Abstract
Integral membrane proteins in bacteria are co‐translationally targeted to the SecYEG translocon for membrane insertion via the signal recognition particle (SRP) pathway. The SRP receptor FtsY and its N‐terminal A domain, which is lacking in any structural model of FtsY, were studied using NMR and fluorescence spectroscopy. The A domain is mainly disordered and highly flexible; it binds to lipids via its N terminus and the C‐terminal membrane targeting sequence. The central A domain binds to the translocon non‐specifically and maintains disorder. Translocon targeting and binding of the A domain is driven by electrostatic interactions. The intrinsically disordered A domain tethers FtsY to the translocon, and because of its flexibility, allows the FtsY NG domain to scan a large area for binding to the NG domain of ribosome‐bound SRP, thereby promoting the formation of the quaternary transfer complex at the membrane.
Keywords: biophysics, intrinsically disordered proteins, NMR spectroscopy, protein–protein interactions, translocon
Co‐translational targeting of nascent membrane proteins to the endoplasmic reticulum of eukaryotic cells or the plasma membrane of bacteria is elicited via the signal recognition particle (SRP) pathway. SRP rapidly scans translating ribosomes and targets those synthesizing membrane proteins to the protein‐conducting channel (SecYEG in bacteria), located in the membrane, by an interaction with the SRP receptor, FtsY in bacteria. FtsY must be bound to the membrane to be functional,1 that is, to recruit SRP and form the transfer complex at the SecYEG translocon.2 Two regions of FtsY, the N‐terminal A domain and a region at the interface between A and N domain (membrane targeting sequence, MTS) (Figure 1 a), are involved in lipid binding as shown by cross‐linking experiments.1a, 3 Cross‐linking experiments also indicated that the A domain binds to the exposed cytosolic C4/C5 loops of SecY.4 Unlike the NG domain, which is fully structured,5 the N‐terminal A domain is lacking in any structural model of FtsY, unbound or in complex with the translocon SecYEG or with SRP bound to a ribosome‐nascent‐chain complex (RNC). Therefore, and based on H/D exchange experiments, the A domain of FtsY was suggested to be intrinsically disordered.3c, 6
Figure 1.
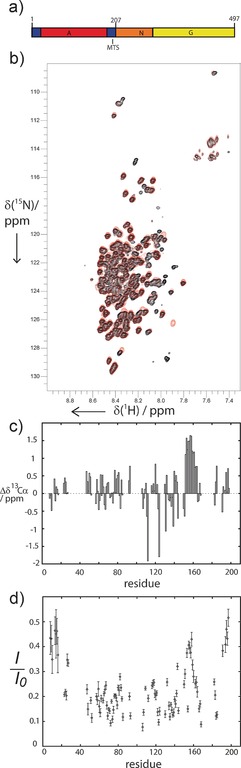
NMR spectroscopy of FtsY and FtsY‐A207. a) Domain structure of FtsY. b) Overlay of 1H–15N 2D TROSY–HSQC spectra of 2H15N13C‐enriched FtsY (red) and FtsY‐A207 (black). c) Cα secondary chemical shifts of FtsY‐A207. d) {1H}‐15N NOE values (I/I 0) for assigned residues of FtsY‐A207.
Herein we use solution NMR spectroscopy and fluorescence measurements to study FtsY and interactions of the A domain with membrane phospholipids and SecYEG embedded in E. coli phospholipids containing nanodiscs. We show with single‐residue resolution that the A domain is highly flexible and exhibits properties of an intrinsically disordered protein (IDP).7 Our data indicate that the positively charged N‐terminal region of the A domain and the C‐terminal membrane targeting sequence (MTS) binds to phospholipids, presumably adopting an α‐helical conformation. Interestingly, we find that the negatively charged central region of the A domain binds to the translocon non‐specifically, most likely mediated by electrostatic interactions with the positively charged cytoplasmic loops of SecY and maintaining intrinsic disorder.
2H15N13C‐labeled FtsY as well as a construct comprising the A domain only, FtsY‐A207, were studied by solution NMR spectroscopy (Supporting Information). A comparison of 1H15N TROSY‐HSQC spectra indicates that only the A domain is visible in the spectra (Figure 1 b, see Supporting Information for further details). Low dispersion of resonances in the 1H dimension points to intrinsic disorder of the A domain. Spectral resonance assignment (Supporting Information, Figure S1) and secondary structure analysis were performed as described in detail in the Supporting Information. Cα secondary chemical shifts (Figure 1 c) overall show close to random‐coil chemical shifts scattering around zero, which clearly indicates intrinsic disorder of the A domain. An exception is the region Asp152 to Ala161 where positive Cα secondary chemical shifts between 1.1 and 1.6 ppm reveal an increased propensity for α helix formation. For a fully structured α helix, positive Cα secondary chemical shifts greater than 3 ppm would be expected, suggesting that α helices in region 152–161 are formed transiently. Strongly negative outliers (positions 112 and 124) can be attributed to nearest‐neighbor effects of neighboring proline residues.8
To investigate the dynamic properties of FtsY‐A207, we performed 15N NMR relaxation experiments using TROSY‐detection,9, 10 including 15N R1, R2 (R1ρ) relaxation experiments and {1H}‐15N NOE measurements, which report on rapid internal dynamics in the pico‐ to nanosecond time range. Low {1H}‐15N NOE values (Figure 1 d) and low 15N R1 and R2 rates (Supporting Information, Figure S2) indicate that residues in the region Ile38 to Ala140 of the A domain are highly dynamic, as expected for an intrinsically disordered region. By contrast, the N‐terminal region (Gly8 to Lys18), the region Asp152 to Ala161, and the visible part of the membrane targeting sequence (MTS) at the C terminus of the A domain (Glu188 to Ala197) exhibit higher {1H}‐15N NOE values and higher 15N R1 and/or R2 rates, indicating the presence of transient secondary structure.
Binding of the A domain to phospholipids in empty nanodiscs lacking SecYEG was quantified by equilibrium titration monitored by the fluorescence change of an NBD label attached to FtsY‐A207 at position 26 (Figure 2 a; Supporting Information). The binding is of low affinity with a K d in the μm range, similar to the binding of full‐length FtsY to empty nanodiscs studied previously.11
Figure 2.

Phospholipid interactions of FtsY‐A207. a) Binding of NBD‐labeled FtsY‐A207 to empty lipid nanodiscs monitored by NBD fluorescence (Supporting Information). Error margins indicate SEM (n=2) and are smaller than the symbols in several cases. b) Resonance intensities in the 1H–15N TROSY–HSQC spectra of 2H13C15N‐labeled FtsY‐A207 measured in the presence of lipid nanodiscs (I ND) (Supporting Information, Figure S3b) are plotted relative to those recorded for the sample without nanodiscs (I free) (Figure S3 a). For the plot of absolute intensities, see Figure S4 a.
To examine whether there are specific effects of lipid binding on individual residues of FtsY‐A207, we recorded 1H–15N TROSY–HSQC spectra of FtsY‐A207 in the presence of empty lipid nanodiscs and compared resonance intensities to a sample without nanodiscs (IND/Ifree, Figure 2 b; Supporting Information, Figures S3 a, b, S4 a). The central part of FtsY‐A207 (Ile38 to Ala161) shows resonance intensity ratios close to 1 (Figure 2 b), which indicates that this part of the A domain does not interact with lipids. However, the N‐terminal region (Gly8 to Lys18) shows reduced resonance intensities for FtsY‐A207 in the presence of nanodiscs, suggesting an immobilization by interaction with lipids. This observation is in line with cross‐linking data.12 The most extensive reduction of resonance intensities, indicating strong binding, is observed for the region around Leu13 at the N terminus, as well as for residues belonging to the MTS at the C terminus of the A domain which disappear completely (Figure 2 b), indicating extensive lipid binding of that region.1a The observed intensity decrease points to an equilibrium between two populations of FtsY‐A207, one bound to lipids (not visible by solution NMR, because of the slow overall tumbling of the lipid nanodisc14 and concomitant fast decay of transverse magnetization) and one unbound (visible by NMR), which are in slow exchange on the millisecond time scale. Lipid binding of the N‐terminus is likely connected to α‐helix formation.12 A population shift towards α‐helical conformation caused by lipid binding was observed for several IDPs.7c, 15
We monitored binding of FtsY‐A207 to the translocon SecYEG, embedded in phospholipid nanodiscs, by FRET between an MDCC label in SecY (position 111) and BODIPY FL (Bpy) at position 167 in FtsY‐A207 (Figure 3 a; Supporting Information); for comparison, the binding of full‐length FtsY(Bpy167) was examined in parallel. For both, FtsY and FtsY‐A207, K d values of 0.18 μm were obtained; the value for FtsY is in accordance with recent measurements.11 The observation of the same affinity for FtsY and FtsY‐A207 binding to SecYEG suggests that the A domain has an important stabilizing role.
Figure 3.
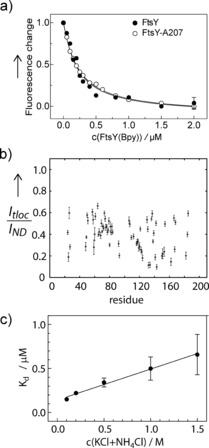
FtsY binding to SecYEG. a) FRET titrations. SecYEG labeled with MDCC at position S111C of SecY was titrated with FtsY‐A207 (○) or full‐length FtsY (•), both labeled with the acceptor fluorophore BODIPY FL (Bpy) at position A167C. The normalized fluorescence change of MDCC is plotted. For visual clarity representative error margins (SEM; n=2) are indicated for the last titration points only. Fitting to equation 1 (Supporting Information) yields K d=0.18±0.02 μm for both FtsY and FtsY‐A207. b) Resonance intensities in 1H–15N TROSY–HSQC spectra of 2H13C15N‐labeled FtsY‐A207 measured in the presence of SecYEG (I tloc; Supporting Information, Figure S3c) are plotted relative to those recorded for the reference sample with empty nanodiscs (I ND) (Figure S3 b). For the plot of absolute intensities, see Figure S4 b. c) Ionic‐strength dependence of FtsY‐A207 binding to SecYEG. Titrations of SecYEG(MDCC) in nanodiscs with FtsY‐A207(Bpy) were performed as in (a), starting with buffer A containing 30 mm NH4Cl plus 70 mm KCl. K d values were determined from titrations performed at increasing concentration of KCl added to buffer A. Error margins are SEM (n=2) and are smaller than the symbols for the points at 0.1 and 0.2 m salt.
15N–1H TROSY‐HSQC spectra of FtsY‐A207 show a strong reduction of resonance intensities for the central and C‐terminal part of the A domain in the presence of SecYEG embedded in lipid nanodicscs (Figure 3 b; Supporting Information, Figures S3 c, S4 b). As this effect was not observed for empty lipid nanodiscs, it indicates binding of the A domain to SecYEG. There is an equilibrium between unbound (visible) and bound (invisible) FtsY‐A207, and slow exchange between the two conformations on a millisecond time scale. As the reduction of resonance intensities varies for different residues, the conformational change most likely does not reflect secondary structure formation upon binding, as a more uniform decrease would be expected if the bound population was helical, experiencing similar dynamics. The binding of the central A domain seems to be transient and non‐specific, with the central disordered A domain sampling an extended interaction surface on SecY. N‐terminal resonances of FtsY‐A207 (Gly8 to Lys18) entirely disappear in the spectra in the presence of the translocon (Figure 3 b; Supporting Information, Figures S3 c, S4 b), indicating enhanced binding, presumably to lipids in the vicinity of the translocon.
The A domain of FtsY is highly charged (pI=4.08). The central region between residues Phe15 and Glu188 is negatively charged (pI=3.72), owing to the high abundance of glutamic acid (25 % of all residues), whereas the N terminus (Met1 to Gly14) is positively charged, containing three lysine residues (positions 3, 5, and 6) and one arginine (position 7; Supporting Information, Figure S5). NMR data indicate that FtsY is anchored on the negatively charged phospholipid bilayer by an interaction with its positively charged N terminus. The same applies for the positively charged C terminus of the A domain (Lys189 to Lys207), in line with previous data.12, 13 Binding of FtsY to anionic lipids has been observed previously.16 In contrast to the negatively charged lipid environment, the translocon SecYEG is highly positively charged (pI around 10), particularly in the exposed C4/C5 loop region (Supporting Information, Figure S6). We observe non‐specific binding of the negatively charged central A domain to the positively charged translocon, suggesting that electrostatic interactions are the driving force for FtsY binding to the translocon. A substantial contribution of electrostatic interactions to the binding affinity is supported by the observed reduction of binding affinity (increase in K d) at increasing ionic strength of the buffer (which will weaken electrostatic interactions between the A domain and the translocon; Figure 3 c).
We propose that the A domain tethers the RNC‐SRP‐FtsY complex to the translocon, promoting the formation of a quaternary transfer complex (Figure 4). The flexibility of the A domain allows the NG domain to scan a large area for binding to the NG domain of RNC‐bound Ffh/SRP, such that it can recruit an RNC to the translocon by forming the heterodimeric complex of the homologous NG domains17, 18 of translocon‐bound FtsY and RNC‐bound SRP. Thus the A domain of FtsY provides an example for an intrinsically disordered protein region that has a tethering function in a multicomponent protein assembly and illustrates how simple physical principles, such as electrostatic interactions, can drive complex cellular processes, such as the assembly of the quaternary transfer complex at the membrane.
Figure 4.
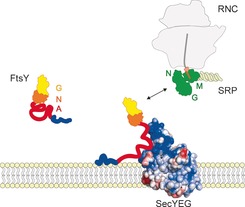
Model of FtsY binding to phospholipids and SecYEG. The positively charged N terminus of the A domain (blue) is attracted to the negatively charged lipids and establishes contact with the membrane by a combination of electrostatic and hydrophobic interactions. Electrostatic attraction between the negatively charged central A domain (red) and the positively charged and exposed C4/C5 loop region (blue) of the translocon SecYEG directs FtsY to the translocon. The NG domain of translocon‐bound FtsY searches for the NG domain of RNC‐bound SRP/Ffh (green) to initiate quaternary transfer complex formation by establishing interactions between the NG domains of FtsY and Ffh.17, 18
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank Franziska Hummel and Anna Pfeifer for expert technical assistance and Wolf Holtkamp for very helpful discussions. This work was supported by the DFG/NIH Research Career Transition award program (to N.‐A.L.), (grant number DFG LA 2724/1‐1).
N.-A. Lakomek, A. Draycheva, T. Bornemann, W. Wintermeyer, Angew. Chem. Int. Ed. 2016, 55, 9544.
Contributor Information
Dr. Nils‐Alexander Lakomek, Email: nils-alexander.lakomek@phys.chem.ethz.ch
Prof. Dr. Wolfgang Wintermeyer, Email: wolfgang.wintermeyer@mpibpc.mpg.de
References
- 1.
- 1a. Weiche B., Burk J., Angelini S., Schiltz E., Thumfart J. O., Koch H. G., J. Mol. Biol. 2008, 377, 761–773; [DOI] [PubMed] [Google Scholar]
- 1b. Bercovich-Kinori A., Bibi E., J. Cell Sci. 2015, 128, 1444–1452. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Neumann-Haefelin C., Schafer U., Muller M., Koch H. G., EMBO J. 2000, 19, 6419–6426; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b. Valent Q. A., Scotti P. A., High S., de Gier J. W., von Heijne G., Lentzen G., Wintermeyer W., Oudega B., Luirink J., EMBO J. 1998, 17, 2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. Angelini S., Boy D., Schiltz E., Koch H. G., J. Cell Biol. 2006, 174, 715–724; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Parlitz R., Eitan A., Stjepanovic G., Bahari L., Bange G., Bibi E., Sinning I., J. Biol. Chem. 2007, 282, 32176–32184; [DOI] [PubMed] [Google Scholar]
- 3c. Stjepanovic G., Kapp K., Bange G., Graf C., Parlitz R., Wild K., Mayer M. P., Sinning I., J. Biol. Chem. 2011, 286, 23489–23497; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3d. Burk J., Weiche B., Wenk M., Boy D., Nestel S., Heimrich B., Koch H. G., J. Bacteriol. 2009, 191, 7017–7026; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3e. Braig D., Bar C., Thumfart J. O., Koch H. G., J. Mol. Biol. 2009, 390, 401–413. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Angelini S., Deitermann S., Koch H. G., EMBO Rep. 2005, 6, 476–481; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. Kuhn P., Weiche B., Sturm L., Sommer E., Drepper F., Warscheid B., Sourjik V., Koch H. G., Traffic 2011, 12, 563–578. [DOI] [PubMed] [Google Scholar]
- 5. Montoya G., Svensson C., Luirink J., Sinning I., Nature 1997, 385, 365–368. [DOI] [PubMed] [Google Scholar]
- 6. Stjepanovic G., Kapp K., Bange G., Graf C., Parlitz R., Wild K., Mayer M. P., Sinning I., J. Biol. Chem. 2011, 286, 23489–23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.
- 7a. Tompa P., Schad E., Tantos A., Kalmar L., Curr. Opin. Struct. Biol. 2015, 25, 49–59; [DOI] [PubMed] [Google Scholar]
- 7b. Wright P. E., Dyson H. J., J. Mol. Biol. 1999, 293, 321–331; [DOI] [PubMed] [Google Scholar]
- 7c. Wright P. E., Dyson H. J., Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y., Jardetzky O., J. Am. Chem. Soc. 2002, 124, 14075–14084. [DOI] [PubMed] [Google Scholar]
- 9. Lakomek N. A., Ying J., Bax A., J. Biomol. NMR 2012, 53, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lakomek N. A., Kaufman J. D., Stahl S. J., Louis J. M., Grishaev A., Wingfield P. T., Bax A., Angew. Chem. Int. Ed. 2013, 52, 3911–3915; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 4003–4007. [Google Scholar]
- 11. Kuhn P., Draycheva A., Vogt A., Petriman N. A., Sturm L., Drepper F., Warscheid B., Wintermeyer W., Koch H. G., J. Cell Biol. 2015, 211, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braig D., Bar C., Thumfart J. O., Koch H. G., J. Mol. Biol. 2009, 390, 401–413. [DOI] [PubMed] [Google Scholar]
- 13. Parlitz R., Eitan A., Stjepanovic G., Bahari L., Bange G., Bibi E., Sinning I., J. Biol. Chem. 2007, 282, 32176–32184. [DOI] [PubMed] [Google Scholar]
- 14. Hagn F., Etzkorn M., Raschle T., Wagner G., J. Am. Chem. Soc. 2013, 135, 1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.
- 15a. Arai M., Sugase K., Dyson H. J., Wright P. E., Proc. Natl. Acad. Sci. USA 2015, 112, 9614–9619; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b. Bah A., Vernon R. M., Siddiqui Z., Krzeminski M., Muhandiram R., Zhao C., Sonenberg N., Kay L. E., Forman-Kay J. D., Nature 2015, 519, 106–109; [DOI] [PubMed] [Google Scholar]
- 15c. Schneider R., Maurin D., Communie G., Kragelj J., Hansen D. F., Ruigrok R. W., Jensen M. R., Blackledge M., J. Am. Chem. Soc. 2015, 137, 1220–1229. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. de Leeuw E., te Kaat K., Moser C., Menestrina G., Demel R., de Kruijff B., Oudega B., Luirink J., Sinning I., EMBO J. 2000, 19, 531–541; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Reinau M. E., Thogersen I. B., Enghild J. J., Nielsen K. L., Otzen D. E., Biopolymers 2010, 93, 595–606. [DOI] [PubMed] [Google Scholar]
- 17. Egea P. F., Shan S. O., Napetschnig J., Savage D. F., Walter P., Stroud R. M., Nature 2004, 427, 215–221. [DOI] [PubMed] [Google Scholar]
- 18. Focia P. J., Shepotinovskaya I. V., Seidler J. A., Freymann D. M., Science 2004, 303, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


