Abstract
Background
Transaminase levels are usually measured as markers of hepatocellular injury following liver resection, but recent evidence was unclear on their clinical value. This study aimed to identify factors that determine peak postoperative transaminase levels and correlated transaminase levels to postoperative complications.
Study design
All liver resections performed at a single center between 2006 and 2015 were included in the analysis. Multivariate analysis was used to identify factors that determine peak ALT and AST levels and postoperative morbidity and mortality. An ALT and AST cutoff for the prediction of mortality was determined using receiver operating characteristic curves analysis.
Results
A total of 539 resections were included. Clavien–Dindo grade III or higher complications, intraoperative transfusion, and operative duration were identified as determinants of peak transaminases. A peak AST cut-off value for predicting mortality was defined at 828 U/L, with an area under the curve of 0.81 (0.73–0.89). The cut-off was an independent predictor of mortality (P < 0.01) along with (intraoperative) transfusion (P < 0.01), fifty–fifty criteria (P < 0.01), and age (P < 0.01).
Conclusion
Postoperative transaminase levels are independent predictors of postoperative morbidity and mortality and therefore clinically relevant. Transaminase levels usually peak during the first 24 h after surgery and thus possess early prognostic power in terms of postoperative mortality.
Introduction
Liver surgery is associated with substantial risks, reflected by morbidity and mortality rates of 43–45% and 1–6%, respectively, depending on the extent of resection and quality of the liver parenchyma.1, 2, 3, 4 Despite the development and implementation of risk-reducing procedures such as preoperative portal vein embolization,5 biliary drainage,6 and vascular inflow occlusion,7 postoperative morbidity remains substantial.8 Of all complications, liver failure remains the most feared complication following major liver resection, with mortality rates up to 80%.9, 10 Early identification of high-risk patients and recognition of potential complications are essential in order to reduce postoperative morbidity and mortality.
Prothrombin index and plasma bilirubin levels as parameters of liver function have been used for the identification of postoperative liver dysfunction and highly correlate with postoperative outcome.10 In addition, plasma transaminase levels (aspartate transaminase, AST, and alanine aminotransferase, ALT) are measured after liver surgery as markers of hepatocellular injury and have been used as endpoints in several randomized controlled trials.11, 12, 13, 14 However, transaminase levels determined in peripheral blood might not adequately represent actual hepatocellular injury inasmuch as elevated levels might have a multifactorial cause.15, 16 Recently it was reported that postoperative peak transaminase levels were not associated with postoperative outcome.16 Furthermore, transaminase levels did not correlate with the intra-operatively applied duration of vascular inflow occlusion (VIO), but rather with operative duration. These findings introduced some uncertainty regarding the clinical validity of plasma transaminase levels in liver resection patients.
In order to evaluate the clinical value of routine postoperative measurement of serum transaminase levels, we retrospectively analyzed the predictive value of postoperative transaminase levels for postoperative morbidity and mortality following liver resection.
Methods
Patients
All consecutive patients who underwent liver resection between January 2006 and September 2015 at the Academic Medical Center (AMC) in Amsterdam were analyzed. Major resections were defined as resection of three or more liver segments.17 Liver resections for both benign and malignant indications were included in the analysis. Patients without postoperative recorded ALT and AST levels were excluded from the analysis. The need for informed consent was waived by the medical ethics committee because of the retrospective nature of the study.
Liver surgery and postoperative outcomes
Preoperative work-up included diagnostic imaging of the liver lesions and staging of disease when necessary. Baseline laboratory parameters including transaminase levels, INR and bilirubin were routinely determined. The indication for liver surgery was always made at a multidisciplinary meeting. In all patients considered for major liver resection future remnant liver volume was determined and in selected patients hepatobiliary scintigraphy was performed for assessment of liver function. In case of insufficient remnant liver volume, portal vein embolization was used to increase the future remnant liver volume. In case of obstructive cholestasis and jaundice, preoperative biliary drainage was performed of at least the future liver remnant.
During surgery, parenchymal transection was performed using ultrasonic dissection (CUSA, Tyco Healthcare, Mansfield, MA). VIO was not routinely performed but was applied when considered indicated by the operating surgeon. When applied intermittently, VIO was performed using cycles of 20 min occlusion and 10 min of subsequent reperfusion. Intraoperative transfusion was defined as transfusion of any blood products during surgery.
Following major liver resection, transaminase levels in the early morning on postoperative day 1, 3, and 5 along with other routine laboratory parameters were routinely measured according to established care pathways. Where available, patients with minor hepatectomies and available transaminase levels were also included. The peak transaminase level corresponded to the highest ALT or AST concentration measured during the first 5 days after surgery.
Postoperative morbidity was scored and graded according to the Clavien–Dindo classification, with complications of at least grade IIIa considered as major morbidity.18 Admission to the intensive care unit was scored without the routine overnight stay at the postoperative recovery unit. Mortality was defined as death within 90 days after surgery.
Statistical analysis
Normal distribution was tested for continuous variables using the Shapiro–Wilk test. Normally distributed data were analyzed with independent samples t-tests or one-way ANOVA and non-Gaussian data with the Mann–Whitney U test or Kruskal–Wallis test. Correlations were tested using Spearman's rank correlation coefficient. Optimal cut-off value for peak transaminase levels for the prediction of major morbidity and mortality were determined using relative operating characteristic (ROC) curve analysis. Positive predictive value (PPV) was defined as the proportion of patients with the endpoint (morbidity or mortality) with a peak transaminase above the set cut-off. Negative predictive value (NPV) was defined as the proportion of patients without the endpoint when peak transaminase level was below the cut-off. Uni and multivariate analysis for determinants of peak transaminase levels were performed using linear regression. Uni and multivariate analyses for postoperative morbidity and mortality were performed using logistic regression. Benign disease was used as reference since mortality was lowest in these patients. A P-value of <0.05 was considered statistically significant. All statistical analysis was performed using SPSS (IBM, Chicago, IL).
Results
Patients
Between 2006 and 2015, 631 consecutive patients underwent liver resection. Postoperative peak ALT and AST levels were available for 539 patients and these patients were therefore included in the analysis. The majority of patients (312, 58%) had undergone major resection. Of the 92 patients without available transaminase levels 90 underwent a minor hepatectomy and 2 a major hepatectomy.
Baseline patient and disease characteristics are provided in Table 1 and a detailed description is provided in the Supplemental information.
Table 1.
Baseline patient and operative characteristics
| All (n = 539) | |
|---|---|
| Age, median (IQR) | 61 (49–69) |
| Male, n (%) | 293 (54) |
| BMI, kg/m2, median (IQR) | 25 (22–28) |
| Diagnosis, n (%) | |
| Colorectal liver metastases | 193 (36) |
| Hepatocellular carcinoma | 67 (12) |
| Perihilar cholangiocarcinoma | 87 (16) |
| Intrahepatic cholangiocarcinoma | 26 (5) |
| Benign | 122 (23) |
| Other | 44 (8) |
| ASA, n (%) | |
| I | 143 (27) |
| II | 315 (58) |
| III | 80 (15) |
| IV | 1 (0) |
| Portal vein embolization, n (%) | 44 (8) |
| Neoadjuvant chemotherapy, n (%) | 152 (28) |
| Cirrhosis, n (%) | 20 (4) |
| Preoperative biliary drainage, n (%) | |
|
36 (7) |
|
12 (2) |
|
32 (6) |
| Resection, n (%) | |
|
69 (13) |
|
45 (8) |
|
125 (23) |
|
56 (10) |
|
4 (1) |
|
149 (28) |
|
91 (17) |
| Major liver resection, n (%) | 312 (58) |
| Laparoscopic procedure, n (%) | 49 (9) |
| Duration of surgery, min, median (IQR) | 282 (195–388) |
| Additional procedures, n (%) | |
|
19 (4) |
|
87 (16) |
|
7 (1) |
|
2 (0) |
| Vascular inflow occlusion, n (%) | |
|
353 (66) |
|
96 (18) |
|
81 (15) |
|
9 (2) |
| Transfusion, n (%) | 176 (32) |
A total of 238 patients (44%) experienced a postoperative complication, of which 168 (31%) were grade IIIa or higher. Severe morbidity was lowest in patients with benign disease (30/122; 25%) and highest in patients with PHC (43/87; 49%). Thirty two patients (6%) died within 90 days after surgery. Mortality varied among diagnoses from 2% (2/122) in patients with benign disease to 17% (15/87) in patients with PHC (Table 2).
Table 2.
Operative outcomes
| All (n = 539) | |
|---|---|
| Fifty–fifty criteria positive, n (%) | 28 (5) |
| Morbidity, n (%) | |
|
238 (44) |
|
168 (31) |
| ICU admission, n (%) | 86 (16) |
| 90 day mortality, n (%) | 32 (6) |
|
2 |
|
2 |
|
1 |
|
8 |
|
6 |
|
13 |
| Hospital stay, days, median (IQR) | 9 (7–14) |
Postoperative transaminase levels
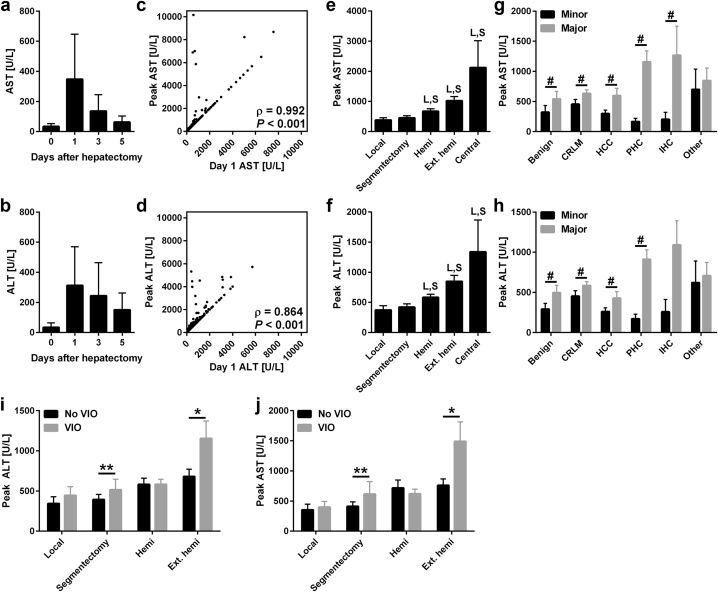
Transaminase levels peaked during the first 24 h after surgery and usually receded towards baseline values 5 days after surgery (Fig. 1a–d). Peak ALT and AST levels depended on the type of resection performed (Fig. 1e and f), the diagnoses (Fig. 1g and H) and the application of VIO (Fig. 1i and j). Postoperative peak ALT and AST levels correlated with intraoperative VIO duration (Spearman's ρ = 0.234 and 0.225, respectively, P < 0.001) and the duration of the operation (Spearman's ρ = 0.442 and 0.498, respectively, P < 0.001). The duration of the operation and VIO duration was also correlated (Spearman's ρ = 0.244, P < 0.001). In multivariate analysis, the duration of the operation, transfusion, and elevated transaminases at baseline predicted peak transaminase levels (Table 3). VIO duration was not a predictor of peak transaminase levels, nor was application of VIO a dichotomous variable in a separate analysis. Separation of intermittent and continuous VIO also did not correlate with peak transaminases.
Figure 1.
Transaminase levels in relation to time, type of resection, diagnosis and vascular inflow occlusion. Postoperative AST (a) and ALT (b) levels before and 1, 3, and 5 days after hepatectomy. Data represent median ± IQR for N = 192–498 per time point. Panels c and d display the correlation of peak AST (c) and ALT (d) with day 1 values. Correlations were tested using Spearman's rank correlation coefficient for N = 539. Panels e and f display the peak AST (e) and ALT (f) according to the extent of resection. L indicates P < 0.05 versus the local resection group and S indicates P < 0.05 compared to the segmentectomy group. Data represent mean ± SEM for N = 4–149 per group. Panels g and h display peak AST (g) and ALT (h) diagnosis and major/minor resection. Data represent mean ± SEM for N = 4–97 per group. # indicates P < 0.05 between major and minor resections within diagnosis groups. Panels i and j demonstrate the peak ALT (i) and AST (j) levels according to the application of VIO in the different resection groups. Central liver resections were left out as N = 2 per group. Differences between groups were analyzed using Kruskal–Wallis or Mann–Whitney U tests. * indicates P < 0.05 and ** indicates P < 0.01. Abbreviations: AST, aspartate transaminase; ALT, alanine aminotransferase; hemi, hemihepatectomy; Ext. hemi, extended hemihepatectomy; CRLM, colorectal liver metastasis; HCC, hepatocellular carcinoma; PHC, perihilar cholangiocarcinoma; IHC, intrahepatic cholangiocarcinoma
Table 3.
Multivariate analysis of factors correlated to peak ALT and AST levels
| Peak ALT |
Peak AST |
|||
|---|---|---|---|---|
| β coefficient | P-value | β coefficient | P-value | |
| Preoperative ALT >100 U/L | 0.12 | 0.006 | – | – |
| Preoperative AST >100 U/L | – | – | 0.18 | 0.001 |
| Major hepatectomy | 0.02 | 0.692 | 0.03 | 0.627 |
| Body surface area | 0.05 | 0.274 | 0.00 | 0.952 |
| Laparoscopic procedure | −0.07 | 0.099 | −0.04 | 0.360 |
| Transfusion | 0.13 | 0.005 | 0.13 | 0.004 |
| Operative duration | 0.22 | 0.001 | 0.19 | 0.001 |
| Pringle duration | 0.05 | 0.238 | 0.06 | 0.214 |
| Neoadjuvant chemotherapy | −0.01 | 0.766 | −0.02 | 0.624 |
| Cirrhosis | −0.02 | 0.640 | −0.01 | 0.851 |
| Preoperative biliary drainage | 0.00 | 0.948 | 0.01 | 0.847 |
Bold P-values indicate statistical significance.
Correlation of peak transaminase levels with postoperative outcomes
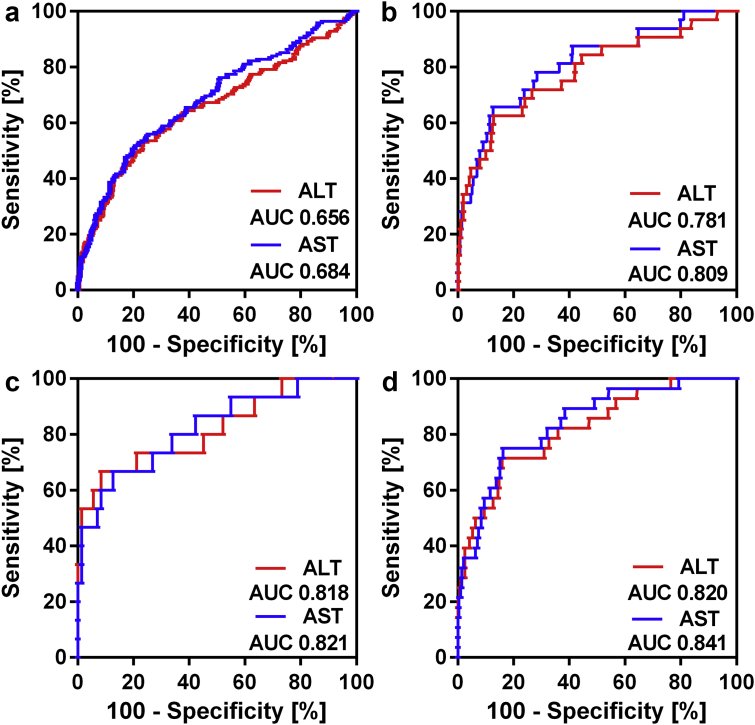
ROC curve analysis was performed to examine the predictive value of peak ALT and AST levels for postoperative morbidity and mortality. AUC values of peak ALT and AST as predictor of major morbidity were 0.656 (0.603–0.709) and 0.684 (0.634–0.734), respectively, which is classified as poor predictive value (Fig. 2). The AUC of peak ALT for 90-day mortality was 0.781 (0.686–0.875), which signifies fair predictive value. The AUC of peak AST for 90-day mortality was 0.809 (0.726–0.893), which signifies good predictive value (Fig. 2).
Figure 2.
Predictive value of transaminases for morbidity and mortality. a: ROC curve for peak ALT and AST for postoperative morbidity. b: ROC curve for peak ALT and AST for postoperative mortality. c: ROC curve for peak ALT and AST for mortality in the PHC patients only. d: ROC curve for peak ALT and AST for mortality in the major resections only. Abbreviations: AST, aspartate transaminase; ALT, alanine aminotransferase
Peak ALT and AST levels were highly predictive for 90-day mortality in several subgroups of patients. In patients with PHC, peak ALT and AST had good predictive value for mortality, with AUC values of 0.82 (0.68–0.95) and 0.82 (0.69–0.95), respectively (Fig. 2). The predictive value of ALT and AST levels was lower for other diagnoses such as CRLM, with AUC levels of 0.75 (0.59–0.91) and 0.77 (0.61–0.93), respectively, albeit at low number of events (N = 5). The predictive value was also better for major resections, with AUC values for peak ALT and AST levels of 0.82 (0.73–0.91) and 0.84 (0.77–0.92), respectively (Fig. 2). Only 4 patients died in the minor resection group and proper analysis could therefore not be performed.
Optimal cut-off values for prediction of postoperative mortality using peak ALT and AST levels in the entire cohort were determined at 812 and 828 U/L, respectively. The PPV of peak ALT level with a cut-off of 812 U/L was 24%, while the NPV was 97%. The cut-off value for AST at 828 U/L performed slightly better with a PPV of 25% and a NPV of 98%. For PHC alone, the cut-off values were 1535 and 1243 U/L for ALT and AST, respectively, resulting in a PPV of 63% and a NPV of 93% for ALT and a PPV of 50% and a NPV of 93% for AST.
Due to the lack of events in the minor resection group, the optimal cut-off values were similar to those for the entire cohort, as for major liver resections alone. Using cut-off values of 812 and 828 U/L for ALT and AST, respectively, the PPV and NPV for mortality were 31% and 97% for ALT, respectively, and 31% and 97% for AST, respectively. Finally, in the group of PHC patients the cut-off values of AST and ALT resulted in a PPV and NPV for mortality of 50% and 93% for AST, respectively, and, 63% and 93% for ALT, respectively.
Considering that peak AST levels had a better predictive value for mortality compared to peak ALT, the cut-off for peak AST was used in the multivariate analysis (Table 4). For the entire cohort, a peak AST level above the cutoff point was an independent predictor for mortality along with age, fifty–fifty criteria, and intraoperative transfusion. The multivariable analysis resulted in a 0.968 significance level using the Hosmer–Lemeshow test, demonstrating good calibration. Despite the poor predictive value of peak ALT and AST for severe morbidity, a peak AST level above 828 U/L was an independent predictor for severe morbidity in conjunction with a major resection and intraoperative transfusion. Hosmer–Lemeshow test yielded a P = 0.421 which again shows good calibration.
Table 4.
Multivariate analysis for postoperative morbidity and mortality
| Morbidity |
Mortality |
|||
|---|---|---|---|---|
| Hazard ratio (95%CI) | P-value | Hazard ratio (95%CI) | P-value | |
| Age | 1.02 (1.00–1.03) | 0.088 | 1.11 (1.06–1.18) | 0.001 |
| Major resection | 2.37 (1.49–3.76) | 0.001 | 1.11 (0.23–4.22) | 0.880 |
| Peak AST >828 U/L | 3.00 (1.75–5.16) | 0.001 | 5.80 (2.2614.93) | 0.001 |
| 50–50 criteria present | 1.65 (0.74–3.65) | 0.221 | 5.76 (2.18–15.18) | 0.001 |
| Transfusion | 1.97 (1.28–3.02) | 0.002 | 5.47 (1.92–15.61) | 0.001 |
| Diagnosis | ||||
|
Reference | 0.716 | Reference | 0.238 |
|
0.70 (0.38–1.31) | 0.269 | 0.44 (0.06–3.36) | 0.435 |
|
0.82 (0.36–1.81) | 0.617 | 0.75 (0.08–6.96) | 0.803 |
|
1.00 (0.48–2.06) | 0.996 | 2.39 (0.37–15.30) | 0.359 |
|
0.75 (0.26–2.12) | 0.584 | 1.26 (0.1213.40) | 0.849 |
|
1.20 (0.54–2.69) | 0.650 | 2.00 (0.24–16.58) | 0.520 |
Bold P-values indicate statistical significance.
Discussion
The present study demonstrated peak AST is a predictor of both postoperative morbidity and mortality and had slightly higher predictive value compared to ALT. Several factors determine peak transaminase levels, including duration of the operation, transfusion, and preoperative elevated transaminase levels. AST levels correlated positively with postoperative mortality, especially in perihilar cholangiocarcinoma and major liver resections.
Plasma transaminase levels are used as postoperative markers of hepatocellular injury and have been used as endpoint in several clinical studies concerning vascular inflow occlusion and liver resection.11, 12, 13, 14 The current analyses validated a recent study demonstrating that VIO is not associated with peak transaminase levels after partial hepatectomy.16 Peak transaminase levels most likely have multifactorial etiology and a single factor such as VIO does probably only have a minor effect on the peak levels. Liver manipulation in itself also can give rise to hepatocellular injury, as previously reported which is most likely a greater determinant of peak levels in longer and major liver surgery since these result in more extensive liver manipulation.19, 20, 21 These could all contribute to the contrasting results in several reports on the predictive value of peak ALT and AST.16, 22
In the present study, postoperative peak ALT and AST levels showed a good discriminative value for postoperative mortality, especially in major liver resections and resections for PHC. Furthermore, the optimal cut-off value for peak AST levels was an independent predictor of 90-day mortality along with major morbidity and age. Therefore, peak transaminase levels seem to have clinical value despite contrasting previous reports.16, 22 In addition, peak AST level was also an independent predictor of postoperative morbidity in the present study, despite a poor positive predictive value of peak AST and ALT for morbidity. AST was more predictive of outcomes compared to ALT, however not significant. Considering operative duration was a major determinant of peak transaminase levels and AST is also present in muscle tissue, overall operative stress rather than liver tissue might be a factor in the predictive value of AST.21 However, higher predictive value of the liver-specific ALT compared to AST in resections for PHC is contradictory to this theory and suggests liver transaminase release is most relevant. Analysis of ALT/AST level ratio did not provide additional insights. The discrepancy could be due to the higher number of events in the PHC patients compared to other diagnoses, which increases the statistical power of the AUC analyses. Alternatively, ALT could be a better predictor compared to AST in PHC patients since most of these patients suffer from obstructive cholestasis and jaundice,23 which could reflect the compromised liver as major determinant of postoperative outcome.24 Most other patients do not suffer from obstructive jaundice and therefore surgical stress itself might be a bigger factor in these patients resulting in higher predictive value of AST over ALT.
The current study does have some limitations. Firstly, the retrospective study design may have introduced selection bias. Although 539 patients between 2006 and 2015 with sufficient study parameters (e.g., ALT and AST) were included, larger cohorts are desired to confirm the current results. Secondly, not every patient was subjected to standardized measurement of peak transaminase levels daily after liver resection. Standardized measurements of transaminase levels, preferably according to structured care pathways, provide more standardized and reliable results. Nevertheless, clear correlations of transaminase levels with postoperative outcomes were presently observed and warrant future studies to confirm the added value of transaminase measurement in the postoperative period. The low costs and minimal invasiveness involved in sampling do provide easy monitoring of high risk surgical patients.
Although peak transaminase levels following liver surgery showed clinical value in the present study, they are most likely not suitable as primary clinical endpoint. Increased transaminase levels have a multifactorial etiology, largely depending on diagnosis, extent of resection, duration of surgery, liver manipulation, and most likely to some extent, on duration of VIO. Although transaminase levels are related to clinical outcomes, the relation is indirect. Endpoints such as postoperative morbidity or hospital stay provide a more solid endpoint and directly relate to patients' outcomes. When taken these observations into account, transaminase levels should be replaced as outcome parameters in order to ensure the relevance of obtained results. Nevertheless, peak ALT and AST levels above 812 and 828 U/L, respectively, have PPV on mortality of 24% and 25%, respectively, and negative predictive values of 97% and 98%, respectively, for 90-day mortality. These estimations potentially have direct clinical value, as these patients can be more intensively observed to adequately anticipate and treat complications. Peak ALT levels might be of special relevance for PHC patients, as a peak value above 1535 U/L had a positive predictive value of 63% for mortality. Considering peak values are most often reached during the first 24 h postoperatively, these values can predict outcome as early as in the first morning after surgery.
Conclusion
Transaminase levels usually peak within 24 h following liver resection and have predictive value for postoperative mortality, especially after major liver resections and resections for perihilar cholangiocarcinoma. Since peak AST above 828 U/L was identified as independent predictor of postoperative morbidity and mortality, this value provides an early window to anticipate potential adverse outcomes and clinically manage these accordingly.
Conflicts of interest
None declared.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2016.07.016.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jarnagin W.R., Gonen M., Fong Y., DeMatteo R.P., Ben-Porat L., Little S. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullen J.T., Ribero D., Reddy S.K., Donadon M., Zorzi D., Gautam S. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862–4. [DOI] [PubMed] [Google Scholar]
- 3.Farges O., Goutte N., Bendersky N., Falissard B., A. CHBT-French Hepatectomy Study Group Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg. 2012;256:697–704. doi: 10.1097/SLA.0b013e31827241d5. discussion 704–5. [DOI] [PubMed] [Google Scholar]
- 4.Andres A., Toso C., Moldovan B., Schiffer E., Rubbia-Brandt L., Terraz S. Complications of elective liver resections in a center with low mortality: a simple score to predict morbidity. Arch Surg. 2011;146:1246–1252. doi: 10.1001/archsurg.2011.175. [DOI] [PubMed] [Google Scholar]
- 5.van Lienden K.P., van den Esschert J.W., de Graaf W., Bipat S., Lameris J.S., van Gulik T.M. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25–34. doi: 10.1007/s00270-012-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacono C., Ruzzenente A., Campagnaro T., Bortolasi L., Valdegamberi A., Guglielmi A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg. 2013;257:191–204. doi: 10.1097/SLA.0b013e31826f4b0e. [DOI] [PubMed] [Google Scholar]
- 7.van Riel W.G., van Golen R.F., Reiniers M.J., Heger M., van Gulik T.M. How much ischemia can the liver tolerate during resection? Hepatobiliary Surg Nutr. 2016;5:58–71. doi: 10.3978/j.issn.2304-3881.2015.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cescon M., Vetrone G., Grazi G.L., Ramacciato G., Ercolani G., Ravaioli M. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995–1002. doi: 10.1097/SLA.0b013e3181a63c74. [DOI] [PubMed] [Google Scholar]
- 9.Shirabe K., Shimada M., Gion T., Hasegawa H., Takenaka K., Utsunomiya T. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304–309. doi: 10.1016/s1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 10.Balzan S., Belghiti J., Farges O., Ogata S., Sauvanet A., Delefosse D. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 828–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scatton O., Zalinski S., Jegou D., Compagnon P., Lesurtel M., Belghiti J. Randomized clinical trial of ischaemic preconditioning in major liver resection with intermittent Pringle manoeuvre. Br J Surg. 2011;98:1236–1243. doi: 10.1002/bjs.7626. [DOI] [PubMed] [Google Scholar]
- 12.Clavien P.A., Selzner M., Rudiger H.A., Graf R., Kadry Z., Rousson V. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850. doi: 10.1097/01.sla.0000098620.27623.7d. discussion 851–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muratore A., Ribero D., Ferrero A., Bergero R., Capussotti L. Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. Br J Surg. 2003;90:17–22. doi: 10.1002/bjs.4055. [DOI] [PubMed] [Google Scholar]
- 14.Park J.B., Joh J.W., Kim S.J., Kwon C.H., Chun J.M., Kim J.M. Effect of intermittent hepatic inflow occlusion with the Pringle maneuver during donor hepatectomy in adult living donor liver transplantation with right hemiliver grafts: a prospective, randomized controlled study. Liver Transpl. 2012;18:129–137. doi: 10.1002/lt.22409. [DOI] [PubMed] [Google Scholar]
- 15.Giovannini I., Chiarla C., Giuliante F., Vellone M., Ardito F., Sarno G. Analysis of the components of hypertransaminasemia after liver resection. Clin Chem Lab Med. 2007;45:357–360. doi: 10.1515/CCLM.2007.078. [DOI] [PubMed] [Google Scholar]
- 16.Boleslawski E., Vibert E., Pruvot F.R., Le Treut Y.P., Scatton O., Laurent C. Relevance of postoperative peak transaminase after elective hepatectomy. Ann Surg. 2014;260:815–820. doi: 10.1097/SLA.0000000000000942. discussion 820–1. [DOI] [PubMed] [Google Scholar]
- 17.Bismuth H. Revisiting liver anatomy and terminology of hepatectomies. Ann Surg. 2013;257:383–386. doi: 10.1097/SLA.0b013e31827f171f. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Broek M.A., Shiri-Sverdlov R., Schreurs J.J., Bloemen J.G., Bieghs V., Rensen S.S. Liver manipulation during liver surgery in humans is associated with hepatocellular damage and hepatic inflammation. Liver Int. 2013;33:633–641. doi: 10.1111/liv.12051. [DOI] [PubMed] [Google Scholar]
- 20.van de Poll M.C., Derikx J.P., Buurman W.A., Peters W.H., Roelofs H.M., Wigmore S.J. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg. 2007;31:2033–2038. doi: 10.1007/s00268-007-9182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannini E.G., Testa R., Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grat M., Holowko W., Lewandowski Z., Kornasiewicz O., Barski K., Skalski M. Early post-operative prediction of morbidity and mortality after a major liver resection for colorectal metastases. HPB. 2013;15:352–358. doi: 10.1111/j.1477-2574.2012.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiggers J.K., Koerkamp B.G., Cieslak K.P., Doussot A., van Klaveren D., Allen P.J. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg. 2016;223:321–331. doi: 10.1016/j.jamcollsurg.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoyama Y., Nagino M., Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepatobiliary Pancreat Surg. 2007;14:159–166. doi: 10.1007/s00534-006-1125-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.