Abstract
Aim
The Danish National Multiple Myeloma Registry (DMMR) is a population-based clinical quality database established in January 2005. The primary aim of the database is to ensure that diagnosis and treatment of plasma cell dyscrasia are of uniform quality throughout the country. Another aim is to support research. Patients are registered with their unique Danish personal identification number, and the combined use of DMMR, other Danish National registries, and the Danish National Cancer Biobank offers a unique platform for population-based translational research.
Study population
All newly diagnosed patients with multiple myeloma (MM), smoldering MM, solitary plasmacytomas, and plasma cell leukemia in Denmark are registered annually; ~350 patients. Amyloid light-chain amyloidosis, POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes syndrome), monoclonal gammopathy of undetermined significance and monoclonal gammopathy of undetermined significance with polyneuropathy have been registered since 2014.
Main variables
The main registered variables at diagnosis are patient demographics, baseline disease characteristics, myeloma-defining events, clinical complications, prognostics, first- and second-line treatments, treatment responses, progression free, and overall survival.
Descriptive data
Up to June 2015, 2,907 newly diagnosed patients with MM, 485 patients with smoldering MM, 64 patients with plasma cell leukemia, and 191 patients with solitary plasmacytomas were registered. Registration completeness of new patients is ~100%. A data validation study performed in 2013–2014 by the Danish Myeloma Study Group showed >95% data correctness.
Conclusion
The DMMR is a population-based data validated database eligible for clinical, epidemiological, and translational research.
Keywords: Multiple Myeloma Registry, population-based, treatment response, progression-free survival, overall survival
Aim of the database
The Danish National Multiple Myeloma Registry (DMMR) is a population-based clinical quality database. DMMR was established on January 1, 2005, to collect clinical data on all newly diagnosed patients with symptomatic multiple myeloma (MM), smoldering MM (SMM), plasma cell leukemia (PCL), and osseous and extraosseous solitary plasmacytoma. Data were collected from all hematological departments in Denmark. The database is funded by the Danish Health Care Regions. The departments of hematology and hospitals are obligated to report data on all diagnosed patients to the database. The Danish Health Care Regions established the registry as a safeguard for the regional governmental health care quality in Denmark. The aim is to ensure that diagnosis and treatment of plasma cell dyscrasia are of uniform quality throughout the country, which has been shown to be the case. Annual reports in Danish are published by the Danish Myeloma Study Group (DMSG). These reports are available at the DMSG website (www.myeloma.dk).
Another aim of the database is to support preclinical, clinical, and epidemiological research, and for these purposes, the database is unique because it is population based and has a high completeness of data. Quality assessment of the registered patients in the database is done by identity match with patients registered in the Danish Pathology Diagnosis Registry, called Patobank, and in the Danish National Hospital Registry,1 which contains information on all hospital admissions for somatic diseases and the Danish Civil Registration System (CRS),2 established in 1968 to register all individuals alive and living in Denmark by a unique personal identification number. Mismatch will be annotated, and the relevant departments will be asked to ensure registration of any missing patients. Treatment of MM has improved by the introduction of high-dose chemotherapy with autologous stem cell support in the 90s3,4 and by the introduction of thalidomide, bortezomib, and new immunomodulatory drugs such as lenalidomide and pomalidomide.5–7 Several new drugs are in the pipeline and are expected to be approved soon.8–10 The clinical trials leading to approval of new drugs are based on the studies of selected myeloma populations with strict exclusion criteria regarding age, performance status, comorbidity, and disease complications, eg, renal insufficiency. The new treatment options are expensive, and there is an increasing demand for documentation of the impact of antineoplastic treatment and supportive care, such as bisphosphonates, vertebroplasty, and erythropoietin. The database will be able to contribute in this context with population-based data from daily clinical practice.
The database does not register comorbidity. However, these data can be captured from the Danish National Hospital Registry, which includes information on all hospital admissions in Denmark since 1977 and all contacts to emergency rooms or outpatient clinics since 1995. Since 1994, diagnostic information on each contact with the hospital has been coded by physicians according to the International Classification of Diseases, Tenth Edition system.1 Data on drug use can be obtained from the Danish prescription registry.11 Status on working capability can be captured from the employment databases, and information on granted disability pension can be obtained from the Danish Register for Evaluation of Marginalization (DREAM), which is based on data from the Danish Ministry of Employment, the Danish Ministry of Education, CRS, and SKAT (the Danish tax authority).12 DREAM includes data on all Danish citizens who have received welfare benefits, since 1991. Each person is registered with a code indicating the type of welfare benefit received. DREAM has 100% coverage of those granted disability pension in Denmark since 2000. Thus, due to the unique Danish personal identification number, the combined use of a number of National registries allows scientific analyses on a number of socioeconomic factors, health care use, comorbidity factors, etc.
The Danish National Cancer Biobank was established in 2009,13 and the hematological cancers became part of this biobank in 2012. From newly diagnosed patients with myeloma, serum, plasma, DNA, bone marrow plasma, and bone marrow mononuclear cells or immunomagnetically separated CD138-positive cells and the CD138 negative fraction of cells have been frozen. Bone marrow and blood have also been sampled from some patients at first relapse or disease progression. Information on biobanked material is registered in the clinical database; thereby the clinical database and cancer biobank form the basis for population-based translational research.
The Danish Cancer Registry registers all malignances and premalignances in Denmark, including the same diagnoses as the specified myeloma registry. This is done in parallel with the MM registry and without any formal collaboration but based on the same data sources. The broad epidemiological cancer registry does not record clinical information on disease complications, given treatment, treatment responses, response duration, etc. Moreover, it does not record information on biobanked material.
Study population
All newly diagnosed patients with MM, SMM, PCL, and osseous and extraosseous solitary plasmacytoma have been registered since January 1, 2005. At progression from SMM or solitary plasmacytomas to MM, patients are re-registered with data from time of transformation. POEMS syndrome (polyneuropathy, organomegali, endocrinopathy, monoclonal gammopathy and skin changes syndrome), amyloid Light-chain amyloidosis, monoclonal gammopathy of undetermined significance (MGUS), and MGUS with polyneuropathy have been registered in the database since January 1, 2014.
The Danish population is 5.5 million. Approximately 290 patients are diagnosed annually with MM, either as de novo or as progression from known MGUS, SMM, or solitary plasmacytoma.
In Denmark, treatment of hematological diseases is centralized to nine hematological departments. Six university hospitals perform high-dose treatment with autologous stem cell support and radiotherapy, and two centers perform allogeneic hematopoietic stem cell transplantation. All treatments are paid for by the public health care system. No hematological patients are treated in private hospitals or clinics.
Since 2009, the DMSG has developed nationwide clinical guidelines for diagnosis and treatment. The guideline for MM is revised annually. Most hematological clinics participate in clinical trials conducted by DMSG, the Nordic Myeloma Study Group (NMSG), or by the European Myeloma Network (EMN). Up to June 30, 2015, the following numbers of patients were registered in the registry: 2,907 MM patients, 485 SMM, 64 primary PCL, 103 solitary bone plasmacytomas, and 88 extraosseous plasmacytomas.
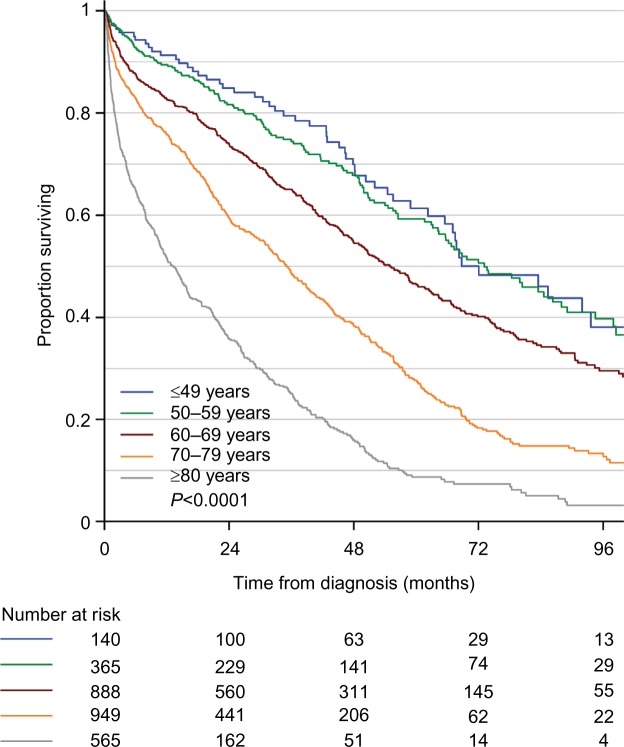
The age-dependent overall survival of MM patients in the registry is shown in Figure 1. As expected, there is a large difference in overall survival according to age. In MM patients <60 years, the median survival exceeds 72 months, whereas it is <24 months for patients >80 years.
Figure 1.
Age-dependent overall survival of 2,907 newly diagnosed Danish patients with symptomatic multiple myeloma.
Notes: The patients were registered in the Danish National Multiple Myeloma Registry between January 1, 2005, and June 30, 2015. Median follow-up is 59.5 months. The completeness of the registry is ~100%, and the survival curves therefore present “real life data” on multiple myeloma survival in the whole population.
Variables
The main registered variables at diagnosis are shown in Table 1. All data are registered electronically on a central server owned by the Danish Health Regions. More than 95% are usually registered in the database within the first year after diagnosis, and after 2 years, the completeness is almost 100%.
Table 1.
Main registered baseline parameters at diagnosis in multiple myeloma, smoldering MM, plasma cell leukemia, and solitary plasmacytoma in the Danish National Multiple Myeloma Registry
| Patient related | Myeloma clonal characteristics | Myeloma complications | Prognostical parameters |
|---|---|---|---|
| Age Sex Personal identification number Postal code Date of diagnosis |
M-component subtype S-M-component concentration U-M-component concentration Light-chain subtype S-free light kappa lambda chain concentration and ratio Bone marrow plasma cell infiltration (%) Cytogenetics Karyotype FISH abnormalities |
Paraclinical findings Bone disease by skeletal survey CT/MRI/PET/DEXA (if performed) Calcium Creatinine Hemoglobin Leukocyte count Thrombocyte count U-protein Clinical complications Medullary compression Bone fractures Hemodialysis-dependent renal failure Amyloidosis Polyneuropathy |
ISS Beta-2-microglobulin Albumin WHO performance status LDH CRP |
Abbreviations: CRP, C-reactive protein; CT, computed tomography; DEXA, dual-energy X-ray absorptiometry; FISH, fluorescence in situ hybridization; ISS, International Staging System; LDH, lactate dehydrogenase; MM, multiple myeloma; MRI, magnetic resonance imaging; PET, positron emission tomography; WHO, World Health Organization.
In 2013–2014, DMSG performed a data validation study of registered data in the database. Ten percent of the registered patients at each department were randomly selected for independent on-site monitoring. A skilled trainee in hematology compared registered data with the patients’ medical records. In cases of inconsistency, a DMSG evaluation committee was consulted. Typical recurrent problems were that a given parameter at diagnosis, eg, serum C-reactive protein, was not taken at the date of diagnosis, which is defined as the date of bone marrow biopsy or tumor biopsy, but 10 days prior and that registered hemoglobin value was the value obtained after blood transfusion. The DMSG evaluation committee deemed these inconsistencies to be of minor importance. Overall, each parameter was correct in >95% of the cases, and the evaluation committee concluded that quality of data is high.
Follow-up
The flow in registrations is illustrated in Table 2. The registration sheet is filled in at diagnosis. It includes a summary of planned treatments and supportive care, eg, planned first-line therapy with cyclophosphamide–bortezomib–dexamethasone, stem cell harvest, and high-dose melphalan with stem cell support combined with radiation therapy and treatment with bisphosphonate.
Table 2.
Main registered parameters concerning treatment, response to treatment and course of disease in multiple myeloma, plasma cell leukemia, and solitary plasmacytoma in the Danish National Multiple Myeloma Registry
| At diagnosis | First-line treatment | Second-line treatment | Follow-up |
|---|---|---|---|
| Planned antimyeloma regimen Planned radiotherapy Planned bisphosphonates |
Received first-line treatment regimen Date for start and end of treatment Best response according to IMWG criteria |
Date of disease progression after first-line therapy (PFS1) Type of progression Received second-line treatment regimen Date for start and end of second-line treatment Best response according to IMWG criteria |
Disease status Number of lines of treatment regimens Date of death Cause of death |
Abbreviations: IMWG, International Myeloma Working Group; PFS1, first progression-free survival.
The actual received first-line regimen and supportive treatment and achieved response to first-line treatment are reported in the first-line treatment form at response evaluation after end of first-line treatment.
Progression-free survival, time to next treatment, second-line treatment regimen, and response to second-line treatment are reported on the second-line treatment form at response evaluation after end of second-line treatment. This scheme also includes information on type of relapse, eg, clinical relapse with progressive bone disease or renal failure, or paraclinical relapse with rise in M-component levels.
A follow-up scheme is reported at time of death or may be reported prior to death at the discretion of the department or physician. It includes a summary of the number of treatment lines administered and cause of death.
Examples of research
The annual DMMR reports from DMSG have documented high early mortality in newly diagnosed frail and elderly patients with MM ineligible for high-dose chemotherapy. Particularly, for the elderly population older than 80 years, the registry documents a high early mortality rate as illustrated in Figure 1. In an analysis of patients registered in 2005–2012, 330 patients (22.0%) out of 1,497 transplant ineligible patients died within 6 months of diagnosis. A skilled trainee reviewed all cases on-site. The majority of early deaths (50.9%) were caused by infections.14 As a consequence of these findings, DMSG started, in 2013, a randomized, controlled clinical trial to study the efficacy of prophylactic antibiotics within the first 6 months of diagnosis in elderly patients with MM (EudraCT number: 2012-004424-38).
Administrative issues and funding
The DMMR is owned and financed by the Danish Health Regions. The server is placed at and run by IT support from Competence Centre East in Copenhagen. Statisticians and clinical epidemiologists are used at the Competence Centre. Scientific hematological expertise and an administrative secretary are connected part time. A DMSG database committee reviews, comments, and writes the annual reports in collaboration with an epidemiologist and a statistician from the Competence Centre.
Conclusion
The DMMR is a population-based database eligible for clinical, epidemiological, and translational research. The database has a very high registration completeness, and a data validation study has shown high-quality data.
Acknowledgments
The authors would like to acknowledge the huge amount of work done by all DMSG members, investigators, and data managers at the Danish Hematological departments. This paper was funded by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by the Danish Regions. The assistance by Vickie Svane Kristensen in preparation of the manuscript is very much appreciated. The data validation study has been supported by a grant from the Danish Cancer Society (grant no R94-A5689-B249).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268. [PubMed] [Google Scholar]
- 2.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 4.Lenhoff S, Hjorth M, Holmberg E, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood. 2000;95(1):7–11. [PubMed] [Google Scholar]
- 5.San Miguel JF, Schlag R, Khuageva NK, et al. VISTA Trial Investigators Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 6.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. FIRST Trial Team Lenalidomide and dexamethasone in transplant ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 7.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 8.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. ASPIRE Investigators Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 9.San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(1):1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 10.Lonial S, Dimopoulos M, Palumbo A, et al. ELOQUENT-2 Investigators Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 11.Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 12.Hedegaard Rasmussen J. DREAM Database. The National Labor Market Authority. Report No. 28. Copenhagen: Ministry of Employment; 2012. [Google Scholar]
- 13.Modin C, Bjerregaard B, Ørntoft T, Høgdall E. Faglig Følgegruppe for Dansk CancerBiobank. [Establishing the Danish CancerBiobank] Ugeskr Laeger. 2010;172(19):1446–1450. Danish. [PubMed] [Google Scholar]
- 14.Holmström MO, Gimsing P, Abildgaard N, et al. Causes of early death in multiple myeloma patients who are ineligible for high-dose therapy with hematopoietic stem cell support: a study based on the nationwide Danish Myeloma Database. Am J Hematol. 2015;90(4):E73–E74. doi: 10.1002/ajh.23932. [DOI] [PubMed] [Google Scholar]