Abstract
Background
Diabetes mellitus (DM) is a common endocrine disease of cats. The prevalence of DM in cats in England is not well‐defined.
Hypothesis/Objectives
To estimate the prevalence and identify risk factors for DM in a large population of cats attending primary‐care practices.
Animals
A cohort of 193,563 cats in the VetCompass Programme attending 118 primary‐care practices in England.
Methods
Cross‐sectional analysis of cohort clinical data. Data were extracted covering September 1st 2009 and August 31st 2014. Period prevalence of DM was calculated. Associations between risk factors and DM were assessed using logistic regression modelling.
Results
Of 1,128 DM cases were identified among 194,563 cats (period prevalence 0.58%; 95% confidence interval [CI] 0.54–0.61). Multivariable modelling indicated that Tonkinese (OR 4.1; 95% CI 1.8–9.6; P = .001), Norwegian Forest (odds ratio [OR] 3.5; 95% CI 1.3–9.6; P = .001) and Burmese (OR 3.0; 95% CI 2.0–4.4; P < .001) cats had increased odds of DM compared with crossbred cats. DM odds increased as bodyweight categories increased above 4 kg (P < .001), as cats aged beyond 6 years old (P < .001) and in insured cats (OR 2.0; 95% CI 1.6–2.4; P < .001) but sex was not significantly associated with DM.
Conclusions and Clinical Importance
Diabetes mellitus is an important component of the primary‐care practice caseload with 1‐in‐200 cats affected. An increased risk of DM in certain cat breeds supports a genetic predisposition. These results can guide future research and preventative healthcare.
Keywords: Endocrine, Feline, Statistical modelling, Surveillance, VetCompass
Abbreviations
- CI
confidence interval
- DM
diabetes mellitus
- DSH
domestic shorthair
- EPR
electronic patient record
- IQR
interquartile range
- OR
odds ratio
- PMS
practice management system
- ROC
receiver operating characteristic
- TNFα
tumor necrosis factor α
- VeNom
veterinary nomenclature
Diabetes mellitus (DM) is one of the most common endocrine diseases of cats, although prevalence estimates have varied between studies that were based on different patient populations and locations.1 The only previous study to estimate the prevalence of DM in UK cats reported a prevalence of 0.43% among an insured population of 14,030 cats,2 whereas estimates from university teaching hospitals range from 0.21% in Sweden3 to 1.24% in the United States.4
The majority of cats with DM resemble Type 2 DM in people, which originates from a combination of decreased β‐cell function, insulin resistance and contributing environmental and genetic factors.1 However, DM in cats can also result from other causes. Hypersomatotropism (acromegaly) has been estimated to cause 25% of diabetes cases in cats in the United Kingdom,5 and other recognized causes include hyperadrenocorticism,6 pancreatic disease7 and diabetogenic drug administration.8
Male sex has often been identified as a risk factor for development of DM in the cat4, 9, 10 and DM in cats also shares several predisposing factors with Type 2 DM in people, including increasing age, physical inactivity and obesity.9, 10, 11 In cats, obesity is proposed to cause insulin resistance through various mechanisms, including altered secretion of adipokine hormones and changes in lipid metabolism.12, 13 Not all obese or older cats develop DM, however, so other genetic or environmental factors might determine whether an individual eventually becomes diabetic. Genetic research into DM in cats is at a preliminary stage, whereas over 70 susceptibility genes have been identified for human Type 2 DM.14 Early work in cats has identified a single nucleotide polymorphism in the melanocortin 4 receptor gene, which is associated with the development of DM in overweight domestic shorthair (DSH) cats15 and several genetic loci associated with the development of DM in lean DSH cats.16 However, it is probable that the heritability of DM in cats is highly complex, as it is in people, and further investigation is required to clarify the role that susceptibility genes might play in the development of DM in cats.
A genetic component for DM in cats is also indicated by specific feline breeds appearing to be at increased or decreased risk of DM. Several studies have identified an increased risk of DM in Burmese cats in the United Kingdom, Europe, and Australia, and a recent Swedish investigation demonstrated an increased risk in Norwegian Forest cats, Russian Blues and Abyssinians, and a decreased risk in Persians.2, 17, 18 An increased risk for DM has not been documented in American Burmese cats, which has been attributed to American Burmese being genetically distinct from Burmese in other parts of the world.19
The only previous study to investigate risk factors for DM in cats in England analysed a modest‐sized insured population of 14,030 cats.2 However, a recent study estimated that only 13.5% of cats in England are insured20 so the management of insured cats is unlikely to be representative of the general owned feline population in England. The aim of this study was to investigate the prevalence of DM and to identify demographic risk factors for DM among a large cohort of cats attending primary‐care practices in England. We hypothesized that the Burmese and Norwegian Forest cat breeds have increased risk of DM diagnosis compared with crossbred cats.
Materials and Methods
The VetCompass Companion Animal Surveillance Programme21 collates de‐identified electronic patient record (EPR) data from primary‐care veterinary practices in the United Kingdom for epidemiological research.22 Collaborating practices were selected based on their willingness to participate and on the condition that they recorded clinical data within an appropriately configured practice management system (PMS). Practitioners recorded summary diagnosis terms from an embedded veterinary nomenclature (VeNom)23 code list during episodes of clinical care. Collected information related mainly to the owned feline population and included patient demographic (species, breed, date of birth, sex, insurance status and bodyweight) and clinical information (free‐form text clinical notes, summary diagnosis terms and treatment with relevant dates) data fields. Electronic patient record data were extracted from PMSs using integrated clinical queries22 and uploaded to a secure VetCompass relational database. Ethical approval of the project was granted by the Royal Veterinary College Ethics and Welfare Committee (reference number 2014/S101).
The study design used a cross‐sectional analysis of cohort clinical data to estimate a period prevalence of DM and to evaluate breed as a risk factor for DM. The study cohort comprised all cats with at least 1 EPR (clinical note, VeNom summary diagnosis, bodyweight or treatment) uploaded to the VetCompass database from September 1st 2009 to August 31st 2014. The study inclusion criterion for a DM case required the cat to have a final diagnosis of DM (or synonym) recorded in the EPR. Once the study cohort had been defined, all study cats were followed over the study period from September 1st 2009 to August 31st 2014 and any cat that met the study inclusion criterion for a DM case at any time during the study period was included as a case. All remaining cats in the study cohort that were not identified as DM cases were included in the analysis as noncases. When identifying breeds at increased risk of DM, sample size calculations using Epi Info 71 estimated a cross‐sectional study would require 1,359 cats of each individual breed being assessed and 135,827 crossbred cats to detect an odds ratio of 2.0 or greater assuming a 0.6% DM prevalence in crossbred cats.
To identify DM cases from the EPRs, all cats in the eligible study population were screened for candidate DM cases by searching the clinical free‐text field (multiple search terms: dm, diab, mellitus, DKA, ketoacid, hyperglyc, glucosur, ketonur), the VeNom term fields (diabetes mellitus), and the treatment field (multiple drug searches: insul, insuv, glargine, prozinc, pzi, diab). The results from these searches were combined and randomized using the RAND function in Microsoft Excel2 to avoid any temporal bias during further evaluation and data extraction because the patient identification number depended on the date of the first EPR entry. The full clinical notes of all candidate DM cases were manually reviewed to confirm the presence of a final diagnosis of DM to decide on case inclusion. For cats confirmed as DM cases, additional data were extracted from the EPR that described whether the case was pre‐existing (first diagnosed prior to September 1st 2009) or incident (first diagnosed during the study period from September 1st 2009 to August 31st 2014), date of diagnosis for incident cases and the date and mechanism of any deaths.
A binary purebred variable was used to group all cats recorded with a breed name recognized by International Cat Care24 as “purebred”, and all other cats as “crossbred”. A breed variable comprised all individual breeds with 300 or more animals in the overall study dataset, a grouping of all remaining purebred cats and a grouping of crossbred cats. Insurance status described whether a cat was insured at any point during the study period and was included as a proxy indicator for both the human‐animal bond and the restriction of financial constraints on clinical care.25 The age values were calculated using the date of first diagnosis for incident DM cases and the mid‐point date between the first and final EPRs for the noncases (termed EPR mid‐point age). Pre‐existing DM cases were included in risk factor analysis as “age not recorded” so the risk factor results could be interpreted as showing the odds at each age group for “becoming a case” rather than for “being a case”. Age (years) was categorized into 7 groups (<3.0, 3.0–5.9, 6.0–8.9, 9.0–11.9, 12–14.9, ≥15.0, not recorded). Bodyweight referred to the maximum bodyweight recorded for cats older than 6 months and was categorized into 8 groups (0.0–2.9 kg, 3.0–3.9 kg, 4.0–4.9 kg, 5.0–5.9, 6.0–6.9, 7.0–7.9, ≥8.0 kg, not recorded). The time contributed to the study for each cat described the period from the dates of the earliest to the latest EPR and gave an indication of the underlying period upon which the period prevalence values were dependent.
After data checking and cleaning in Microsoft Excel2, analyses were conducted using Stata 13.3 A period prevalence with 95% confidence intervals (CI) for DM was reported that described the prevalence of DM (including pre‐existing and incident cases) overall and by breed. The CI estimates were derived from standard errors based on approximation to the normal distribution.26 Descriptive statistics characterized purebred, breed, sex, insurance, age, bodyweight, and time contributed to the study for the case and noncase cats. Mortality data were summarized for the DM cases alone. The Mann–Whitney U test was used to compare bodyweight values between male and female cats.26
Binary logistic regression modeling was used to evaluate univariable associations between risk factors (purebred, breed, bodyweight category, age category, sex, and insurance) and DM. Both pre‐existing and incident DM cases were included in risk factor analysis. Risk factors with liberal associations in univariable modeling (P < .2) were taken forward for multivariable evaluation. Collinearity was assessed between all variables taken forward for multivariable modeling using the Stata corr and collin commands.27 Model development used manual backwards stepwise elimination. To deal with complete separation of data for some breeds that had zero‐cells (ie, a breed with no DM cases), the Stata firthlogit program2 allowed inference based on the profile penalized likelihood.28 Clinic attended was parameterized as a random effect and pair‐wise interaction effects were evaluated for the final model.29 The area under the ROC curve was used to evaluate the discriminatory ability of the model to predict DM‐positive and DM‐negative cats (nonrandom effect model).29 Statistical significance was set at P < .05.
Results
The overall dataset comprised 194,563 cats attending 118 clinics in England between 1st September 2009 and 31st August 2014. The search strategies for possible DM cases revealed 4,031 candidate animals. After manual verification, 1,128 cats met the study inclusion criterion for DM, yielding an apparent prevalence of 0.58% (95% CI: 0.54–0.61%) for DM in cats attending primary‐care veterinary practices in England. Of these DM cases, 504 (44.7%) were pre‐existing and 624 (55.3%) were incident cases. The median time contributed to the study across all cats in the dataset was 0.3 years (interquartile range [IQR]: 0.0–1.8 years, range 0.0–5.0 years). Breeds with the highest prevalence of DM within‐breed included the Burmese (2.27%, 95% CI 1.63–3.06), Norwegian Forest Cat (2.21%, 95% CI 0.96–4.31) and Tonkinese (2.17%, 95% CI 0.88–4.41), while the prevalence in crossbred cats was 0.58% (95% CI 0.54–0.61) (Table 1).
Table 1.
Prevalence of diabetes mellitus diagnosed across cat breeds attending primary‐care veterinary practices in England (September 1st 2009 to August 31st 2014)
| Breed Category | Total No. Cats | No. DM Cases | Period Prevalence (%) | 95% CI |
|---|---|---|---|---|
| Burmese | 1810 | 41 | 2.27 | 1.63–3.06 |
| Norwegian Forest | 362 | 8 | 2.21 | 0.96–4.31 |
| Tonkinese | 323 | 7 | 2.17 | 0.88–4.41 |
| Oriental | 312 | 3 | 0.96 | 0.20–2.78 |
| Russian | 428 | 4 | 0.93 | 0.26–2.38 |
| Crossbred | 173,578 | 1006 | 0.58 | 0.54–0.61 |
| Maine Coon | 1259 | 7 | 0.56 | 0.22–1.14 |
| Rex | 355 | 2 | 0.56 | 0.07–2.02 |
| Siamese | 1826 | 9 | 0.49 | 0.23–0.93 |
| Other breed‐types | 2737 | 13 | 0.47 | 0.25–0.81 |
| Persian | 2465 | 7 | 0.28 | 0.11–0.58 |
| Birman | 1103 | 3 | 0.27 | 0.06–0.79 |
| British short hair | 3804 | 9 | 0.24 | 0.11–0.45 |
| Bengal | 2110 | 5 | 0.24 | 0.08–0.55 |
| Ragdoll | 1692 | 4 | 0.24 | 0.06–0.60 |
| Exotic | 399 | 0 | 0 | 0.0–0.92 |
| Overall | 194,563 | 1128 | 0.58 | 0.54–0.61 |
DM, diabetes mellitus; 95% CI, 95% confidence interval.
Completeness of data varied between the factors assessed: breed 99.9%, sex 99.1%, insurance 55.9%, age 99.5%, and bodyweight 70.8%. Of the DM cats with information available, 121 (10.7%) were purebred, 674 (59.8%) were male and 360 (41.6%) were insured. Median bodyweight was 5.4 kg (IQR: 4.5–6.5, range 2.3–22.9) and the median age at diagnosis for incident cases was 13.0 years (IQR: 10.3–15.0, range 1.0–21.8) (Fig 1). The most commonly affected breeds overall were crossbred cats (n = 1,006, proportion of case cats 89.2%), Burmese (n = 41, 3.6%), British Shorthair (n = 9, 0.8%), Siamese (n = 9, 0.8%) and Norwegian Forest cats (n = 8, 0.7%) (Table 2).
Figure 1.
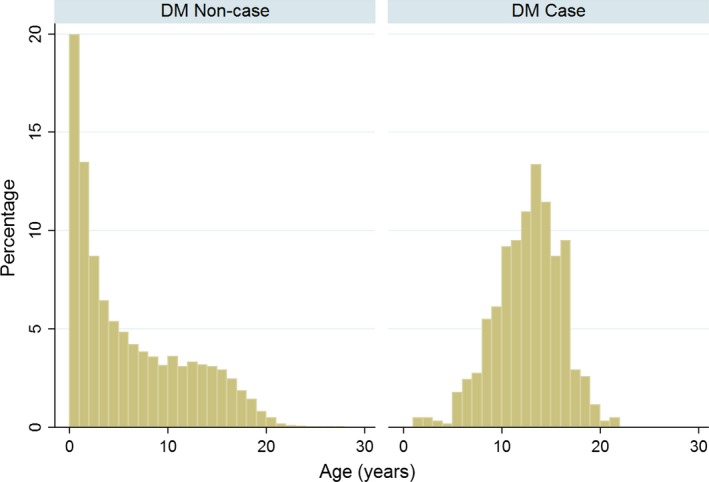
Ages of cats without (DM noncase; n = 192,505) and with (DM case; n = 621) diabetes mellitus attending primary‐care veterinary practices in England. The age was calculated for the DM noncases at the center‐date of the available clinical records and for the DM cases was at the date of first diagnosis.
Table 2.
Descriptive and univariable logistic regression results for risk factors associated with presence of diabetes mellitus in cats attending primary‐care veterinary practices in England. Data on both pre‐existing and incident DM cases were included
| Variable | Category | Case No. (%) | Noncase No. (%) | Odds Ratio | 95% CI | P‐Value |
|---|---|---|---|---|---|---|
| Purebred status | Crossbred | 1006 (89.2) | 172,572 (89.2) | Base | ||
| Purebred | 121 (10.7) | 20,709 (10.7) | 1.0 | 0.8–1.2 | .98 | |
| Not recorded | 1 (0.1) | 154 (0.1) | 1.1 | 0.2–8.0 | .91 | |
| Breed | Crossbred | 1006 (89.2) | 172,572 (89.2) | Base | ||
| Tonkinese | 7 (0.6) | 316 (0.2) | 3.8 | 1.8–8.1 | <.001 | |
| Norwegian Forest | 8 (0.7) | 354 (0.2) | 3.9 | 1.9–7.8 | <.001 | |
| Burmese | 41 (3.6) | 1769 (0.9) | 4.0 | 2.9–5.5 | <.001 | |
| Russian | 4 (0.4) | 424 (0.22) | 1.6 | 0.6–4.3 | .34 | |
| Oriental | 3 (0.3) | 309 (0.2) | 1.7 | 0.5–5.2 | .38 | |
| Rex | 2 (0.2) | 353 (0.2) | 1.0 | 0.2–3.9 | .97 | |
| Siamese | 9 (0.8) | 1817 (0.9) | 0.8 | 0.4–1.6 | .63 | |
| Maine Coon | 7 (0.6) | 1252 (0.7) | 1.0 | 0.5–2.0 | .91 | |
| Birman | 3 (0.3) | 1100 (0.6) | 0.5 | 0.2–1.5 | .19 | |
| Persian | 7 (0.6) | 2458 (1.3) | 0.5 | 0.2–1.0 | .059 | |
| British Shorthair | 9 (0.8) | 3795 (2.0) | 0.4 | 0.2–0.8 | .007 | |
| Ragdoll | 4 (0.4) | 1688 (0.9) | 0.4 | 0.2–1.1 | .073 | |
| Bengal | 5 (0.4) | 2105 (1.1) | 0.4 | 0.2–1.0 | .045 | |
| Exotic | 0 (0.0) | 399 (0.2) | 0.2 | 0.0–3.4 | .28 | |
| Other breed‐types | 13 (1.2) | 2724 (1.4) | 0.8 | 0.5–1.4 | .478 | |
| Bodyweight (kg) | <3.0 | 28 (2.5) | 16,681 (8.6) | Base | ||
| 3.0–3.9 | 118 (10.5) | 39,938 (20.7) | 1.8 | 1.2–2.7 | .007 | |
| 4.0–4.9 | 273 (24.2) | 42,249 (21.8) | 3.8 | 2.6–5.7 | <.001 | |
| 5.0–5.9 | 257 (22.8) | 24,790 (12.8) | 6.2 | 4.2–9.1 | <.001 | |
| 6.0–6.9 | 189 (16.8) | 9317 (4.8) | 12.1 | 8.1–18.0 | <.001 | |
| 7.0–7.9 | 118 (10.5) | 2664 (1.4) | 26.4 | 17.4–39.9 | <.001 | |
| ≥8.0 | 56 (5.0) | 1029 (0.5) | 32.4 | 20.5–51.3 | <.001 | |
| No bodyweight available for >6 month of age | 89 (7.9) | 56,767 (29.4) | 0.9 | 0.6–1.4 | .75 | |
| Age (years)a | <3.0 | 6 (0.5) | 81,075 (41.9) | 0.2 | 0.1–0.4 | <.001 |
| 3.0–5.9 | 14 (1.2) | 32,034 (16.6) | Base | |||
| 6.0–8.9 | 66 (5.9) | 22,330 (11.5) | 6.8 | 3.8–12.0 | <.001 | |
| 9.0–11.9 | 154 (13.7) | 18,892 (9.8) | 18.7 | 10.8–32.2 | <.001 | |
| 12.0–14.9 | 222 (19.7) | 18,422 (9.5) | 27.6 | 16.1–47.3 | <.001 | |
| ≥15.0 | 159 (14.1) | 19,752 (10.2) | 18.4 | 10.7–31.8 | <.001 | |
| Not recorded | 507 (45.0) | 930 (0.5) | 1247.4 | 730.6–2129.9 | <.001 | |
| Sex | Female | 450 (39.9) | 98,436 (50.9) | Base | ||
| Male | 674 (59.8) | 93,333 (48 3) | 1.6 | 1.4–1.8 | <.001 | |
| Not recorded | 4 (0.4) | 1666 (0.9) | 0.5 | 0.2–1.4 | .20 | |
| Insurance status | Noninsured | 506 (44.9) | 78,642 (40.7) | Base | ||
| Insured | 360 (31.9) | 29,253 (15.1) | 1.9 | 1.7–2.2 | <.001 | |
| Not recorded | 262 (23.2) | 85,540 (44.2) | 0.5 | 0.4–0.6 | <.001 |
95% CI, 95% confidence interval; EPR, electronic patient record.
Incident cases: age at diagnosis, pre‐existing cases: no age included, noncases: age at mid‐point of clinical records.
Of the noncase cats with information available, 20,709 (10.7%) were purebred, 93,333 (48.3%) were male and 29,253 (27.1%) were insured. Median bodyweight was 4.2 (IQR: 3.5–5.1) kg and the median EPR mid‐point age was 4.1 (IQR: 1.2–10.2) years. The most common breeds among the noncase cats were crossbreds, British shorthair, Persian, Bengal, and Siamese (Table 2).
There were 301 (48.3%) deaths among the 624 incident DM cases during the 5‐year study period. Of the 291 deceased cats with a recorded mechanism of death, 270 (92.8%) of the deaths were assisted (euthanasia), with the remaining 21 (7.2%) being unassisted. The median age at death for 298 incident DM cases with information available was 14.1 (IQR 11.5–16.3, range 1.0–21.5) years. Of the 301 cats that died during the study period, 220 (73.1%) died within 1 year from the date of diagnosis, 50 (16.6%) died between 1 and 2 years later, 25 (8.3%) died between 2 and 3 years later and 6 (2.0%) died after 3 years. In the overall study population, the median bodyweight of male cats (4.8 kg, IQR 4.0–5.6) was higher than for female cats (3.8 kg, IQR 3.1–4.5) (P < .001).
Univariable logistic regression modeling identified five variables with liberally significant (P < .20) association with DM diagnosis: breed, bodyweight, age, sex, and insurance status (Table 2) which were further evaluated using multivariable regression modeling. The final multivariable model (Table 3) retained four significant risk factors (breed, bodyweight, age, and insurance status) and showed good discrimination (area under the ROC curve: 0.944). No biologically significant interactions were identified. Including the clinic attended as a random effect improved the final model (P < .001); the clinic attended accounted for 4.2% of the variation in the data. After accounting for the effects of the other variables in the model, 3 breeds showed increased odds of DM diagnosis compared with the crossbred cats: Tonkinese (odds ratio [OR] 4.1; 95% CI 1.8–9.6; P = .001), Norwegian Forest (OR 3.5; 95% CI 1.3–9.6; P = .001) and Burmese (OR 3.0; 95% CI 2.0–4.4; P < .001). Odds of DM increased as maximum recorded bodyweight increased. Compared with cats weighing under 3 kg, cats weighing 4.0–4.9 kg had 3.2 times the odds (95% CI 2.0–5.2, P < .001) and cats weighing 5.0–5.9 kg had 5.1 times the odds (95% CI 3.1–8.2, P < .001) of DM. Increasing age was associated with increasing odds of DM diagnosis; cats aged 6.0–8.9 years showed 5.6 times the odds (95% CI 3.1–10.0, P < .001) and cats aged 9.0–11.9 years showed 17.1 (95% CI 9.9–29.6, P < .001) times the odds of DM diagnosis compared with cats aged 3.0–5.9 years. Insured cats had 2.0 (95% CI 1.6–2.4, P < .001) times the odds of DM diagnosis compared with uninsured cats. Sex was not significantly associated with the odds of DM in the final multivariable model.
Table 3.
Final multivariable logistic regression model for risk factors associated with diabetes mellitus diagnosis in cats attending primary‐care veterinary practices in England. Data on both pre‐existing and incident DM cases were included
| Variable | Category | OR | 95% CI | P‐Value |
|---|---|---|---|---|
| Breed | Crossbred | Base | ||
| Tonkinese | 4.1 | 1.8–9.6 | .001 | |
| Norwegian Forest | 3.5 | 1.3–9.6 | .016 | |
| Burmese | 3.0 | 2.0–4.4 | <.001 | |
| Russian | 2.5 | 0.8–8.1 | .12 | |
| Oriental | 1.8 | 0.4–7.3 | .43 | |
| Rex | 1.2 | 0.2–8.5 | .88 | |
| Siamese | 1.1 | 0.5–2.3 | .77 | |
| Maine Coon | 0.9 | 0.4–2.1 | .74 | |
| Birman | 0.8 | 0.3–2.6 | .73 | |
| Persian | 0.5 | 0.2–1.4 | .20 | |
| British Short Hair | 0.5 | 0.2–1.1 | .080 | |
| Ragdoll | 0.4 | 0.1–2.2 | .31 | |
| Bengal | 0.3 | 0.1–1.3 | .11 | |
| Exotic | 0.8 | 0.1–13.7 | .91 | |
| Other breed‐types | 0.6 | 0.3–1.1 | .11 | |
| Bodyweight (kg) | <3.0 | Base | ||
| 3.0–3.9 | 1.4 | 0.8–2.3 | .19 | |
| 4.0–4.9 | 3.2 | 2.0–5.2 | <.001 | |
| 5.0–5.9 | 5.1 | 3.1–8.2 | <.001 | |
| 6.0–6.9 | 10.2 | 6.3–16.8 | <.001 | |
| 7.0–7.9 | 19.3 | 11.5–32.6 | <.001 | |
| > or =8 | 20.0 | 11.0–36.3 | <.001 | |
| No bodyweight available for >6 month of age | 0.0 | 0.0–0.1 | <.001 | |
| Age (years)a | <3.0 | 0.4 | 0.2–1.0 | .060 |
| 3.0–5.9 | Base | |||
| 6.0 to <9.0 | 5.6 | 3.1–10.0 | <.001 | |
| 9.0 to <12.0 | 17.1 | 9.9–29.6 | <.001 | |
| 12.0 to <15.0 | 31.8 | 18.5–54.7 | <.001 | |
| > or =15.0 | 39.1 | 22.5–67.9 | <.001 | |
| No age available | 59093.7 | 29330.8–119058.1 | <.001 | |
| Insurance status | Noninsured | Base | ||
| Insured | 2.0 | 1.6–2.4 | <.001 | |
| Unknown | 1.0 | 0.8–1.2 | .90 |
95% CI, confidence interval.
Incident cases: age at diagnosis, pre‐existing cases: no age included, noncases: age at mid‐point of clinical records.
Discussion
This study used the largest primary‐care population of cats in England assembled to date to report the prevalence and risk factors for DM in cats. Our results reaffirm DM as a relatively common condition diagnosed in UK primary‐care practice; the 0.58% prevalence of DM recorded in this study is similar, although slightly higher, than previous prevalence estimates from insured feline populations in the United Kingdom (0.43%)2 and from veterinary hospital populations in the United States (0.42%)4 and slightly lower than estimates in Australia (0.74%).18 The study identified an increased risk of DM in Norwegian Forest, Burmese, and Tonkinese cats compared with crossbred cats. A greater risk of DM was also identified with increasing age and bodyweight, and among insured cats.
The findings of this investigation support several previous studies that revealed an increased risk of DM in Burmese cats30 and a recent study that identified increased risk in Norwegian Forest Cats.17 However, this is the first study to identify an increased risk of DM in Tonkinese cats and these results are informative in that breeds with a high within‐breed prevalence but that are not common in the general population, such as the Tonkinese, may be overlooked without access to large merged datasets such as the VetCompass database. The Tonkinese breed was created in the 1950s through cross‐breeding of Burmese and Siamese cats. This makes the Tonkinese and Burmese cat breeds genetically similar and it is possible that this genetic similarity could contribute to a shared predisposition for DM.31 A genetic component to DM in Burmese cats could also be supported by the increased DM risk shown for Australian and European Burmese, whereas an increased risk has not yet been demonstrated in the genetically distinct American Burmese population.2, 18, 19 However, it should be noted that, although several studies have investigated breed predispositions for DM in North American cats,4, 9 none of these have specifically assessed the Burmese breed. It is likely that genetic predisposition for DM among cats is polygenic in origin, as it is people with Type 2 DM.14 Genetic studies into human Type 2 DM have identified a large number of susceptibility loci, many of which are thought to increase a patient's risk of developing DM by altering β‐cell function or insulin sensitivity in body tissues.32 Cats and people with insulin resistance can maintain normoglycemia through a compensatory increase in β‐cell mass and insulin secretion, but will eventually develop DM if this hypersecretory response fails.33, 34, 35 In cats, insulin hypersecretion can be accompanied by hyperamylinemia and it is thought that amyloid deposition within pancreatic islets contributes to eventual β‐cell failure in DM in cats.36 However, in vitro and rodent models have shown that even mild increases in blood glucose concentration can lead to altered gene expression in pancreatic β‐cells and this is thought to contribute to eventual β‐cell failure in Type 2 diabetic people.34, 37 Genotype is therefore an important determinant of a patient's ability to withstand insulin resistance, and their susceptibility to β‐cell failure. Initial work in cats with DM has identified a number of genetic loci associated with DM development, including several loci associated with DM in lean DSH cats16 and a genetic polymorphism in the melanocortin 4 receptor, which is associated with DM in obese domestic shorthair cats.15 This initial work supports genotype in cats as an influence on DM susceptibility, and may explain why certain purebred cat breeds show increased, or decreased, risk of DM compared with crossbred cats.
This study revealed progressively increasing risk of DM as adult bodyweight increased above 2.9 kg. Although body weight and body condition are not synonymous, the increased risk of DM shown by the highest weight categories in this study could be partially caused by the presence of overweight and obese cats in these groups. Obesity is a well‐recognized risk factor for insulin resistance and DM in both cats and humans.38, 39 Chronic adipose tissue inflammation is a major cause of insulin resistance in obese people, but has not been documented in obese cats.13, 38 However, other mechanisms for obesity‐related insulin resistance might be similar between humans and cats. Derangements in the production of adipokine hormones have been documented in both obese cats and obese people. Adiponectin is a prevalent adipokine that has insulin‐sensitising and anti‐inflammatory properties. Reduced adiponectin concentrations have been demonstrated in obese people and obese cats, and are associated with increased risk of DM in people.40, 41, 42 In both humans and cats, obesity is also associated with increased concentrations of the adipokine leptin, which promotes energy utilization, satiety, and decreased serum glucose concentrations.13, 43 It is thought that hyperleptinemia in obese people develops in response to “leptin resistance” in which patients have an impaired biological response to leptin, and a similar reduced response to leptin may therefore contribute to insulin resistance in obese cats.44 Obesity is also associated with dyslipidemia in both cats and people.45, 46 Increased concentrations of lipid metabolites can contribute to insulin resistance by impairing insulin signaling and inducing production of inflammatory mediators.47 Previous work has shown that plasma profiles of lean Burmese cats show similar adipokine and lipid abnormalities to those shown by obese DSH cats suggesting that lean Burmese cats are in a metabolic state that resembles naturally occurring obesity in cats.45
Inclusion of cats with hypersomatotropism (acromegaly) in the diabetic group could also contribute to the increased risk of DM identified with increasing bodyweight category. Nearly 25% of DM cases in cats in the United Kingdom have been estimated to be caused by hypersomatotropism, so it is likely that a substantial proportion of diabetic cases in the current study were also affected by this condition.5 Acromegalic cats can show progressive weight gain and have higher bodyweights than nonacromegalic diabetic cats so their presence could have contributed to the increased risk of DM seen with increasing bodyweight in this study.5 Information on whether diabetic cats received screening for acromegaly was unfortunately not available for the current investigation. Finally, it is also possible that the increased risk of DM in higher weight categories in this study might reflect the influence of other factors that affect bodyweight, such as patient age and breed. Future studies which extract data on body condition score as well as bodyweight will help to disentangle these various effects.
The study identified an increasing risk of DM as patient age increased above 6 years. This finding agrees with other studies showing increased risk of DM in middle‐aged to geriatric cats4, 17 and resembles how prevalence of Type 2 DM increases with age in human patients.48 In people, an age‐related decline in insulin sensitivity and β‐cell function are thought to contribute to the increased risk of DM in older patients49 and similar mechanisms might contribute to DM in cats. Aging may also allow more time for significant amyloid deposition in the pancreas of cats and this could contribute to the development of DM in some cats.50 Finally, many comorbid diseases that promote DM in cats, such as hypersomatotropism and, admittedly more rarely, hyperadrenocorticism, typically affect middle‐aged or older cats and thus increase risk of DM in these age groups.5, 6
Our study showed that 48% of incident diabetic cases died or were euthanized during the study period with the highest proportion of these deaths occurring within 1 year of diagnosis. This result is similar to the 1‐year mortality rate of 41% in diabetic cats examined at Swiss and American referral hospitals51, 52 but is lower than the 1‐year mortality rate of 68% found by a small UK study that examined a primary‐practice population.54 Data on cause of death or reason for euthanasia was not available for cats in our study, but previous reports have found that concurrent diseases, rather than DM itself, are the most common cause of mortality in diabetic cats.53, 54 However, the emotional, time, and financial demands of managing a diabetic cat can be high for some pet owners and trigger a decision to euthanize their pet.55
Insured cats showed twice the odds of DM diagnosis compared with noninsure cats in this study. This may partially reflect that insured cats may have more bonded owners and are more likely to undergo investigations and have DM diagnosed.56 However, animals with health problems, such as DM, might be more likely to have their insurance status recorded, which could bias the study results toward detecting an increased association between insurance and DM diagnosis.
Although male cats showed 1.6 times the odds of DM compared with female cats in the univariable analysis of the current study, sex did not significantly contribute to a patient's risk of DM after accounting for the effects of other risk factors, including bodyweight in the multivariable analysis. In contrast, many previous studies that reported an increased risk of DM in male cats.2, 10 Male cats are more prone to obesity, than female cats57 and some studies have also reported that male cats are more likely to develop hypersomatotropism, which can cause DM alongside increasing bodyweight.58 Several previous studies that reported increased risk for DM in males did not have access to detailed individual bodyweight data2, 17 or did not adjust for other confounding effects, such as bodyweight, when assessing sex as a risk factor for DM.9, 10 The current study revealed no association between sex and DM; this may have been because a substantial proportion of the male risk was explained by the significantly greater median bodyweight of males (4.8 kg) compared with females (3.8 kg) in the study. It is also possible that any remaining association between sex and risk of DM was only weak and that the current study had insufficient power to detect this.
This study had some limitations. Several previous studies have examined neuter status as a risk factor for DM.2, 9 However, this was not evaluated in the current study because the vast majority of study cats were neutered early in life and neuter status would therefore mainly reflect patient age. The median time contributed to the study by each cat was short (0.3 years) but this was a conservative estimate that covered just the period between the first and final EPRs and did not also account for the phases both before and after these records when cats may also have presented to the veterinary practices had they been ill. Electronic clinical records are not primarily recorded for research purposes and therefore may vary in quality across the participating practices.59 Classification of cases as diabetic relied greatly on the clinical acumen and resources of the participating veterinarians. Information on how individual DM cases were diagnosed, and what treatment was given was not included in the current study but will be explored in a follow‐on study. Insurance status described whether an animal was insured at any point during the study period and it was possible that some cats became insured following the initial DM diagnosis. Finally, the practices that contributed to this study were all private, primary‐care practices mainly based in central and south‐eastern England and it is possible that the patient population at charity clinics or practices based elsewhere in the country might differ in their management from the population represented in the current study. This may affect generalizability of the descriptive prevalence results which are more heavily dependent on prevailing levels of clinical care but should have had minimal impact on the risk factor results which are more dependent on basic physiology which will be more constant across all cats in England.60
In conclusion, the findings of this investigation confirm that DM is a relatively common disease among cats in England and is associated with high mortality. The investigation supports previously identified risk factors for DM in cats, including a greater risk with increasing age and bodyweight, and an increased risk in Burmese and Norwegian Forest cats. However, the study is the first to report an increased risk of DM in Tonkinese cats, which are derived from the Burmese breed. This finding supports the theory that a genetic component contributes to increased susceptibility for DM in Burmese cats and related breeds. After accounting for bodyweight and other risk factors, male gender was not confirmed as a significant independent risk factor. Findings from this study can improve understanding of DM and diagnosis rates for this disease, and improve welfare in cats by alerting clinicians to demographic subsets of cats at increased risk.
Acknowledgments
Thanks to Noel Kennedy (RVC) for VetCompass software and programming development and Peter Dron (RVC) for database development. We acknowledge the Medivet Veterinary Partnership, Vets4Pets/Companion Care, Blythwood Vets, Vets Now and the other UK practices who collaborate in VetCompass. We are grateful to The Kennel Club, The Kennel Club Charitable Trust and Dogs Trust for supporting VetCompass. Dan O'Neill was supported by a grant from The Kennel Club Charitable Trust and the PhD of Ruth Gostelow is funded by Evetts Luff Animal Welfare Trust.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This research was performed at The Department of Production and Population Health at The Royal Veterinary College. The work in this manuscript has not been presented at any scientific meeting.
Footnotes
Epi Info 7, Centers for Disease Control and Prevention, Atlanta, GA
Excel, Microsoft Corporation, Redmond, WA
Stata 13, StataCorp LP, College Station, TX
References
- 1. Nelson RW, Reusch CE. Animal models of disease: classification and etiology of diabetes in dogs and cats. J Endocrinol 2014;222:T1–T9. [DOI] [PubMed] [Google Scholar]
- 2. McCann TM, Simpson KE, Shaw DJ, et al. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire‐based putative risk factor analysis. J Feline Med Surg 2007;9:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sallander M, Eliasson J, Hedhammar A. Prevalence and risk factors for the development of diabetes mellitus in Swedish cats. Acta Vet Scand 2012;54:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prahl A, Guptill L, Glickman NW, et al. Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J Feline Med Surg 2007;9:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niessen SJ, Forcada Y, Mantis P, et al. Studying cat (Felis catus) diabetes: beware of the acromegalic imposter. PLoS One 2015;10:e0127794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valentin SY, Cortright CC, Nelson RW, et al. Clinical findings, diagnostic test results, and treatment outcome in cats with spontaneous hyperadrenocorticism: 30 cases. J Vet Intern Med 2014;28:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linderman MJ, Brodsky EM, de Lorimier LP, et al. Feline exocrine pancreatic carcinoma: a retrospective study of 34 cases. Vet Comp Oncol 2013;11:208–218. [DOI] [PubMed] [Google Scholar]
- 8. Lien YH, Huang HP, Chang PH. Iatrogenic hyperadrenocorticism in 12 cats. J Am Anim Hosp Assoc 2006;42:414–423. [DOI] [PubMed] [Google Scholar]
- 9. Panciera DL, Thomas CB, Eicker SW, et al. Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986). J Am Vet Med Assoc 1990;197:1504–1508. [PubMed] [Google Scholar]
- 10. Slingerland LI, Fazilova VV, Plantinga EA, et al. Indoor confinement and physical inactivity rather than the proportion of dry food are risk factors in the development of feline type 2 diabetes mellitus. Vet J 2009;179:247–253. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen CT, Pham NM, Lee AH, et al. Prevalence of and risk factors for type 2 diabetes mellitus in Vietnam: a systematic review. Asia Pac J Public Health 2015;27:588–600. [DOI] [PubMed] [Google Scholar]
- 12. Hoenig M, Wilkins C, Holson JC, et al. Effects of obesity on lipid profiles in neutered male and female cats. Am J Vet Res 2003;64:299–303. [DOI] [PubMed] [Google Scholar]
- 13. Hoenig M, Pach N, Thomaseth K, et al. Cats differ from other species in their cytokine and antioxidant enzyme response when developing obesity. Obesity (Silver Spring) 2013;21:E407–E414. [DOI] [PubMed] [Google Scholar]
- 14. Sun X, Yu W, Hu C. Genetics of type 2 diabetes: insights into the pathogenesis and its clinical application. Biomed Res Int 2014;2014:926713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forcada Y, Holder A, Church DB, et al. A polymorphism in the melanocortin 4 receptor gene (MC4R:c.92C>T) is associated with diabetes mellitus in overweight domestic shorthaired cats. J Vet Intern Med 2014;28:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forcada Y, Boursnell M, Catchpole B, Church DB. A genome‐wide association study identifies novel candidate genes for the susceptibility to diabetes mellitus in DSH cats [abstract]. 25th ECVIM‐CA Congress, Lisbon, Portugal. [DOI] [PMC free article] [PubMed]
- 17. Ohlund M, Fall T, Strom Holst B, et al. Incidence of diabetes mellitus in insured Swedish cats in relation to age, breed and sex. J Vet Intern Med 2015;29(5):1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lederer R, Rand JS, Jonsson NN, et al. Frequency of feline diabetes mellitus and breed predisposition in domestic cats in Australia. Vet J 2009;179:254–258. [DOI] [PubMed] [Google Scholar]
- 19. Alhaddad H, Khan R, Grahn RA, et al. Extent of linkage disequilibrium in the domestic cat, Felis silvestris catus, and its breeds. PLoS One 2013;8:e53537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg 2015;17:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. http://www.rvc.ac.uk/vetcompass. Accessed October 2, 2015.
- 22. O'Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in dogs attending primary‐care veterinary practices in England. PLoS One 2014;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. http://www.venomcoding.org/VeNom/Welcome.html. Accessed October 2, 2015.
- 24. International Cat Care [Internet]. In. http://icatcare.org/advice/cat-breeds: 2015.
- 25. Coe JB, Adams CL, Bonnett BN. A focus group study of veterinarians' and pet owners' perceptions of the monetary aspects of veterinary care. J Am Vet Med Assoc 2007;231:1510–1518. [DOI] [PubMed] [Google Scholar]
- 26. Kirkwood BR, Sterne JAC. Essential Medical Statistics, 2nd ed Oxford: Blackwell Science; 2003. [Google Scholar]
- 27. Dormann CF, Elith J, Bacher S, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013;36:27–46. [Google Scholar]
- 28. Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002;21:2409–2419. [DOI] [PubMed] [Google Scholar]
- 29. Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research, 2nd ed Charlottetown: VER Inc; 2009. [Google Scholar]
- 30. Rand JS, Bobbermien LM, Hendrikz JK, et al. Over representation of Burmese cats with diabetes mellitus. Aust Vet J 1997;75:402–405. [DOI] [PubMed] [Google Scholar]
- 31. Kurushima JD, Lipinski MJ, Gandolfi B, et al. Variation of cats under domestication: genetic assignment of domestic cats to breeds and worldwide random‐bred populations. Anim Genet 2013;44:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohlke KL, Boehnke M. Recent advances in understanding the genetic architecture of type 2 diabetes. Hum Mol Genet 2015;24:R85–R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoenig M, Hall G, Ferguson D, et al. A feline model of experimentally induced islet amyloidosis. Am J Pathol 2000;157:2143–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weir GC, Bonner‐Weir S. Five stages of evolving beta‐cell dysfunction during progression to diabetes. Diabetes 2004;53(Suppl 3):S16–S21. [DOI] [PubMed] [Google Scholar]
- 35. Gal A, Hoenig M, O'Brien TD, Wallig M. Histopathology of pancreata from life‐long dietary induced pre‐diabetic obese cats and lean controls [abstract]. Vet Pathol 2010;47(6S):150. [Google Scholar]
- 36. Martin LJ, Siliart B, Lutz TA, et al. Postprandial response of plasma insulin, amylin and acylated ghrelin to various test meals in lean and obese cats. Br J Nutr 2010;103:1610–1619. [DOI] [PubMed] [Google Scholar]
- 37. Sharma PR, Mackey AJ, Dejene EA, et al. An islet‐targeted genome‐wide association scan identifies novel genes implicated in cytokine‐mediated islet stress in type 2 diabetes. Endocrinology 2015;156:3147–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity‐induced insulin resistance. Biochim Biophys Acta 2014;1842:446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc 1998;212:1725–1731. [PubMed] [Google Scholar]
- 40. Spranger J, Kroke A, Mohlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003;361:226–228. [DOI] [PubMed] [Google Scholar]
- 41. Muranaka S, Mori N, Hatano Y, et al. Obesity induced changes to plasma adiponectin concentration and cholesterol lipoprotein composition profile in cats. Res Vet Sci 2011;91:358–361. [DOI] [PubMed] [Google Scholar]
- 42. Bjornvad CR, Rand JS, Tan HY, et al. Obesity and sex influence insulin resistance and total and multimer adiponectin levels in adult neutered domestic shorthair client‐owned cats. Domest Anim Endocrinol 2014;47:55–64. [DOI] [PubMed] [Google Scholar]
- 43. Antuna‐Puente B, Feve B, Fellahi S, et al. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab 2008;34:2–11. [DOI] [PubMed] [Google Scholar]
- 44. Crujeiras AB, Carreira MC, Cabia B, et al. Leptin resistance in obesity: an epigenetic landscape. Life Sci 2015;140:57–63. [DOI] [PubMed] [Google Scholar]
- 45. Lee P, Mori A, Coradini M, et al. Potential predictive biomarkers of obesity in Burmese cats. Vet J 2013;195:221–227. [DOI] [PubMed] [Google Scholar]
- 46. Ruotolo G, Howard BV. Dyslipidemia of the metabolic syndrome. Curr Cardiol Rep 2002;4:494–500. [DOI] [PubMed] [Google Scholar]
- 47. Piya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol 2013;216:T1–T15. [DOI] [PubMed] [Google Scholar]
- 48. Cowie CC, Rust KF, Byrd‐Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 2006;29:1263–1268. [DOI] [PubMed] [Google Scholar]
- 49. De Tata V. Age‐related impairment of pancreatic beta‐cell function: pathophysiological and cellular mechanisms. Front Endocrinol 2014;5:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herndon AM, Breshears MA, McFarlane D. Oxidative modification, inflammation and amyloid in the normal and diabetic cat pancreas. J Comp Pathol 2014;151:352–362. [DOI] [PubMed] [Google Scholar]
- 51. Callegari C, Mercuriali E, Hafner M, et al. Survival time and prognostic factors in cats with newly diagnosed diabetes mellitus: 114 cases (2000–2009). J Am Vet Med Assoc 2013;243:91–95. [DOI] [PubMed] [Google Scholar]
- 52. Kraus MS, Calvert CA, Jacobs GJ, et al. Feline diabetes mellitus: a retrospective mortality study of 55 cats (1982–1994). J Am Anim Hosp Assoc 1997;33:107–111. [DOI] [PubMed] [Google Scholar]
- 53. Little CJ, Gettinby G. Heart failure is common in diabetic cats: findings from a retrospective case‐controlled study in first‐opinion practice. J Small Anim Pract 2008;49:17–25. [DOI] [PubMed] [Google Scholar]
- 54. Niessen S, Powney S, Guitian J, et al. Diabetes mellitus and euthanasia: how often and why. J Vet Intern Med 2010;24:1568. [DOI] [PubMed] [Google Scholar]
- 55. Niessen SJ, Powney S, Guitian J, et al. Evaluation of a quality‐of‐life tool for cats with diabetes mellitus. J Vet Intern Med 2010;24:1098–1105. [DOI] [PubMed] [Google Scholar]
- 56. Egenvall A, Nødtvedt A, Penell J, et al. Insurance data for research in companion animals: benefits and limitations. Acta Vet Scand 2009;51:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Appleton DJ, Rand JS, Sunvold GD. Insulin sensitivity decreases with obesity, and lean cats with low insulin sensitivity are at greatest risk of glucose intolerance with weight gain. J Feline Med Surg 2001;3:211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Niessen SJ, Petrie G, Gaudiano F, et al. Feline acromegaly: an underdiagnosed endocrinopathy? J Vet Intern Med 2007;21:899–905. [DOI] [PubMed] [Google Scholar]
- 59. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol 2015;44(3):827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Elwood M. Critical Appraisal of Epidemiological Studies and Clinical Trials. 3rd ed. Oxford: Oxford University Press; 2007. [Google Scholar]


