0.74 |
| Neoadjuvant chemotherapy for PDAC | 57 (13%) | 21 (9%) | 0.13 |
| Neoadjuvant chemoradiation for PDAC | 30 (7%) | 11 (5%) | 0.31 |
| Open PD | 417 (94%) | 214 (91%) | 0.12 |
| Pancreatic malignancy | 361 (81%) | 180 (77%) | 0.19 |
| PRBC transfusion | 103 (23%) | 48 (20%) | 0.44 |
| Mean estimated blood loss, ml, range | 824 (50–10,000) | 808 (50–6200) | 0.82 |
| Mean operative time, minutes, range | 399 (218–706) | 401 (218–735) | 0.69 |
| Use of superficial wound vacuum dressing | 37 (8%) | 13 (5.5%) | 0.21 |
| Concomitant vascular resection | 61 (14%) | 24 (10%) | 0.22 |
| Postoperative SSI | 77 (17%) | 40 (17%) | 0.92 |
| Postoperative biliary leak | 93 (21%) | 43 (18%) | 0.42 |
| Postoperative pancreatic fistula | 10 (2.2%) | 9 (3.8%) | 0.33 |
In the derivation cohort, 77 patients (17%) had a post-operative SSI. Univariate logistic regression was utilized to identify factors associated with the development of a postoperative SSI (Table 2). Obstructive jaundice, neoadjuvant chemotherapy or radiation for PDAC, placement of a pre-operative biliary stent/drain, operative time greater than 7 h, intraoperative transfusion of packed red blood cells, a concomitant vascular resection, and the absence of placement of a superficial wound vacuum dressing were all associated with SSI after PD (all, p < 0.05). These factors were incorporated into a multivariate logistic regression model, in which neoadjuvant chemotherapy, placement of a preoperative biliary stent/drain, and the absence of a superficial wound vacuum dressing remained significant for risk of SSI after PD (all, p < 0.05) (Table 3). However, by AIC, the model utilizing only neoadjuvant chemotherapy and the placement of a preoperative biliary stent/drain was superior to a model incorporating all three factors (p = 0.002). As such, both variables were normalized to a score based on a rounded-off odds ratio; neoadjuvant chemotherapy and placement of a preoperative biliary stent or drain were each normalized to 1 point. Predicted risk of SSI using this score was 0%, 32%, and 64% for 0, 1, and 2 points, respectively (Table 4). Actual versus predicted rate of SSI correlated strongly for this risk model with a coefficient of determination (R2) of 0.93 (Fig. 1a) and corresponded to an area under the curve (AUC) of 0.73.
Table 2.
Univariable regression analysis of predictors of SSI in the derivation cohort
| Characteristic | Odds ratio (OR) | 95% Confidence interval | p-value |
|---|---|---|---|
| Age > 60 | 1.14 | 0.65–2.0 | 0.47 |
| Male gender | 1.1 | 0.69–1.9 | 0.63 |
| Diabetes | 1.1 | 0.60–2.1 | 0.73 |
| Current or prior smoking | 0.69 | 0.38–1.25 | 0.23 |
| BMI > 25 | 1.01 | 0.62–1.66 | 0.95 |
| BMI > 30 | 0.86 | 0.48–1.58 | 0.64 |
| Obstructive jaundice | 3.1 | 1.8–5.1 | <0.001 |
| Neoadjuvant chemotherapy for PDAC | 5.5 | 3.0–10.0 | <0.001 |
| Neoadjuvant chemoradiation for PDAC | 3.0 | 1.4–6.7 | 0.006 |
| Open surgery | 2.6 | 0.61–11.4 | 0.20 |
| Preoperative biliary stent/drain | 5.1 | 2.89–8.7 | <0.001 |
| Pancreatic malignancy | 1.3 | 0.66–2.5 | 0.47 |
| PRBC transfusion during operation | 1.8 | 1.04–3.04 | 0.04 |
| EBL > 500 ml | 1.5 | 0.91–2.6 | 0.11 |
| EBL > 1000 ml | 1.3 | 0.8–2.3 | 0.26 |
| Operative time > 7 h | 1.8 | 1.08–2.9 | 0.02 |
| Absence of superficial wound vacuum dressing | 8.3 | 1.1–61.4 | 0.04 |
| Concomitant vascular resection | 2.3 | 1.2–4.2 | 0.008 |
| Postoperative biliary leak | 0.52 | 0.06–4.2 | 0.54 |
| Postoperative pancreatic fistula | 1.65 | 0.95–2.9 | 0.08 |
Bold values are considered significant, with a p value < 0.05.
Table 3.
Multivariable regression analysis of predictors of SSI in the derivation cohort
| Characteristic | Odds ratio (OR) | 95% Confidence interval | p-value |
|---|---|---|---|
| Obstructive jaundice | 1.22 | 0.77–1.9 | 0.38 |
| Neoadjuvant chemotherapy for PDAC | 5.4 | 2.6–10.9 | <0.001 |
| Neoadjuvant chemoradiation for PDAC | 0.59 | 0.22–1.6 | 0.30 |
| Preoperative biliary stent/drain | 5.9 | 3.7–9.4 | <0.001 |
| PRBC transfusion during operation | 1.2 | 0.73–2.0 | 0.47 |
| Operative time > 7 h | 1.3 | 0.82–2.1 | 0.26 |
| Absence of superficial wound vacuum dressing | 6.7 | 1.3–24.9 | 0.02 |
| Concomitant vascular resection | 1.47 | 0.72–3.0 | 0.28 |
Bold values are considered significant, with a p value < 0.05.
Table 4.
Risk model for SSI demonstrating the actual and predicted SSI rates for each score
| Score | No SSI within 30 days (Actual) | SSI within 30 days (Actual) | Predicted SSI within 30 days by score |
|---|---|---|---|
| 0 | 239 (92%) | 20 (8%) | 0% |
| 1 | 118 (76%) | 38 (24%) | 32% |
| 2 | 9 (32%) | 19 (68%) | 64% |
Figure 1.
Weighted bubble plots of predicted versus actual SSI rates in the derivation cohort (a) and validation cohort (b)
Validation of risk score for SSI after pancreaticoduodenectomy
The risk stratification score was then applied to the validation cohort. For the validation cohort, the observed SSI rates for risk scores of 0, 1, and 2 points were 5%, 30.5% and 61.5%, respectively. These were similar to the observed SSI rates for the same scores in the derivation cohort. The model performed equivalently in the validation group (R2 = 0.99) (Fig. 1b), and corresponded to an AUC of 0.77.
Discussion
Pancreatic adenocarcinoma is often a lethal disease that requires a multimodality approach. At this time, the best opportunity for cure includes surgical resection.26, 27 Although mortality has improved significantly for patients undergoing pancreatic resection, postoperative morbidity remains high after PD, and SSI is one of the most common postoperative complications.2, 4, 5 In a study of 1144 patients undergoing PD at Johns Hopkins Hospital, postoperative complications were associated with delayed time to adjuvant therapy and decreased disease-free and overall survival.6 Furthermore, studies of SSIs in postsurgical patients have found an estimated cost of up to $1400 per patient secondary to prolonged hospitalization, wound care, and wound complications in patients with procedures complicated by a SSI.28 It is imperative that we understand factors that can increase the risk of post-operative complications, specifically SSI, and implement mechanisms to reduce if not prevent their occurrence. However, few studies to date have identified risk factors associated with the development of an SSI after PD.
In our study, on univariate analysis, pre-operative factors for SSI after PD included obstructive jaundice, neoadjuvant chemotherapy or radiation for PDAC, placement of a pre-operative biliary stent/drain, operative time greater than 7 h, and transfusion of one or more packed red blood cells. In a subsequent multivariate analysis, neoadjuvant chemotherapy and placement of a preoperative biliary stent/drain remained significant. Our study is the first to demonstrate a potential relationship between neoadjuvant chemotherapy and SSI for PDAC.29, 30, 31, 32 Additionally, our study demonstrated a correlation between neoadjuvant radiation therapy and SSI in PDAC, a relationship that has been previously shown for rectal and breast cancer.33, 34 However, it is important to note that neoadjuvant radiation was not a risk factor by multivariate modeling. This is perhaps due to the fact that radiation is often administered concurrently with chemotherapy, which likely confounded the relationship between neoadjuvant radiation and SSI. Our finding that pre-operative biliary stent or drain placement was associated with SSI is consistent with findings in other recent studies, and is believed to be related to bacterial colonization related to the stenting device.19, 20, 21, 22, 23 In patients with obstructive jaundice who require neoadjuvant chemotherapy, preoperative stenting or drainage may be a necessary bridge to surgical intervention. However, in those who can undergo immediate resection of the pancreas mass, limiting stent or drain placement could potentially decrease SSI risk.22
Intraoperatively, on univariate analysis, intraoperative red blood cell transfusion, operative time greater than 7 h, a concomitant vascular resection and the absence of a superficial wound vacuum dressing were associated with SSI after PD. In a subsequent multivariate analysis, only the absence of placement of a superficial wound vacuum dressing remained significant. Interestingly, open compared to laparoscopic surgery was not associated with an increased risk of SSI. This is contrary to multiple previous studies in open abdominal surgery showing that open surgery is associated with increased risk of SSI compared to laparoscopic surgery. This includes a 2005 Cochrane review of 25 randomized control trials (RCTs), which found laparoscopic colectomy was associated with a decreased risk of SSI compared to open surgery, and a 2009 Cochrane review of 34 RCTs, which found laparoscopic hysterectomy was associated with a similar decreased risk of SSI compared to open surgery.35, 36 In our study, the majority of patients in both the derivation (94%) and validation (91%) cohort underwent open resection, and only a small percentage of patients underwent minimally invasive laparoscopic or robotic (MIS) resection. As such, our current study was underpowered to look at the effect of laparoscopic versus open PD on SSI. Given the overwhelming evidence for decreased SSI rates for MIS versus open approach for other abdominal procedures, using this approach in selected patients may help to decrease SSIs.
We also determined that the use of a superficial vacuum closure (VAC) device markedly decreased the SSI rate. In a previous study of patients undergoing ventral hernia repair, superficial VAC decreased the rate of SSI from 32% to 9% (p < 0.001) and surgical site occurrence (SSO) from 42% to 17%, respectively (p < 0.001).24 Furthermore, when used in grade 3 ventral hernias, superficial VAC had an SSI rate of 5.2% at 90 days, which is markedly lower than previously published rates using standard closure in these complex hernia repairs.25 Based on these findings, the application of a superficial VAC may also be an important adjunct to decreased SSI rates in patients undergoing open PD, especially those determined preoperative to be at high risk. Further assessment of the utility of the superficial wound VAC for decreasing wound infection rate in this population using a properly powered prospective randomized trial will be needed to more conclusively determine its ability to prevent SSI in this population.
In addition to identifying pre-operative and perioperative risk factors for a postoperative wound infection, we developed a novel and internally validated risk stratification score was created that accurately predicts the risk of SSI after pancreaticoduodenectomy. The risk score is based upon neoadjuvant chemotherapy and biliary stent/drain placement. Using this stratification score, a patient who received neoadjuvant chemotherapy and a preoperative biliary stent or drain had an estimated risk of SSI of 64% utilizing our model, similar to the rates observed in both the derivation and validation cohorts. Interestingly, 42.8% (291) of patients in this study had one or both of these risk factors. The presence of one or both risk factors may help to explain the high rates of SSI after pancreaticoduodenectomy and underscores the need for improved perioperative management.
Further study of this novel risk score for SSI in patients undergoing PD is needed before it can be applied to a larger population. While we were able to validate this risk score in a separate cohort of patients, the study was performed using data that was collected retrospectively and potentially limits its application to a wider population. Additional validation with prospectively collected data will be needed to avoid underestimating the true incidence of SSI after PD. In addition, while we determined that the use of a postoperative wound vacuum closure device is associated with a decreased risk of SSI after PD, this device is not standard practice after PD at our institution or elsewhere and its use was partly influenced by physician bias. Therefore, a properly powered randomized control trial to assess the utility of a postoperative wound vacuum closure device will be needed before recommending more widespread use after PD.
In conclusion, neoadjuvant chemotherapy and radiation, operative time greater than 7 h, intraoperative red blood cell transfusion, preoperative placement of a biliary drain or stent, concomitant vascular resection, and the absence of a superficial wound vacuum dressing at surgery were individual risk factors for the development of a SSI by univariate analysis. However, only neoadjuvant chemotherapy, the absence of a postoperative wound vacuum dressing, and preoperative placement of a bile stent/drain were independent risk factors by multivariate analysis. A risk stratification model was created by multivariate modeling which was able to accurately predict the risk of SSI based on neoadjuvant chemotherapy and preoperative biliary stent or drain placement. Identifying patients with the highest risk of SSI can help target interventions to reduce SSI, such as the application of a superficial wound vacuum dressing.
Funding
Funding for this study was provided by National Institutes of Health grant CA126607-06A1 (KEP), Sol Goldman Cancer Research Grant (KEP).
Conflicts of interest
None declared.
Footnotes
This manuscript will be presented as an e-poster at the 12th World Congress of the International Hepato-Pancreato-Biliary Association (IHPBA) in April 2016.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Winter J.M., Cameron J.L., Campbell K.A., Arnold M.A., Chang D.C., Coleman J. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1211. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeOliveira M.L., Winter J.M., Schafer M., Cunningham S.C., Cameron J.L., Yeo C.J. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassi C., Falconi M., Salvia R., Mascetta G., Molinari E., Pederzoli P. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients/with invited commentary. Dig Surg. 2001;18:453–458. doi: 10.1159/000050193. [DOI] [PubMed] [Google Scholar]
- 6.Wu W., He J., Cameron J.L., Makary M., Soares K., Ahuja N. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. 2014;21:2873–2881. doi: 10.1245/s10434-014-3722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventral Hernia Working G., Breuing K., Butler C.E., Ferzoco S., Franz M., Hultman C.S. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Kusachi S., Kashimura N., Konishi T., Shimizu J., Kusunoki M., Oka M. Length of stay and cost for surgical site infection after abdominal and cardiac surgery in Japanese hospitals: multi-center surveillance. Surg Infect (Larchmt) 2012;13:257–265. doi: 10.1089/sur.2011.007. [DOI] [PubMed] [Google Scholar]
- 9.Kashimura N., Kusachi S., Konishi T., Shimizu J., Kusunoki M., Oka M. Impact of surgical site infection after colorectal surgery on hospital stay and medical expenditure in Japan. Surg Today. 2012;42:639–645. doi: 10.1007/s00595-012-0126-8. [DOI] [PubMed] [Google Scholar]
- 10.Coello R., Charlett A., Wilson J., Ward V., Pearson A., Borriello P. Adverse impact of surgical site infections in English hospitals. J Hosp Infect. 2005;60:93–103. doi: 10.1016/j.jhin.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura T., Uesaka K., Ohmagari N., Kanemoto H., Mizuno T. Risk factor of surgical site infection after pancreaticoduodenectomy. World J Surg. 2012;36:2888–2894. doi: 10.1007/s00268-012-1742-6. [DOI] [PubMed] [Google Scholar]
- 12.Valero G., Lujan J., Hernandez Q., De Las Heras M., Pellicer E., Serrano A. Neoadjuvant radiation and chemotherapy in rectal cancer does not increase postoperative complications. Int J Colorectal Dis. 2003;18:495–499. doi: 10.1007/s00384-003-0520-1. [DOI] [PubMed] [Google Scholar]
- 13.Vilar-Compte D., Jacquemin B., Robles-Vidal C., Volkow P. Surgical site infections in breast surgery: case-control study. World J Surg. 2004;28:242–246. doi: 10.1007/s00268-003-7193-3. [DOI] [PubMed] [Google Scholar]
- 14.Lai E., Mok F., Fan S., Lo C., Chu K., Liu C. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195–1198. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- 15.Greig J., Krukowski Z., Matheson N. Surgical morbidity and mortality in one hundred and twenty-nine patients with obstructive jaundice. Br J Surg. 1988;75:216–219. doi: 10.1002/bjs.1800750309. [DOI] [PubMed] [Google Scholar]
- 16.Lygidakis N., Van der Heyde M., Lubbers M. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir Scand. 1986;153:665–668. [PubMed] [Google Scholar]
- 17.Pisters P.W., Hudec W.A., Hess K.R., Lee J.E., Vauthey J.-N., Lahoti S. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234:47. doi: 10.1097/00000658-200107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sewnath M.E., Birjmohun R.S., Rauws E.A., Huibregtse K., Obertop H., Gouma D.J. The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am Coll Surg. 2001;192:726–734. doi: 10.1016/s1072-7515(01)00819-5. [DOI] [PubMed] [Google Scholar]
- 19.Heslin M.J., Brooks A.D., Hochwald S.N., Harrison L.E., Blumgart L.H., Brennan M.F. A preoperative biliary stent is associated with increased complications after pancreatoduodenectomy. Arch Surg. 1998;133:149–154. doi: 10.1001/archsurg.133.2.149. [DOI] [PubMed] [Google Scholar]
- 20.Hochwald S.N., Burke E.C., Jarnagin W.R., Fong Y., Blumgart L.H. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261–266. doi: 10.1001/archsurg.134.3.261. [DOI] [PubMed] [Google Scholar]
- 21.Sohn T.A., Yeo C.J., Cameron J.L., Pitt H.A., Lillemoe K.D. Do preoperative biliary stents increase postpancreaticoduodenectomy complications? J Gastrointest Surg. 2000;4:258–268. doi: 10.1016/s1091-255x(00)80074-8. [DOI] [PubMed] [Google Scholar]
- 22.van der Gaag N.A., Rauws E.A., van Eijck C.H., Bruno M.J., van der Harst E., Kubben F.J. Preoperative biliary drainage for cancer of the head of the pancreas. NEJM. 2010;362:129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 23.Sewnath M.E., Karsten T.M., Prins M.H., Rauws E.J., Obertop H., Gouma D.J. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17. doi: 10.1097/00000658-200207000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares K.C., Baltodano P.A., Hicks C.W., Cooney C.M., Olorundare I.O., Cornell P. Novel wound management system reduction of surgical site morbidity after ventral hernia repairs: a critical analysis. Am J Surg. 2015;209:324–332. doi: 10.1016/j.amjsurg.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Unda N., Soares K.C., Azoury S.C., Baltodano P.A., Hicks C.W., Burce K.K. Negative-pressure wound therapy in the management of high-grade ventral hernia repairs. J Gastrointest Surg. 2015;19:2054–2061. doi: 10.1007/s11605-015-2894-0. [DOI] [PubMed] [Google Scholar]
- 26.Crile G., Jr. The advantages of bypass operations over radical pancreatoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970;130:1049–1053. [PubMed] [Google Scholar]
- 27.Barugola G., Partelli S., Marcucci S., Sartori N., Capelli P., Bassi C. Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 28.Dimick J.B., Chen S.L., Taheri P.A., Henderson W.G., Khuri S.F., Campbell D.A., Jr. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–537. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 29.Decker M.R., Greenblatt D.Y., Havlena J., Wilke L.G., Greenberg C.C., Neuman H.B. Impact of neoadjuvant chemotherapy on wound complications after breast surgery. Surgery. 2012;152:382–388. doi: 10.1016/j.surg.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S.A., Wong Y.K., Wang C.P., Wang C.C., Jiang R.S., Ho H.C. Surgical site infection after preoperative neoadjuvant chemotherapy in patients with locally advanced oral squamous cell carcinoma. Head Neck. 2011;33:954–958. doi: 10.1002/hed.21560. [DOI] [PubMed] [Google Scholar]
- 31.Meric F., Milas M., Hunt K.K., Hess K.R., Pisters P.W., Hildebrandt G. Impact of neoadjuvant chemotherapy on postoperative morbidity in soft tissue sarcomas. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18:3378–3383. doi: 10.1200/JCO.2000.18.19.3378. [DOI] [PubMed] [Google Scholar]
- 32.Song J., Zhang X., Liu Q., Peng J., Liang X., Shen Y. Impact of neoadjuvant chemotherapy on immediate breast reconstruction: a meta-analysis. PLoS One. 2014;9:e98225. doi: 10.1371/journal.pone.0098225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konishi T., Watanabe T., Kishimoto J., Nagawa H. Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg. 2006;244:758–763. doi: 10.1097/01.sla.0000219017.78611.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen M., Ball K., Fraser V.J. Fifth Decennial International Conference on Health-Care Related Infections. 2010. Risk of surgical site infection due to chemotherapy and radiotherapy in patients undergoing mastectomy. [Google Scholar]
- 35.Schwenk W., Haase O., Neudecker J., Muller J.M. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD003145.pub2. CD003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieboer T.E., Johnson N., Lethaby A., Tavender E., Curr E., Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009 CD003677. [Google Scholar]