Abstract
Objective
To determine the proportion of patients with axial spondyloarthritis (SpA) among those with chronic back pain and ≥1 of 3 SpA features in the US.
Methods
The study was conducted at rheumatology practices in the US. Patients were required to have chronic back pain for ≥3 months beginning at <45 years of age, no prior SpA diagnosis, and ≥1 of the following 3 SpA features: HLA–B27 positivity, current inflammatory back pain, and magnetic resonance imaging (MRI) or radiographic evidence of sacroiliitis. Medical history and physical examination findings, pelvic radiographs, MRIs of sacroiliac joints, C‐reactive protein (CRP) level, and HLA–B27 status were obtained. Investigators were asked if a clinical diagnosis of axial SpA could be made based on the results. Data were also analyzed separately to determine if patients fulfilled the Assessment of SpondyloArthritis international Society (ASAS) criteria for axial SpA and/or modified New York criteria for ankylosing spondylitis (AS).
Results
A total of 751 patients were enrolled (46% were existing patients in rheumatology practices, 40% were new referrals, and 14% were self referred). Among patients with available data, 319 of 697 (46%) were diagnosed as having axial SpA by the investigator, and 348 of 744 (47%) fulfilled the ASAS criteria, of whom 238 were classified as having nonradiographic axial SpA and 108 as having AS; 2 had missing data. Using investigator's clinical diagnosis as the gold standard, the specificity and sensitivity of the ASAS criteria were 79% and 81%, respectively.
Conclusion
Our findings indicate that among patients with chronic back pain for ≥3 months beginning at ages younger than 45 years, the presence of ≥1 of 3 SpA features is an effective way to identify those with possible axial SpA.
Axial spondyloarthritis (SpA) is a chronic inflammatory disease that primarily affects the sacroiliac joints and spine but can also involve entheses and peripheral joints. It encompasses both ankylosing spondylitis (AS) and nonradiographic axial SpA, which are differentiated based upon the presence or absence of radiographic sacroiliitis, respectively, fulfilling the 1984 modified New York criteria for AS 1, 2. Both AS and nonradiographic axial SpA typically go undiagnosed for many years; however, although AS and nonradiographic axial SpA have comparable clinical manifestations, AS is more easily identified by the presence of sacroiliitis on radiographs 2, 3. Nonradiographic axial SpA may affect as many women as men, whereas AS more often affects men, which also contributes to a delay in diagnosis in women who have axial SpA 4, 5, 6.
There is limited information on the epidemiology of axial SpA in the US because most of the published literature has focused mainly on AS 7, and there has been only one other study using the Assessment of SpondyloArthritis international Society (ASAS) criteria 8, 9. Based on a recent National Health and Nutrition Examination Survey, the age‐adjusted prevalence of axial SpA in the US has been estimated to be 0.9–1.4% using the Amor criteria 10 and European Spondylarthropathy Study Group criteria 11, 12. These analyses did not use the classification criteria developed by ASAS for patients with axial SpA 13, 14, and available data did not allow for prevalence information to be broken down for AS versus nonradiographic axial SpA.
The delay to diagnosis of axial SpA can be partly attributed to delayed referral to rheumatologists 4, 15, 16, 17. The Multicenter Ankylosing Spondylitis Survey Trial to Evaluate and Compare Referral Parameters in Early SpA (MASTER) study, which was conducted in Germany, demonstrated that among undiagnosed patients with chronic back pain starting before the age of 45 years, the presence of inflammatory back pain, HLA–B27, and/or sacroiliitis on imaging is a reliable screening method for axial SpA for orthopedists and primary care physicians 18. Based on these results, there was interest in understanding if this would also apply to patients in the US. The present study, the Prevalence of Axial SpA (PROSpA) study, was then conducted to determine the proportion of patients with nonradiographic axial SpA among those with chronic back pain for ≥3 months with age at onset of <45 years, and ≥1 of the following features: HLA–B27 positivity, current inflammatory back pain, and imaging (magnetic resonance imaging [MRI] or radiographic) evidence of sacroiliitis. The proportions of patients with AS and those with axial SpA (AS and nonradiographic axial SpA) were also evaluated. Data were collected to descriptively characterize axial SpA patients and to compare US rheumatologists’ expert diagnosis of axial SpA with fulfillment of the ASAS criteria.
PATIENTS AND METHODS
Patients
Patients were new referrals to rheumatologists from other physicians, self referred, or existing patients at the investigative site and had not previously been diagnosed as having any type of SpA (Figure 1). Eligible patients were 18 years of age or older, had chronic back pain for ≥3 months with age at onset of <45 years, and had ≥1 of the following SpA‐related features: HLA–B27 positivity, current inflammatory back pain, and prior imaging (MRI or radiographic) evidence of sacroiliitis. Current inflammatory back pain was defined as the presence of at least 4 of the following 5 parameters: age at onset <40 years, insidious onset, improvement with exercise, no improvement with rest, and pain at night with improvement upon getting up 19. HLA–B27 or imaging results were not required at study entry if the patient fulfilled the inflammatory back pain criteria. Patients were excluded if they had previously been diagnosed as having any type of SpA (e.g., AS, psoriatic arthritis, or reactive arthritis). All prior medications used to treat back pain were recorded, including the maximum dose and duration of therapy, if known.
Figure 1.
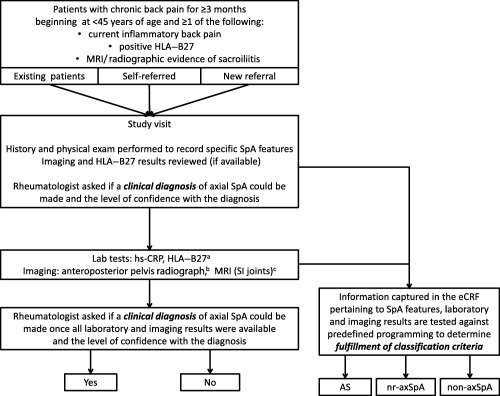
Prevalence of Axial SpA (PROSpA) study methodology. a = Laboratory tests for high‐sensitivity C‐reactive protein (hsCRP) level and HLA–B27 status were conducted if they had not been done previously. b = Anteroposterior pelvis radiographs were obtained if they had not been obtained within 180 days prior to the study visit. c = Magnetic resonance imaging (MRI) of the sacroiliac (SI) joints was performed if not done within 180 days prior to the study visit or if MRIs were not available for review, and the anteroposterior pelvis radiograph was negative for sacroiliitis consistent with the modified New York criteria for ankylosing spondylitis. eCRF = electronic case report form; nr‐axSpA = nonradiographic axial spondyloarthritis.
Study design
PROSpA was a multicenter, non–drug treatment, single‐visit study conducted at rheumatology practices in the US. A central or local institutional review board or independent ethics committee approved the study at each site, and the study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. Before any study‐related procedures, the informed consent statement was reviewed, signed, and dated by the patient.
A single study visit was required to obtain the patient's medical history, conduct a physical examination, assess disease activity (using the Bath AS Disease Activity Index [BASDAI] [20] and patient's and physician's global assessments of disease activity), and obtain samples for laboratory tests for C‐reactive protein (CRP) level and HLA–B27 status, if not previously done (both performed at the site's local laboratory) (Figure 1). Patients underwent radiography of the anteroposterior pelvis (if not previously done within 180 days before the study visit) and MRI of the sacroiliac joints (if not previously done within 180 days before the study visit or if MRIs were not available for review and the anteroposterior pelvis radiograph was negative for sacroiliitis that was consistent with the modified New York criteria for AS). MRIs and radiographs were evaluated by a designated radiologist at each site who viewed a mandatory training video on what specific findings need to be identified on the imaging films for the study. Information obtained during the study visit and the laboratory and imaging results included all data necessary to evaluate for fulfillment of the ASAS axial SpA criteria and/or modified New York criteria for AS (Figure 1).
Rheumatologist's clinical diagnosis and determination of fulfillment of classification criteria
The rheumatologist was asked if a diagnosis of axial SpA could be made and the level of confidence in the diagnosis (range 0 [not confident at all] to 10 [very confident]) at two time points: the time of the study visit and when results of all pending laboratory and imaging tests had been reviewed (Figure 1). Independent of the rheumatologist's diagnosis, study data were used to classify patients. Information was uploaded to a database, and prespecified programming based on the published application of the ASAS axial SpA and modified New York criteria was used to determine which patients met these classification criteria. Patients were classified as having axial SpA (fulfilled the ASAS criteria), subdivided into nonradiographic axial SpA (fulfilled the ASAS criteria but not the modified New York criteria for AS) and AS (fulfilled both the ASAS criteria and the modified New York criteria for AS), or non–axial SpA (did not fulfill either criteria).
Statistical analysis
Assuming a 10% prevalence of nonradiographic axial SpA among eligible patients, a target sample size of 750 would have a 95% confidence interval of ∼7.9–12.5%. Baseline demographics, disease characteristics, and disease activity were summarized based on the disease categories AS, nonradiographic axial SpA, and non–axial SpA.
RESULTS
Disposition of the patients
A total of 751 patients were enrolled in the study at 68 rheumatology sites in the US; 343 (46%) were existing patients at the practice, 303 (40%) were new referrals, and 105 (14%) were self referred. Of the new referrals, 163 (54%) were referred by their primary care physician, while 35 (12%), 16 (5%), 2 (1%), and 87 (29%) were referred by orthopedic physicians, physical medicine and rehabilitation health care professionals, chiropractors, or another source of referral, respectively. A total of 705 patients (93.9%) completed the study, while 46 patients (6.1%) prematurely withdrew from the study (did not complete all protocol‐specified procedures).
At study entry, of the 751 patients enrolled, 295 (39.3%), 153 (20.4%), and 20 (2.7%) had prior information available on HLA–B27 status, anteroposterior pelvis radiographs, and MRIs of the sacroiliac joints respectively. Among these patients, 162 were positive for HLA–B27, 30 had positive findings on anteroposterior pelvis radiographs, and 8 had positive findings on MRI of the sacroiliac joints. Fulfillment of inflammatory back pain criteria was used for study inclusion for 703 patients.
Relationship of rheumatologist's clinical diagnosis to fulfillment of classification criteria
Following the single study visit, at which the clinical assessments and laboratory tests were performed, 697 patients were given a clinical diagnosis of “axial SpA” or “non‐axial SpA.” After review of all clinical, laboratory, and imaging results, 319 patients (46%) were diagnosed as having axial SpA by the investigators.
Among the 744 patients who had sufficient data to allow for determination of fulfillment of the ASAS criteria, 348 patients (47%) fulfilled the criteria. Of these patients, 108 (15%) were classified as having AS based on fulfillment of the modified New York criteria, 238 (32%) did not fulfill the modified New York criteria and were categorized as having nonradiographic axial SpA (123 fulfilled the ASAS criteria through the imaging arm and 115 through the clinical arm), and 2 patients had missing data that did not allow for evaluation of fulfillment of the modified New York criteria (Figure 2). Additionally, 396 patients did not fulfill the ASAS criteria or the modified New York criteria for AS and were classified as having non–axial SpA.
Figure 2.
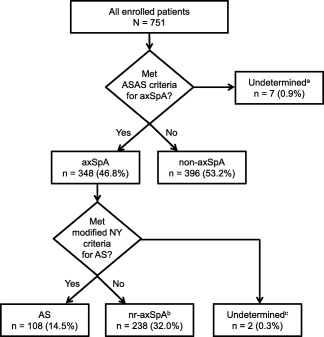
Disease classification based on Assessment of Spondylo‐Arthritis international Society (ASAS) criteria for axial spondyloarthritis (axSpA) and modified New York criteria for ankylosing spondylitis (AS). a = The “undetermined” category included patients with at least 1 missing variable that did not allow for evaluation of the ASAS axial SpA criteria. b = The “nonradiographic axial SpA (nr‐axSpA)” group included 10 patients with radiographic evidence of sacroiliitis fulfilling the radiologic criterion but none of the 3 clinical criteria of the modified New York criteria and who were therefore not included in the subgroup of patients with AS. c = The “undetermined” category included patients with either a missing radiograph or at least 1 clinical variable that did not allow evaluation of the modified New York criteria.
Analysis of clinical diagnosis versus fulfillment of classification criteria was done for the 697 patients who had available data. Of the 319 patients who were diagnosed as having axial SpA by the investigator, 101 (32%) were classified as having AS (fulfilled the ASAS and modified New York criteria) and 157 (49%) were classified as having nonradiographic axial SpA (fulfilled the ASAS criteria but not the modified New York criteria). However, 61 of the patients (19%) did not fulfill the ASAS criteria. Of the 378 patients who were not diagnosed as having axial SpA by the investigator, 78 (21%) fulfilled the ASAS criteria for axial SpA; 6 patients (2%) also met the modified New York criteria for AS, and the 72 (19%) who did not were classified as having nonradiographic axial SpA. Therefore, using the investigator's clinical diagnosis as the gold standard, the specificity of the ASAS criteria was 79% (95% confidence interval [95% CI] 75–83%) and the sensitivity of the ASAS criteria was 81% (95% CI 77–85%).
When a clinical diagnosis of axial SpA was made, the investigator's overall level of confidence in the diagnosis was lower for patients who were subsequently found to not fulfill the ASAS criteria compared with those who had more SpA manifestations and fulfilled the ASAS and/or modified New York criteria (Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39612/abstract). Among 157 patients classified as having nonradiographic axial SpA and diagnosed by the investigator as having axial SpA, 71% fulfilled the imaging arm of the ASAS criteria; among 72 patients not diagnosed by the investigator, 86% fulfilled only the clinical arm (Table 1).
Table 1.
Patients with and those without an investigator diagnosis of axial SpA who fulfilled the ASAS criteria for axial SpA but not the modified New York criteria for ASa
| Fulfilled ASAS criteria for axial SpA | ||
|---|---|---|
| Investigator diagnosis of axial SpA | Imaging arm | Clinical arm |
| Yes (n = 157) | 112 (71.3) | 45 (28.7) |
| No (n = 72) | 10 (13.9) | 62 (86.1) |
Values are the number (%) of patients. Patients were classified as having nonradiographic axial spondyloarthritis (SpA) according to the Assessment of SpondyloArthritis international Society (ASAS) criteria. AS = ankylosing spondylitis.
Baseline demographic characteristics of the patients
Among patients who were diagnosed as having axial SpA by a rheumatologist, more men than women met the modified New York criteria for AS, and there were slightly more women in the nonradiographic axial SpA subgroup at baseline. These patients had experienced their symptoms for an average of 14 years with age at onset in their 20s (Table 2). Mean age, duration of chronic back pain, and age at onset of back pain were generally comparable for all subgroups regardless of the rheumatologist's clinical diagnosis of axial SpA, with the exception of the subgroup of 6 patients who were not diagnosed by a rheumatologist as having axial SpA but fulfilled the modified New York criteria for AS.
Table 2.
Baseline demographic characteristics of the patients
| Rheumatologist's clinical diagnosis of axial SpA and fulfillment of classification criteria | ||||||
|---|---|---|---|---|---|---|
| Yes by clinical diagnosis | No by clinical diagnosis | |||||
| AS(n = 101)a | Nonradiographic axial SpA(n = 157)b | Non–axial SpA(n = 61) | AS(n = 6)a | Nonradiographic axial SpA(n = 72)b | Non–axial SpA(n = 300) | |
| Female, no. (%) | 43 (43) | 84 (54) | 33 (54) | 5 (83) | 43 (60) | 179 (60) |
| Age, mean ± SD years | 41.5 ± 12.4 | 40.6 ± 11.9 | 42.6 ± 11.4 | 49.5 ± 17.1 | 41.8 ± 12.6 | 41.8 ± 12.6 |
| Race, no. (%) | ||||||
| White | 85 (84) | 143 (91) | 55 (90) | 6 (100) | 65 (90) | 265 (88) |
| African American | 11 (11) | 7 (4) | 2 (3) | 0 | 3 (4) | 24 (8) |
| Asian | 2 (2) | 6 (4) | 2 (3) | 0 | 2 (3) | 6 (2) |
| Ethnicity, no. (%) Hispanic orLatino | 11 (10.9) | 19 (12.1) | 9 (14.8) | 1 (16.7) | 6 (8.3) | 39 (13.0) |
| Duration of chronic back pain, mean yearsc | 14.0 | 13.8 | 14.2 | 27.9 | 14.2 | 13.7 |
| Age at onset of chronic back pain, mean ± SD yearsc | 28.1 ± 8.9 | 27.3 ± 9.3 | 28.6 ± 8.5 | 22.2 ± 11.0 | 28.2 ± 9.0 | 28.7 ± 8.9 |
Fulfilled the Assessment of SpondyloArthritis international Society (ASAS) criteria and modified New York criteria for ankylosing spondylitis (AS).
Fulfilled the ASAS criteria but not the modified New York criteria for AS.
Data were available for 100 patients classified as having AS by disease classification criteria who had a clinical diagnosis of axial spondyloarthritis (SpA), 155 patients classified as having nonradiographic axial SpA by disease classification criteria who had a clinical diagnosis of axial SpA, 58 patients classified as having non–axial SpA by disease classification criteria who had a clinical diagnosis of axial SpA, and 298 patients classified as having non–axial SpA by disease classification criteria who did not have a clinical diagnosis of axial SpA.
Baseline disease characteristics and SpA features
Among patients classified as having nonradiographic axial SpA, a greater proportion of patients who were diagnosed by a rheumatologist as having axial SpA had positive MRI findings than patients who were not diagnosed by a rheumatologist as having axial SpA. All other SpA features were reported with similar frequency among patients with nonradiographic axial SpA regardless of the rheumatologist's clinical diagnosis of axial SpA (Table 3).
Table 3.
Baseline SpA featuresa
| Rheumatologist's clinical diagnosis of axial SpA and fulfillment of classification criteria | ||||||
|---|---|---|---|---|---|---|
| Yes by clinical diagnosis | No by clinical diagnosis | |||||
| AS(n = 101)b | Nonradiographic axial SpA(n = 157)c | Non–axial SpA(n = 61) | AS(n = 6)b | Nonradiographic axial SpA(n = 72)c | Non–axial SpA(n = 300) | |
| HLA–B27 positive | 49 (49) | 99 (63) | 8 (13) | 1 (17) | 64 (89) | 13 (4) |
| Number of patients with MRI results available | 19 | 150 | 61 | 1 | 71 | 300 |
| MRI evidence of sacroiliitisd | 16 (84) | 105 (70) | 0 (0) | 1 (100) | 9 (13) | 0 (0) |
| Radiographic evidence of sacroiliitise | 101 (100) | 9 (6)f | 0 (0) | 6 (100) | 1 (1)f | 0 (0) |
| Inflammatory back pain | 95 (94) | 149 (95) | 53 (87) | 6 (100) | 62 (86) | 292 (97) |
| Arthritis, past or present | 26 (26) | 52 (33) | 19 (31) | 2 (33) | 31 (43) | 60 (20) |
| Heel enthesitis, past or present | 27 (27) | 45 (29) | 20 (33) | 1 (17) | 22 (31) | 78 (26) |
| Dactylitis, past or present | 3 (3) | 11 (7) | 5 (8) | 0 | 2 (3) | 5 (2) |
| Anterior uveitis confirmed by an ophthalmologist | 12 (12) | 21 (13) | 2 (3) | 1 (17) | 13 (18) | 6 (2) |
| Psoriasis, past or present | 7 (7) | 12 (8) | 9 (15) | 1 (17) | 6 (8) | 33 (11) |
| Crohn's disease or ulcerative colitis,past or present | 8 (8) | 2 (1) | 3 (5) | 0 | 4 (6) | 13 (4) |
| Family history of SpAg | 18 (18) | 37 (24) | 8 (13) | 1 (17) | 28 (39) | 45 (15) |
| Good response to NSAIDsh | 51 (50) | 94 (60) | 26 (43) | 3 (50) | 40 (56) | 122 (41) |
| Elevated CRPi | 47 (47) | 57 (36) | 20 (33) | 2 (33) | 24 (33) | 82 (27) |
Except where indicated otherwise, values are the number (%) of patients. MRI = magnetic resonance imaging; CRP = C‐reactive protein.
Fulfilled the Assessment of SpondyloArthritis international Society (ASAS) criteria and modified New York criteria for ankylosing spondylitis (AS).
Fulfilled the ASAS criteria but not the modified New York criteria for AS.
Active inflammatory lesions of sacroiliac joints with definite bone marrow edema/osteitis, suggestive of sacroiliitis associated with spondyloarthritis (SpA).
Sacroiliitis of grade ≥2 bilaterally or grade 3–4 unilaterally, consistent with AS.
Includes patients with radiographic evidence of sacroiliitis fulfilling the radiologic criterion but none of the 3 clinical criteria of the modified New York criteria and who were, therefore, not captured in the subgroup of patients classified as having AS.
Presence in first‐ or second‐degree relative of any of the following: AS, psoriasis, anterior uveitis, reactive arthritis, or inflammatory bowel disease.
Back pain no longer present or much better 24–48 hours after a full dose of nonsteroidal antiinflammatory drugs (NSAIDs).
Values greater than the upper limit of normal for the local laboratory where testing was performed.
Overall, baseline disease activity measures were similar between patients classified as having AS and those classified as having nonradiographic axial SpA. Mean ± SD BASDAI scores were 5.9 ± 2.0 and 5.9 ± 2.1, respectively, and AS Disease Activity Scores (ASDAS) 21 were 2.6 ± 1.0 and 2.4 ± 0.8, respectively. Functional impairment was slightly greater in the AS subgroup than in the nonradiographic axial SpA subgroup, as reflected by the mean ± SD Bath AS Functional Index 22 score (4.9 ± 2.4 versus 4.3 ± 2.5). Global assessments of disease activity (on a 0–10 numeric rating scale) were similar between patients classified as having AS and those classified as having nonradiographic axial SpA, whether assessed by a physician (mean ± SD 5.6 ± 2.2 and 4.9 ± 2.0, respectively) or by the patient (6.0 ± 2.4 and 6.0 ± 2.4, respectively). Additionally, a numerically greater proportion of patients classified as having AS had elevated CRP levels or high‐sensitivity CRP levels at baseline compared with patients classified as having nonradiographic axial SpA (46% versus 35%).
Lower than expected proportions of men and HLA–B27–positive subjects were noted regardless of the clinical setting in which patients were identified for the study (Table 4). The proportion of patients subsequently diagnosed as having axial SpA by the investigator was greatest among new referrals.
Table 4.
Characteristics of the patients according to the clinical setting in which they were identified for the studya
| Existing patients (n = 343) | Self referred (n = 105) | New referrals (n = 303) | |
|---|---|---|---|
| Investigator clinical diagnosis of axial SpA, no. (%) | 136 (39.7) | 30 (28.6) | 153 (50.5) |
| Male, % | 43 | 63 | 54 |
| Age, mean years | 41.8 | 41.2 | 40.7 |
| HLA–B27 positive, % | 55 | 43 | 44 |
SpA = spondyloarthritis.
DISCUSSION
An important challenge in obtaining a timely and accurate diagnosis is the delay in referral to the appropriate clinical expert. Two prior studies, one conducted in Germany (MASTER) and one conducted in 16 countries (Recognising and Diagnosing Ankylosing Spondylitis Reliably [RADAR]), showed that a simple referral strategy based on the presence of chronic back pain for ≥3 months with age at onset of <45 years and ≥1 of 3 SpA‐related features (HLA–B27 positivity, current inflammatory back pain, or evidence of sacroiliitis on imaging) was reliable for identifying patients with axial SpA 15, 18. The present study (the PROSpA study) confirmed this conclusion, with 46% of patients who were enrolled in the study subsequently diagnosed as having axial SpA by a US rheumatologist when using a similar strategy. Similarly, the MASTER study 18 reported that 42% of patients referred using the same criteria were diagnosed as having “definite” axial SpA by a rheumatologist.
However, in contrast to the MASTER study, in which there were 1.5 times as many patients with AS as patients with nonradiographic axial SpA, in the PROSpA study there were more than twice as many patients with nonradiographic axial SpA as patients with AS among those who fulfilled the ASAS criteria for axial SpA. This difference may be because there were fewer men (44% versus 53%) and a lower prevalence of HLA–B27 (33% versus 55%) among patients included in the PROSpA study than in the MASTER study 18. The lower proportion of men and HLA–B27–positive patients enrolled in the present study may have occurred because HLA–B27–positive men with axial SpA are more readily identified and diagnosed in clinical practice, and were therefore not eligible for inclusion.
Approximately 80% of the patients who were diagnosed as having axial SpA also fulfilled the ASAS criteria for axial SpA. The reported specificity and sensitivity from this study were 79% and 81%, respectively, which are close to the reported 84% and 83% for the validation study of the ASAS criteria 14. For comparison, the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA have a specificity and sensitivity of 61% and 82% 23.
Existing patients (n = 343) in rheumatology practices comprised 46% of enrolled patients, of which 136 (39.7%) were eventually diagnosed as having axial SpA during the study. Information was not collected about why such patients were not previously diagnosed as having axial SpA; we can only speculate on possible scenarios. Patients who were HLA–B27 negative may not have received further testing and could have been followed up as nonspecific back pain patients. Some patients may have developed additional SpA features over time, leading to a subsequent diagnosis during the study. During participation in the PROSpA study, access to and availability of imaging results such as MRI may have facilitated the diagnosis for some patients. Data from our study (Table 1) suggest a greater reliance on positive MRI results than on the presence of HLA–B27 and additional SpA features when making a diagnosis of axial SpA. The majority of patients who were diagnosed as having axial SpA by a rheumatologist fulfilled the imaging arm of the ASAS criteria, whereas the majority of those who were not diagnosed as having axial SpA fulfilled the clinical arm. This observation highlights the need for accurate interpretation of MRIs in clinical practice given the importance of MRI in the evaluation of patients for axial SpA.
Published reports indicate a delay of as much as 10 years between symptom onset and diagnosis 16, 17. The baseline demographic and disease characteristics of the patients in the present study (PROSpA) indicate a long duration of symptoms (an average of 14 years before diagnosis) in this previously undiagnosed population, for both the AS and nonradiographic axial SpA subgroups. This indicates that more education is needed in the US to increase awareness, appropriate referral, and timely diagnosis for patients with axial SpA.
Although the percentages for individual SpA features were generally similar between patients who were diagnosed by investigators as having axial SpA and those who were not, it is the presence of a combination of these various manifestations that leads to a clinical diagnosis. It appears that imaging evidence of sacroiliitis differentiates between patients who are eventually diagnosed as having axial SpA and those who are not. Of 131 patients with positive MRI findings, 121 were diagnosed by the rheumatologist as having axial SpA; of 117 patients with radiographic evidence of sacroiliitis (according to the modified New York criteria), 110 were diagnosed as having axial SpA. There are 2 possible reasons why 7 patients who had findings on anteroposterior pelvis radiographs consistent with the radiologic criterion of the modified New York criteria were not diagnosed as having axial SpA by the rheumatologist (including 1 patient who met radiologic but not clinical modified New York criteria and therefore was classified as having nonradiographic axial SpA). First, the rheumatologist might have disagreed with the radiologist's interpretation of the radiograph. Disagreement in reading radiographs for AS has been well described in the literature 24, 25, 26, 27. Second, the radiographic changes, viewed in the context of the patient's clinical presentation, may have been considered to be more consistent with another diagnosis.
Several limitations related to the study design should be mentioned. First, for the patients enrolled in the study who were already established patients at the site, there was no systematic collection of information regarding their previous disease course. Thus, we cannot accurately describe the reasons for delayed diagnosis. Second, radiographs and MRIs were interpreted by the local reader and not centrally read. However, this was consistent with the goal of the study to reflect real‐life practice, and the radiologists were required to undergo mandatory video training to standardize the interpretation of the images. Finally, investigators were required to complete imaging studies, test for HLA–B27 and/or CRP level, and inquire about specific clinical manifestations. Conducting this series of mandatory procedures might have biased the rheumatologists toward making a diagnosis of axial SpA.
Overall, these findings emphasize the need to improve the identification and diagnosis of both AS and nonradiographic axial SpA among patients already receiving care in rheumatology practices and those newly referred to rheumatologists. Increased disease awareness and the use of appropriate referral criteria can reduce the delay to diagnosis and provide a better understanding of the prevalence of axial SpA. These patients experience a similar burden of disease and their disease can remain undiagnosed and, therefore untreated, for many years.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Deodhar had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Deodhar, Mease, Reveille, Curtis.
Acquisition of data
Deodhar, Mease, Reveille.
Analysis and interpretation of data
Deodhar, Mease, Reveille, Curtis, Chen, Malhotra, Pangan.
ROLE OF THE STUDY SPONSOR
AbbVie sponsored the study, contributed to its design, and participated in the collection, analysis, and interpretation of the data, and in the writing, review, and approval of the final manuscript. AbbVie provided medical writing assistance (performed by Jessica L. Suboticki, PhD, and Kathleen V. Kastenholz, PharmD, MS). Publication of this article was contingent upon approval by AbbVie.
Supporting information
Supplementary Figure 1. Level of confidence (0 = not confident; 10 = very confident) with clinical diagnosis of axial SpA categorized by fulfillment of classification criteria (AS, nr‐axSpA, or non‐axSpA) after completion of all laboratory procedures and imaging. Only patients with available data for disease classification and a rheumatologist's clinical diagnosis (“axial SpA” or “no axial SpA”) were included. AS, ankylosing spondylitis; ASAS, Assessment of SpondyloArthritis international Society; axSpA, axial SpA; nr‐axSpA, non‐radiographic axial SpA; SpA, spondyloarthritis.
Supported by AbbVie.
Dr. Deodhar has received consulting fees and/or speaking fees from AbbVie and Novartis (more than $10,000 each), Amgen, Janssen, Pfizer, and UCB (less than $10,000 each) and research grants from AbbVie, Amgen, Janssen, Novartis, Pfizer, and UCB.
Dr. Mease has received consulting fees and/or speaking fees from AbbVie, Amgen, Bristol‐Myers Squibb, Eli Lilly, Novartis, and Pfizer (more than $10,000 each) and from Genentech, Janssen, Merck, and UCB (less than $10,000 each) and research grants from AbbVie, Amgen, Bristol‐Myers Squibb, Eli Lilly, Novartis, Novo Nordisk, Pfizer, and UCB.
Dr. Reveille has received consulting fees from AbbVie and UCB (less than $10,000 each).
Dr. Curtis has received consulting fees from Roche/Genentech, UCB, Janssen, the Consortium of Rheumatology Researchers of North America (CORRONA), and Amgen (more than $10,000 each) and from Pfizer, BMS, Crescendo, and AbbVie (less than $10,000 each).
Dr. Chen owns stock in AbbVie.
Dr. Malhotra owns restricted stock units in AbbVie. Dr. Pangan owns stock and restricted stock units in AbbVie.
REFERENCES
- 1. Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 2. Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005;52:1000–8. [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, Haibel H, Baraliakos X, Listing J, Marker‐Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 4. Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA‐B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6. [DOI] [PubMed] [Google Scholar]
- 5. Sieper J, van der Heijde D. Nonradiographic axial spondyloarthritis: new definition of an old disease? [review]. Arthritis Rheum 2013;65:543–51. [DOI] [PubMed] [Google Scholar]
- 6. Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369:1379–90. [DOI] [PubMed] [Google Scholar]
- 7. Helmick CG, Felson DT, Lawrence RC, Gabriel C, Hirsch R, Kwoh CK, et al, for the National Arthritis Data Workgroup . Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum 2008;58:15–25. [DOI] [PubMed] [Google Scholar]
- 8. Rudwaleit M, van der Heijde D, Landewe R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25 – 31. [DOI] [PubMed] [Google Scholar]
- 9. Strand V, Rao SA, Shillington AC, Cifaldi MA, McGuire M, Ruderman EM. Prevalence of axial spondyloarthritis in United States rheumatology practices: Assessment of SpondyloArthritis International Society criteria versus rheumatology expert clinical diagnosis. Arthritis Care Res (Hoboken) 2013;65:1299–306. [DOI] [PubMed] [Google Scholar]
- 10. Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic 1990;57:85–9. In French. [PubMed] [Google Scholar]
- 11. Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al, and the European Spondylarthropathy Study Group . The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34:1218–27. [DOI] [PubMed] [Google Scholar]
- 12. Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross‐sectional survey. Arthritis Care Res (Hoboken) 2012;64:905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rudwaleit M, Landewe R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- 14. Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 15. Sieper J, Srinivasan S, Zamani O, Mielants H, Choquette D, Pavelka K, et al. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: the Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann Rheum Dis 2013;72:1621–7. [DOI] [PubMed] [Google Scholar]
- 16. Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non‐radiographic axial spondyloarthritis: results of a randomised placebo‐controlled trial (ABILITY‐1). Ann Rheum Dis 2013;72:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiltz U, Baraliakos X, Karakostas P, Igelmann M, Kalthoff L, Klink C, et al. The degree of spinal inflammation is similar in patients with axial spondyloarthritis who report high or low levels of disease activity: a cohort study. Ann Rheum Dis 2012;71:1207–11. [DOI] [PubMed] [Google Scholar]
- 18. Poddubnyy D, Vahldiek J, Spiller I, Buss B, Listing J, Rudwaleit M, et al. Evaluation of 2 screening strategies for early identification of patients with axial spondyloarthritis in primary care. J Rheumatol 2011;38:2452–60. [DOI] [PubMed] [Google Scholar]
- 19. Sieper J, van der Heijde D, Landewe R, Brandt J, Burgos‐Vagas R, Collantes‐Estevez E, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis 2009;68:784–8. [DOI] [PubMed] [Google Scholar]
- 20. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 21. Lukas C, Landewe R, Sieper J, Dougados M, Davis J, Braun J, et al. Development of an ASAS‐endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. [DOI] [PubMed] [Google Scholar]
- 22. Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 23. Radner H, Neogi T, Smolen JS, Aletaha D. Performance of the 2010 ACR/EULAR classification criteria for rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2014;73:114–23. [DOI] [PubMed] [Google Scholar]
- 24. Van den Berg R, Lenczner G, Feydy A, van der Heijde D, Reijnierse M, Saraux A, et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs: results from the DESIR cohort. Arthritis Rheumatol 2014;66:2403–11. [DOI] [PubMed] [Google Scholar]
- 25. Hollingsworth PN, Cheah PS, Dawkins RL, Owen ET, Calin A, Wood PH. Observer variation in grading sacroiliac radiographs in HLA‐B27 positive individuals. J Rheumatol 1983;10:247–54. [PubMed] [Google Scholar]
- 26. Taylor HG, Wardle T, Beswick EJ, Dawes PT. The relationship of clinical and laboratory measurements to radiological change in ankylosing spondylitis. Br J Rheumatol 1991;30:330–5. [DOI] [PubMed] [Google Scholar]
- 27. Yazici H, Turunc M, Ozdogan H, Yurdakul S, Akinci A, Barnes CG. Observer variation in grading sacroiliac radiographs might be a cause of ‘sacroiliitis' reported in certain disease states. Ann Rheum Dis 1987;46:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Level of confidence (0 = not confident; 10 = very confident) with clinical diagnosis of axial SpA categorized by fulfillment of classification criteria (AS, nr‐axSpA, or non‐axSpA) after completion of all laboratory procedures and imaging. Only patients with available data for disease classification and a rheumatologist's clinical diagnosis (“axial SpA” or “no axial SpA”) were included. AS, ankylosing spondylitis; ASAS, Assessment of SpondyloArthritis international Society; axSpA, axial SpA; nr‐axSpA, non‐radiographic axial SpA; SpA, spondyloarthritis.


