Abstract
Background
Intrahepatic lesions of mixed hepatocellular (HCC) and intrahepatic cholangiocellular carcinoma (ICC) histology are rare. The aim was to describe the natural history of these tumors relative to monomorphic ICC or HCC utilizing the National Cancer Data Base (NCDB).
Methods
Patients with ICC, HCC, and mixed histology (cHCC-CCA) were identified in the NCDB (2004–2012). Inter-group comparisons were made. Kaplan–Meier and multivariable Cox Proportional Hazards analyzed overall survival.
Results
The query identified 90,499 patients with HCC; 14,463 with ICC; and 1141 with cHCC-CCA histology. Patients with cHCC-CCA histology were relatively young (61 vs. 62 (HCC, p = 0.877) and 67 (ICC, p < 0.001) years) and more likely to have poorly differentiated tumor (29.2% vs. 10.3% (HCC) and 17.2% (ICC) p < 0.001). Median overall survival for cHCC-CCA was 7.9 months vs. 10.8 (HCC) and 8.2 (ICC, all p < 0.001). Stage-specific survival for mixed histology tumors was most similar to that of HCC for all stages. cHCC-CCA were transplanted at a relatively high rate, and transplant outcomes for mixed tumors were substantially worse than for HCC lesions.
Discussion
cHCC-CCA demonstrate stage-specific survival similar to HCC, but post-surgical survival more consistent with ICC. Patients with a pre-operative diagnosis of cHCC-CCA should undergo resection when appropriate.
Abbreviations: ICC, intrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma; cHCC-CCA, combined hepatocellular and intrahepatic cholangiocarcinoma; NCDB, National Cancer Data Base; PUF, participant user file; AJCC, American Joint Committee on Cancer; CoC, Commission on Cancer; ACS, American College of Surgeons; SEER, surveillance epidemiology and end results; US, United States
Introduction
Combined hepatocellular and cholangiocarcinoma (cHCC-CCA) is a rare tumor with poor prognosis that arises in the liver. This entity is described as demonstrating histologic features of both the hepatocellular (HCC) and intrahepatic cholangiocarcinoma (ICC) components which comprise it. It is a clinical conundrum due to behavior being a mixture of its components.1 Classifications for these tumors include the Allen & Lisa classification2 and the Goodman classification.3 These pathologic classifications define a spectrum ranging from independent occurrence of both tumor histologies in the same liver to mingling tumors and a distinct subtype resembling fibrolamellar HCC histology. The diagnosis of cHCC-ICC is usually made at pathologic evaluation after either resection or transplantation, and the pre-operative likelihood of identifying a mixed-histology tumor is low.
The largest series suggests that cHCC-CCA represent less than 1% of all primary hepatic malignancies.4, 5, 6, 7 Most literature on this topic is descriptive in nature, consisting of case reports and small series. Consequently, data regarding surgical outcomes for cHCC-CCA are limited. Staging criteria for these tumors are particularly controversial, and due to their mixture of phenotypic characteristics it is unclear whether they should be treated more like HCC or ICC. Currently, clinical consensus opposes the use of transplantation as a therapeutic option for patients with these tumors.8, 9, 10 However, data regarding optimum management of cHCC-ICC remain murky.
This project sought to improve understanding of this topic by comparing the survival of these patients to patients with isolated HCC and ICC. In order to thoroughly investigate this question, the National Cancer Data Base – a national hospital-based datasource in the United States (US) – was utilized.2, 3
Methods
This retrospective review was based on the National Cancer Data Base (NCDB) participant user file (PUF) from 1998 to 2011. The query was focused on 3 patient cohorts: those with (1) intrahepatic cholangiocarcinoma (ICC), (2) hepatocellular carcinoma (HCC), and (3) biphenotypic cHCC-CCA mixed morphology tumors. The Mayo Clinic Institutional Review Board has deemed analysis of the NCDB PUF exempt from review. The NCDB contains over 30 million records of individual cancer patients collected by more than 1500 Commission on Cancer (CoC) approved facilities across the US. The NCDB is estimated to capture approximately 70% of all newly diagnosed patients of cancer in the US.11
Patients were identified using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography (C22.0–22.1) and histology codes (8170–8175 for HCC, 8160–8162 for ICC, and 8180 for cHCC-CCA). Curative intent surgery included surgery of primary site codes 20–26 – wedge resection; 30–38 – Lobectomy; 50–59 extended lobectomy; 60–61 hepatectomy; 65–66 – bile duct excision; and 75 – hepatectomy with transplantation. Patients with surgical codes 0 (no surgery), 10–17 (local tumor destruction), 90 (Surgery, NOS), and 99 (Unknown) were classified as not having curative intent surgery. Only patients who were diagnosed and treated at the performing facility were included. Pathologic TNM staging information is recorded in the NCDB PUF using the American Joint Commission on Cancer (AJCC) 6th and 7th edition staging manuals dependent on year of diagnosis, and the AJCC summary stage variable was used for staging purposes. Patients who developed cancer at more than one site in their lifetime were excluded as were patients with missing follow up or final pathological staging data. Fibrosis was not assessed due to large amounts of missing data on this parameter within the database.
Statistical analysis
For inter-group comparisons, normally distributed continuous data were expressed as mean and standard deviation and examined with the two-tailed student's t-test. Non-normally distributed continuous data were expressed as median with inter-quartile range and were examined with the Mann–Whitney U test. The Pearson's chi-squared was used to examine uniformly distributed categorical variables and Fisher's exact test was used for categorical variables with non-uniform distribution. Missing data were handled with indicator variables as shown in the tables.
Survival analysis was performed using the method of Kaplan and Meier with survival defined as time from diagnosis to death or censor. Survival curves were compared with the log-rank test. NCDB neither provides data on recurrence nor cause of death; therefore overall survival was the primary outcome. To estimate the independent effect on survival, a multivariable Cox proportional hazards model was developed which included age, race, comorbidity score, tumor size, node status (N0/N1), lymphovascular invasion, tumor grade (high/low), pathologic stage (localized/locally advanced/metastatic), CA 19-9 elevation, positive margins, chemotherapy, radiotherapy and surgical intervention (none/local/resection/transplant). A p value <0.05 was considered statistically significant for all comparisons. Statistical analysis was performed with R version 3.2.2 (R Foundation for Statistical Computing – Vienna, Austria www.r-project.org).
Results
The query identified in total 106,103 patients, including 90,499 (85%) patients with HCC, 14,463 (14%) with ICC, and 1141 (1%) with mixed histology. Patients with mixed histology were of similar median [IQR] age compared with HCC (61 [54–71] vs. 62 [53–71] years) but younger than those with ICC (67 [57–76] years p < 0.001). cHCC-ICC and HCC were found more often in male patients, but ICC had approximately equivalent gender distribution. ICC had the greatest prevalence among Caucasian patients (83.8%) whereas cHCC-ICC and HCC had greater prevalence among African Americans (12.4% and 15.4% respectively). The proportion of patients with high comorbidity scores (19.2% with two or more comorbidities – Table 1) was greatest in patients with HCC.
Table 1.
Cohort demographic and surgical characteristics and overall outcomes
| Mixed | HCC | p | ICC | p | |
|---|---|---|---|---|---|
| n | 1141 | 90,499 | 14,463 | ||
| Median [IQR] age | 62 [53–71] | 61 [54–71] | 0.877 | 67 [57–76] | <0.001 |
| Age ≥65 | 445 (39.0%) | 33,789 (37.4%) | 0.267 | 7726 (53.4%) | <0.001 |
| Female gender | 395 (34.6%) | 21,664 (23.9%) | <0.001 | 7200 (49.8%) | <0.001 |
| Race | <0.001 | <0.001 | |||
| White | 885 (77.6%) | 65,235 (72.1%) | 12,127 (83.8%) | ||
| Black | 141 (12.4%) | 13,906 (15.4%) | 1179 (8.2%) | ||
| Other | 115 (10.1%) | 11,358 (12.6%) | 1157 (8.0%) | ||
| Charlson–Deyo score | 0.007 | <0.001 | |||
| 0 | 458 (40.1%) | 33,482 (37.0%) | 6895 (47.7%) | ||
| 1 | 224 (19.6%) | 18,283 (20.2%) | 2025 (14.0%) | ||
| 2+ | 176 (15.4%) | 17,372 (19.2%) | 1047 (7.2%) | ||
| Missing | 283 (24.8%) | 21,362 (23.6%) | 4496 (31.1%) | ||
| Year of diagnosis (%) | 0.04 | <0.001 | |||
| 1998–2002 | 283 (24.8%) | 21,362 (23.5%) | 4496 (31.0%) | ||
| 2003–2011 | 858 (75.2%) | 69,137 (76.5%) | 9967 (69.0%) | ||
| Median [IQR] tumor size (cm) | 5.7 [3.3–9.2] | 5 [3.0–8.3] | <0.001 | 6 [3.5–9.0] | 0.362 |
| Tumor size breakdown (%) | 0.001 | <0.001 | |||
| <3 cm | 156 (13.7%) | 15,846 (17.5%) | 1228 (8.5%) | ||
| 3–5 cm | 180 (15.8%) | 15,849 (17.5%) | 1542 (10.7%) | ||
| 5–10 cm | 279 (24.5%) | 21,862 (24.2%) | 3101 (21.4%) | ||
| >=10 cm | 181 (15.9%) | 12,419 (13.7%) | 1446 (10.0%) | ||
| Missing | 345 (30.2%) | 24,523 (27.1%) | 7146 (49.4%) | ||
| CA 19-9 elevated (% of cases with measured level) | 63 (46.0%) | NA | 1980 (66.3%) | <0.001 | |
| Nodes harvested (% of cases) | 157 (13.6%) | 4268 (4.7%) | <0.001 | 1939 (13.1%) | 0.224 |
| N1 status (% of available) | 34 (19.70%) | 365 (6.50%) | <0.001 | 798 (36.80%) | <0.001 |
| High grade tumor (%) | 333 (29.2%) | 9356 (10.3%) | <0.001 | 2491 (17.2%) | <0.001 |
| Stage (%) | <0.001 | <0.001 | |||
| Localized (Stage 0–2) | 327 (28.7%) | 32,345 (35.7%) | 2744 (19.0%) | ||
| Locally advanced (Stage 3) | 228 (20.0%) | 19,484 (21.5%) | 2758 (19.1%) | ||
| Metastatic (Stage 4) | 337 (29.5%) | 20,256 (22.4%) | 5161 (35.7%) | ||
| Unknown | 249 (21.8%) | 18,414 (20.3%) | 3800 (26.3%) | ||
| Lymphovascular invasion | 26 (2.3%) | 1086 (1.2%) | 0.004 | 261 (1.8%) | 0.24 |
| Margins positive (% of resected) | 22 (5.8%) | 854 (4.8%) | <0.001 | 501 (19.1%) | <0.001 |
| Surgical treatment procedure | <0.001 | <0.001 | |||
| No surgery | 722 (63.3%) | 69,390 (76.7%) | 11,475 (79.3%) | ||
| Local tumor destruction | 20 (1.8%) | 3554 (3.9%) | 108 (0.7%) | ||
| Resection | 245 (21.5%) | 8394 (9.3%) | 2429 (16.8%) | ||
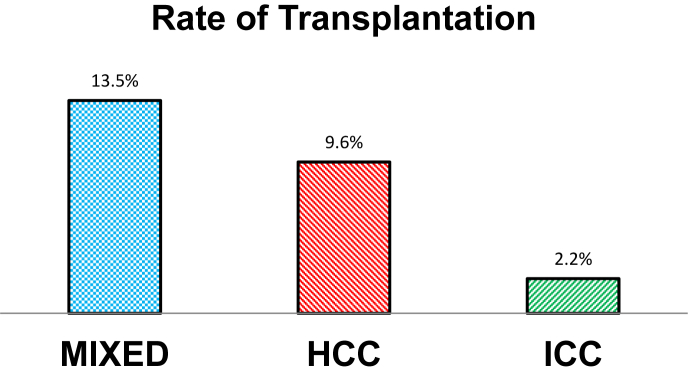
| Transplant | 149 (13.5%) | 8654 (9.6%) | 312 (2.2%) | ||
| Unknown | 5 (0.4%) | 507 (0.6%) | 139 (1.0%) | ||
| Any radiation (%) | 88 (7.7%) | 6076 (6.7%) | 0.082 | 2221 (15.4%) | <0.001 |
| Any chemotherapy (%) | 405 (35.5%) | 30,223 (33.4%) | 0.046 | 5725 (39.6%) | 0.024 |
| Reason for no surgery (% of non-surgical cases) | <0.001 | <0.001 | |||
| Not recommended | 605 (83.6%) | 56,516 (81.2%) | 9226 (80.2%) | ||
| Contraindicated | 63 (8.7%) | 6173 (8.9%) | 1042 (9.1%) | ||
| Patient died prior to surgery | 3 (0.4%) | 388 (0.6%) | 46 (0.4%) | ||
| No reason provided | 13 (1.8%) | 1806 (2.6%) | 285 (2.5%) | ||
| Patient refused surgery | 7 (1.0%) | 1013 (1.5%) | 169 (1.5%) | ||
| Unknown | 2 (0.3%) | 564 (0.8%) | 46 (0.4%) | ||
| Autopsy only | 31 (4.3%) | 3175 (4.6%) | 692 (6.0%) | ||
| Facility type (%) | 0.002 | ||||
| Community cancer program | 86 (7.5%) | 5710 (6.3%) | 1114 (7.7%) | ||
| Comprehensive community cancer program | 403 (35.3%) | 32,732 (36.2%) | 5871 (40.6%) | ||
| Academic/research program | 652 (57.1%) | 52,014 (57.5%) | 7467 (51.6%) | ||
| Other cancer program | (0.0%) | 43 (0.0%) | 11 (0.1%) | ||
| 30 day readmission (%) | 45 (5.3%) | 1853 (2.7%) | <0.001 | 248 (2.5%) | <0.001 |
| Median Length of stay in days [IQR] | 7 [5–11] | 6 [4–10] | 0.010 | 7 [5–10] | 0.719 |
| 30 Day mortality (%) | 26 (6.5%) | 878 (5.1%) | 0.270 | 149 (5.2%) | 0.282 |
| 90 Day mortality (%) | 41 (10.3%) | 1514 (8.7%) | 0.231 | 277 (9.7%) | 0.305 |
| Median OS (months) | |||||
| Stage I | 28.6 | 31.8 | 0.538 | 22.9 | 0.007 |
| Stage II | 24.2 | 22.7 | 0.480 | 14.8 | <0.001 |
| Stage III | 7.5 | 6 | 0.791 | 10.3 | 0.003 |
| Stage IV | 3.1 | 2.8 | 0.123 | 4.6 | 0.078 |
cHCC-ICC had the highest proportion of very large tumors (15.9% > 10 cm). cHCC-ICC and ICC had approximately equal likelihood of node harvests and both were greater than HCC. The rate of node positivity was greatest among patients with ICC (36.8% vs. 19.7% for mixed and 6.5% of HCC lesions). Patients with cHCC-ICC lesions were less likely than those with ICC to have elevated carbohydrate antigen 19-9 (CA 19-9 elevated in 46.0% vs. 66.3%, p < 0.001). cHCC-ICC had the highest frequency of poorly differentiated tumor (29.2% vs. 10.3% (HCC) and 17.2% (ICC), p < 0.001). Stage IV disease was more frequent with ICC than either HCC or cHCC-ICC (35.7% vs. 22.4% and 29.5% respectively, both p < 0.001 – Table 1). The rate of margin positive resections was greatest for ICC (19.1% vs. 5.8% for cHCC-ICC and 4.8% for HCC – Table 1).
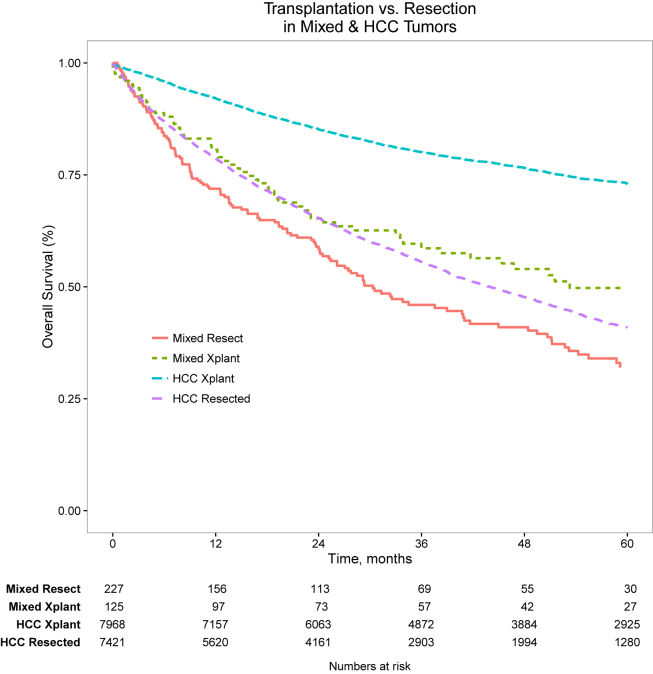
Interestingly liver transplantation was employed more frequently for patients with cHCC-CCA than either ICC or HCC (Fig. 1). Non-operative management was least frequent for patients with cHCC-ICC. Radiation therapy was used most frequently in patients with ICC. The frequency of chemotherapy receipt was similar for all groups. Patients with cHCC-ICC and HCC had treatment in academic medical centers slightly more often than patients with ICC. 30 and 90 day mortality rate and length of stay were similar across groups. The rate of 30-day unplanned readmission was greater for patients with cHCC-ICC than those with either ICC or HCC (Table 1). Median unadjusted overall survival for cHCC-ICC was 7.9 months vs. 10.8 months for HCC and 8.2 months for ICC (p < 0.001). Unadjusted stage-specific survival for cHCC-ICC was similar to that of HCC for all stages (Fig. 2). However, after adjustment for patient, tumor, and treatment factors, the mortality hazard for cHCC-ICC was approximately equivalent to that of ICC and both were greater than for HCC (Table 2). For patients with cHCC-ICC undergoing curative intent surgical resection or transplantation, survival was substantially worse than for patients with HCC (Fig. 3).
Figure 1.
Rate of transplantation by tumor morphology
Figure 2.
Stage-specific survival by tumor type
Table 2.
Cox proportional hazards model
| HR | 95% CI | p | |
|---|---|---|---|
| Morphology (HCC referent) | |||
| Mixed | 1.16 | [1.07, 1.24] | <0.001 |
| ICC | 1.15 | [1.12, 1.18] | <0.001 |
| Age ≥ 65 | 1 | [0.86, 1.15] | 0.95 |
| Race | |||
| White | Referent | ||
| Black | 1.14 | [0.93, 1.39] | 0.21 |
| Other | 0.74 | [0.58, 0.95] | 0.02 |
| Charlson-Deyo Score | |||
| 0 | Referent | ||
| 1 | 1.3 | [1.08, 1.56] | 0.006 |
| 2 | 1.73 | [1.42, 2.12] | <0.001 |
| Tumor Size | |||
| 0–3 cm | Referent | ||
| 3–5 cm | 1.27 | [0.95, 1.70] | 0.11 |
| 5–10 cm | 1.52 | [1.15, 2.01] | <0.001 |
| ≥10 cm | 1.86 | [1.37, 2.54] | <0.001 |
| N1 status | 0.8 | [0.51, 1.27] | 0.35 |
| High grade tumor | 1.21 | [1.00, 1.47] | 0.05 |
| Lymphovascular invasion present | 1.41 | [0.69, 2.87] | 0.34 |
| Stage | |||
| Localized (Stage 0–2) | Referent | ||
| Locally advanced (Stage 3) | 1.5 | [1.20, 1.87] | <0.001 |
| Metastatic (Stage 4) | 1.64 | [1.32, 2.04] | <0.001 |
| Elevated CA 19-9 | 1.33 | [0.76, 2.32] | 0.32 |
| Margins positive | 1.91 | [1.18, 3.09] | 0.009 |
| Did not receive radiation | 1.2 | [0.95, 1.53] | 0.13 |
| Did not receive chemotherapy | 1.67 | [1.44, 1.94] | <0.001 |
| Surgical procedure | |||
| No surgery | Referent | ||
| Local tumor destruction | 0.38 | [0.21, 0.67] | <0.001 |
| Resection | 0.48 | [0.30, 0.77] | 0.002 |
| Transplant | 0.38 | [0.23, 0.63] | <0.001 |
Figure 3.
Transplantation vs. resection for mixed and HCC morphology tumors
Discussion
The present analysis has investigated similarities and differences between patients with biphenotypic liver cancers and patients with isolated HCC or ICC. Patients with biphenotypic cancers have stage-specific unadjusted survival similar to HCC. After adjustment for patient, tumor, and treatment factors, however, their mortality hazard is similar to ICC which may be likely due to adjustment for differences in therapy and the notion that survival for patients with cHCC-ICC is affected more by tumor biology than liver function. According to the National Cancer Data Base, patients with biphenotypic cancers undergo liver transplantation at a greater rate per case than patients with either HCC or ICC. Moreover, the long-term survival outcomes after liver transplantation for cHCC-ICC were greater than after surgical resection. However, long-term outcomes after transplantation for cHCC-CCA remain substantially worse than for HCC.12, 13, 14, 15
Most cancers of the liver are diagnosed based on a combination of clinical and radiographic criteria, without the need for biopsy. Both HCC and ICC have characteristic imaging features.16, 17, 18, 19 The imaging features of cHCC-ICC are often only subtly different9, 20 from HCC and ICC, precluding reliable imaging diagnosis. Moreover, even percutaneous biopsy of cHCC-ICC may be misleading because only the dominant HCC or ICC component of cHCC-ICCC is confirmed pathologically, and the true histopathology of cHCC-ICC can only be confirmed after evaluation of the resected surgical specimen. The most likely reason for the high rate of transplantation among patients with biphenotypic cancers in this study is that the diagnosis is made post-operatively upon evaluation of the complete hepatic specimen. Indeed, given that often the pathologic diagnosis is based only on a biopsy, many patients in the NCDB dataset with a diagnosis of HCC or ICC may never have a resected surgical specimen pathologically reviewed, contributing to under-diagnosis of the disease. One way in which this study provides new information is the fact that a smaller proportion of patients with cHCC-ICC lesions have CA 19-9 elevation, compared to patients with monomorphic ICC tumors. This realization may aid in pre-operative distinction of patients with mixed tumors compared to ICC lesions.
Despite apparent similarity between HCC and cHCC-CCA overall, patients with cHCC-CCA undergoing surgical treatment did significantly worse than those with HCC after both transplantation and resection. This finding underscores the importance of identifying patients with cHCC-CCA pre-operatively and avoiding transplantation as a therapy given their dramatically worse expected survival than HCC after transplantation. The finding of decreased long-term overall survival in patients with cHCC-CCA after equivalent surgical therapy suggests that outcomes are more likely affected by specific cancer biology in these patients than underlying liver dysfunction which would be expected for patients with HCC.
Limitations
This study is limited by its retrospective and non-randomized nature. Use of NCDB data does not allow for re-review of pathologic specimens or cross-sectional imaging. In many patients where the diagnosis of mixed morphology is made after transplantation, it is likely that there was no pre-operative knowledge of the diagnosis. An attempt has been made to adjust for selection bias and confounding through utilization of multivariable modeling and subgroup analysis of cohorts which are as homogeneous as possible. However, unobservable confounding likely remains. Finally, there may be some misclassification bias in that some patients with a non-surgical diagnosis of HCC or ICC may have had occult mixed morphology tumors which were not identified pathologically. The NCDB lacks granularity to further differentiate mixed tumors into the subsets suggested by Allen et al.2 and Goodman et al.,3 so a separate analysis of collision vs. transitional tumors was not possible in this study. Finally, given the fact that the vast majority of cHCC-CCA lesions are identified only after surgical resection, it remains unclear how many non-surgical cHCC-CCA patients may have been clinically classified as either HCC or ICC lesions with no pathologic diagnosis.
Conclusions
Mixed morphology hepatic tumors demonstrate unadjusted stage-specific survival more similar to HCC than to ICC, suggesting that HCC staging criteria may be most useful. However, after adjustment for patient, tumor, and treatment factors, survival for mixed morphology lesions more closely approximated that of ICC lesions. Transplantation outcomes for mixed morphology lesions remain well below expectations for transplantation in HCC. In patients where a pre-operative diagnosis of mixed tumor is known, the present analysis suggests that patients be treated with liver resection when possible, and otherwise could be considered for transplantation where clinical protocols for multimodal treatment of ICC are being undertaken.
Funding sources
The Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery provides salary support for Drs. Habermann and Ivanics and in kind material support for Drs. Bergquist and Shubert. Drs. Bergquist and Shubert have received salary support from the Mayo Clinic Clinician Investigator Training program. No specific grants are associated with this work.
Conflicts of interest
None declared.
Acknowledgment
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used are derived from a de-identified NCDB participant user file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methods or the conclusions drawn from these data by the investigators.
The authors gratefully acknowledge the support of the Mayo Clinic Department of Surgery and the Kern Center for the Science of Health Care Delivery as substantial contributors of in-kind resources to the project. Additionally, Dr. Shubert and Dr. Bergquist acknowledge the support of the Mayo Clinic Clinician Investigator Training Program for their salary support. Finally, we would like to thank the American College of Surgeons for affording us the opportunity to present this work at their Clinical Congress in 2015.
Footnotes
This work has not previously or concurrently been submitted for publication. The work was presented as a poster during the Scientific Forum at the American College of Surgeons Clinical Congress in Chicago, IL October 2015.
References
- 1.Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system.
- 2.Allen R.A., Lisa J.R. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949 Jul;25:647–655. [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman Z.D., Ishak K.G., Langloss J.M., Sesterhenn I.A., Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985 Jan 1;55:124–135. doi: 10.1002/1097-0142(19850101)55:1<124::aid-cncr2820550120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Kim K.H., Lee S.G., Park E.H., Hwang S., Ahn C.S., Moon D.B. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann Surg Oncol. 2009;16:623–629. doi: 10.1245/s10434-008-0278-3. [DOI] [PubMed] [Google Scholar]
- 5.Liver Cancer Study Group of Japan Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 6.Wachtel M.S., Zhang Y., Xu T., Chiriva-Internati M., Frezza E.E. Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin Med Pathol. 2008;1:43–47. doi: 10.4137/cpath.s500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Wang F., Kessinger A. Outcome of combined hepatocellular and cholangiocarcinoma of the liver. J Oncol. 2010;2010:15–19. doi: 10.1155/2010/917356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groeschl R.T., Turaga K.K., Gamblin T.C. Transplantation versus resection for patients with combined hepatocellular carcinoma-cholangiocarcinoma. J Surg Oncol. 2013 May 1;107:608–612. doi: 10.1002/jso.23289. [DOI] [PubMed] [Google Scholar]
- 9.Panjala C., Senecal D.L., Bridges M.D., Kim G.P., Nakhleh R.E., Nguyen J.H.H. The diagnostic conundrum and liver transplantation outcome for combined hepatocellular-cholangiocarcinoma. Am J Transplant. 2010 May;10:1263–1267. doi: 10.1111/j.1600-6143.2010.03062.x. [DOI] [PubMed] [Google Scholar]
- 10.Vilchez V., Shah M.B., Daily M.F., Pena L., Tzeng C.-W.D., Davenport D. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB. 2016;18:29–34. doi: 10.1016/j.hpb.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raval M.V., Bilimoria K.Y., Stewart A.K., Bentrem D.J., Ko C.Y. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 12.Meyer C.G., Penn I., James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 13.Shimoda M., Farmer D.G., Colquhoun S.D., Rosove M., Ghobrial R.M., Yersiz H. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl. 2001 Dec;7:1023–1033. doi: 10.1053/jlts.2001.29419. [DOI] [PubMed] [Google Scholar]
- 14.De Jong M.C., Nathan H., Sotiropoulos G.C., Paul A., Alexandrescu S., Marques H. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen K.T., Steel J., Vanounou T., Tsung A., Marsh J.W., Geller D.A. Initial presentation and management of hilar and peripheral cholangiocarcinoma: is a node-positive status or potential margin-positive result a contraindication to resection? Ann Surg Oncol. 2009;16:3308–3315. doi: 10.1245/s10434-009-0701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruix J., Sherman M., Llovet J.M., Beaugrand M., Lencioni R., Burroughs A.K. Clinical management of hepatocellular carcinoma. Conclusions of the barcelona-2000 EASL conference. J Hepatol. 2001:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 17.Torzilli G., Minagawa M., Takayama T., Inoue K., Hui A.M., Kubota K. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 18.Blechacz B., Gores G.J. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008 Jul;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimola J., Forner A., Reig M., Vilana R., de Lope C.R., Ayuso C. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791–798. doi: 10.1002/hep.23071. [DOI] [PubMed] [Google Scholar]
- 20.Kassahun W.T., Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008:1271–1278. doi: 10.1111/j.1742-1241.2007.01694.x. [DOI] [PubMed] [Google Scholar]