Abstract
Background
Despite the paucity of data available, stall‐side serum amyloid (SAA) assays are commonly used to make diagnostic and treatment decisions in foals with bronchopneumonia.
Hypothesis
Measurement of SAA concentrations can accurately differentiate pneumonic from healthy foals.
Animals
Fifty‐four pneumonic foals between 3 weeks and 5 months of age were compared to 44 healthy controls. In addition, 47 foals on a farm endemic for R. equi infections were studied.
Methods
Serum samples were collected from pneumonic foals at hospital admission. Foals were categorized as having pneumonia caused by R. equi or by other microorganisms based on culture of a tracheobronchial aspirate. In addition, serum samples were obtained at 2‐week intervals from foals born at a farm endemic for R. equi. SAA concentrations were measured by a point‐of‐care assay. Diagnostic performance of SAA was assessed by use of receiver operating characteristic curves.
Results
Concentrations of SAA in foals with bronchopneumonia were significantly (P < 0.001) higher than those of healthy foals, but 15 of 54 pneumonic foals (28%) had SAA concentrations <5 μg/mL. There was no correlation between SAA concentrations and radiographic score in foals with R. equi pneumonia. The ability of SAA to predict development of R. equi pneumonia at the endemic farm was limited with a sensitivity of 64% and a specificity of 77%.
Conclusion and clinical importance
Overall, SAA concentrations are significantly higher in pneumonic than in healthy foals. However, performance of SAA in detecting pneumonic foals is limited by the high proportion of false‐positive and false‐negative results.
Keywords: Pneumonia, Rhodococcus equi, Streptococcus zooepidemicus
Abbreviations
- APP
acute phase protein(s)
- CI
confidence intervals
- ROC
receiver operating characteristic
- SAA
serum amyloid A
Pneumonia is the leading cause of disease and death in foals in Texas1 and ranks 3rd as a cause of morbidity and 2nd (after a combined category of trauma, injury, and wounds) as a cause of mortality in the United States.2 Gram‐positive bacteria, such as Streptococcus equi subspecies zooepidemicus (S. zooepidemicus) and Rhodococcus equi, are the most common causes of pneumonia in foals between 3 weeks and 6 months of age.3, 4 R. equi is endemic at many horse‐breeding farms with cumulative incidence of clinical disease often exceeding 20% of the foal crop.5 At farms where the disease is endemic, the costs resulting from veterinary care, long‐term treatment, and mortality of some foals are very high. Early recognition of R. equi pneumonia before development of clinical signs, along with appropriate treatment of infected foals, would likely reduce losses and limit the costs associated with long‐term treatment of severely affected animals.
Acute phase proteins (APP) are defined as proteins whose plasma concentration increases or decreases by at least 25% after an inflammatory stimulus.6 Fibrinogen, one of the earliest recognized APP, has historically been the most commonly measured APP in horses.7 Fibrinogen concentrations are commonly measured in foals with bronchopneumonia as a marker for severity of disease and to monitor response to treatment.8 Foals with pneumonia caused by R. equi have significantly higher plasma fibrinogen concentrations than foals with pneumonia caused by other bacterial agents, although there is considerable overlap between these groups.9 SAA offers several advantages over fibrinogen as an APP including low or undetectable concentrations in plasma of healthy individuals, rapid and marked (>10‐fold) increase during an inflammatory process, and rapid decrease with disease resolution.7 Several studies have evaluated SAA as an aid in the detection and management of inflammatory disorder in foals and adult horses.10, 11, 12 The recent availability of stall‐side point‐of‐care assays has resulted in the widespread use of SAA to make treatment decisions in foals with bronchopneumonia and to detect foals suspected of having subclinical pneumonia at farms endemic for R. equi despite the lack of objective information regarding the value of these approaches.
The objectives of this study were to determine if pneumonic foals have significantly higher SAA concentrations as measured with a point‐of care assay than clinically healthy foals, to determine if foals with pneumonia caused by R. equi have significantly higher SAA concentrations than foals with pneumonia caused by other bacteria, and to determine if there is an association between SAA concentration and lesion severity in foals with pneumonia caused by R. equi. An additional objective of this study was to investigate the diagnostic performance of a point‐of‐care SAA assay for early identification of foals with R. equi pneumonia at a farm where the disease is endemic.
Materials and Methods
SAA at the time of diagnosis in foals with bronchopneumonia
Archived serum samples collected at admission from foals between 3 weeks and 5 months of age referred to the veterinary teaching hospital for diagnosis and treatment of lower respiratory tract disease were used. Foals were diagnosed with pneumonia based on three criteria: (i) clinical signs of respiratory disease such as cough, bilateral purulent nasal discharge, fever, tachypnea, or respiratory distress; (ii) septic airway inflammation as defined by a predominance of degenerate neutrophils with or without bacteria on cytological examination of a tracheobronchial aspirate; and (iii) evidence of bronchopneumonia based on thoracic ultrasonography or radiography. Pneumonic foals from which R. equi was recovered from culture of tracheobronchial aspirate fluid were considered as having pneumonia caused by R. equi (n = 31). Pneumonic foals from which R. equi was not cultured were considered as having pneumonia caused by other infectious agents (n = 23). When radiographs were available for foals with R. equi, a radiographic scoring system ranging from 0 (no abnormalities) to 18 (most severe lesions) was used as a measure of disease severity.13 Archived serum samples from foals between 3 weeks and 5 months of age housed at a farm with no history of endemic infections caused by R. equi and that remained clinically healthy from birth to at least 6 months of age were used as controls (n = 44). The healthy foals received a physical examination before sample collection, but were not subjected to other diagnostic tests. The study was approved by the institutional Clinical Research Review Committee.
Use of SAA for early detection of pneumonia on a farm endemic for R. equi
Archived serum samples from a prior study conducted at a thoroughbred farm endemic for infections caused by R. equi were used.14 Only samples from foals that had not received hyperimmune plasma were used. Foals were monitored daily for clinical signs of illness by experienced farm personnel. During the first 6 months of life, blood samples were obtained from each foal at 2–3‐week intervals. The first blood sample was collected between 2 and 3 weeks of age. Foals with clinical signs of illness received a complete physical examination, including thorough auscultation of the lungs, by the farm veterinarian. A tracheobronchial aspirate was obtained from every foal with clinical signs of disease of the lower respiratory tract such as cough, bilateral nasal discharge, tachypnea, fever, or abnormal lung sounds. Diagnosis of pneumonia caused by R. equi was made on the basis of bacteriologic culture of the organism from a tracheobronchial aspirate. Foals diagnosed with R. equi pneumonia were treated orally with erythromycin ethylsuccinate (25 mg/kg q 8 h) and rifampin (5 mg/kg q 12 h) for a minimum of 3 weeks. Foals that remained clinically healthy during the entire breeding season were used as negative controls.
Measurement of SAA and fibrinogen concentrations
Fibrinogen concentrations were measured on fresh plasma samples by means of the heat precipitation method.14, 15 Concentrations of SAA were measured on archived serum samples stored at −80°C by the point‐of‐care assay1 and quantitative results were obtained by a handheld reader2 by following the instructions of the manufacturer. As per data generated by the manufacturer, the assay was quantitative at concentrations ranging between 7 and 3,000 μg/mL with a mean intra‐assay coefficient of variation of 7.8% (range 0.9–13%) at concentrations ranging between 50 and 2,000 μg/mL.
Data analysis
Normality of the data and equality of variances were assessed by the Shapiro‐Wilk and Levene's tests, respectively. Data were not normally distributed despite transformation. Concentrations of SAA between foals with R. equi pneumonia, foals with pneumonia caused by other bacteria, and clinically healthy foals were compared by the Kruskal‐Wallis one‐way ANOVA on ranks. Multiple pair‐wise comparisons between groups were done by Dunn's method. Differences in SAA concentrations between survivors and non‐survivors were compared by the Mann‐Whitney U‐test. SAA concentrations at time of diagnosis were compared to concentrations approximately 2 weeks after initiation of treatment by the Wilcoxon signed rank test. The diagnostic performance of SAA to identify pneumonia was assessed by use of receiver operating characteristic (ROC) curve analysis. Foals with pneumonia were considered true positives and clinically healthy foals were considered true negatives. Kendall's tau was used to assess correlation between SAA concentration and radiographic score in foals with pneumonia caused by R. equi.
The diagnostic performance of SAA or fibrinogen for detection of R. equi pneumonia before or at the same time of clinical diagnosis of R. equi pneumonia was also assessed by ROC curve analysis. Foals with culture‐confirmed R. equi pneumonia were considered true positives and foals that remained clinically healthy during the entire breeding season were considered true negatives. In foals diagnosed with R. equi pneumonia, the highest SAA or fibrinogen concentration available before or on the day of clinical diagnosis was used in the analysis. In foals that remained clinically healthy, the highest SAA or fibrinogen concentration obtained during the 6‐month monitoring period was used in the analysis. The method of Delong et al16 was used for the calculation of the standard error of the area under the curve (AUC) and of the difference between two AUCs. The AUC is a summary statistic of overall diagnostic performance. By use of AUC, a categorical distinction of test performance can be made between non‐informative (AUC, 0.50), less accurate (0.51–0.70), moderately accurate (0.71–0.90), and highly accurate (0.90–1.00) tests.9, 17 Data are presented as median (25th and 75th percentiles) unless otherwise specified. For all analyses, values of P ≤ 0.05 were considered significant.
Results
SAA at the time of diagnosis in foals with bronchopneumonia
R. equi was cultured from a tracheobronchial aspirate in 31 pneumonic foals. A total of 23 foals had pneumonia from which R. equi was not cultured. Bacterial isolates cultured from foals from which R. equi was not identified included Streptococcus equi subspecies zooepidemicus (n = 13), other beta‐hemolytic streptococci (n = 2), Actinobacillus spp. (n = 3), Pasteurella spp. (n = 2), Escherichia coli (n = 2), and Bordetella bronchiseptica (n = 1). There was a significant (P < 0.001) effect of group on SAA concentrations (Fig 1). SAA concentrations (median, 25th and 75th percentiles) of foals with pneumonia caused by R. equi (212 μg/mL; 7–781 μg/mL) and of foals with pneumonia caused by other bacteria (27 μg/mL; 0–604 μg/mL) were significantly higher than that of clinically healthy foals (0 μg/mL; 0–5 μg/mL). SAA concentrations of foals with R. equi pneumonia were not significantly different from those of foals with pneumonia caused by other bacteria. Survival data were available for 28 foals with R. equi pneumonia. Concentration of SAA in 7 non‐survivors (221 μg/mL; 129–1,185 μg/mL) was not significantly different (P = 0.135) from that of 21 survivors (108 μg/mL; 0–606 μg/mL).
Figure 1.
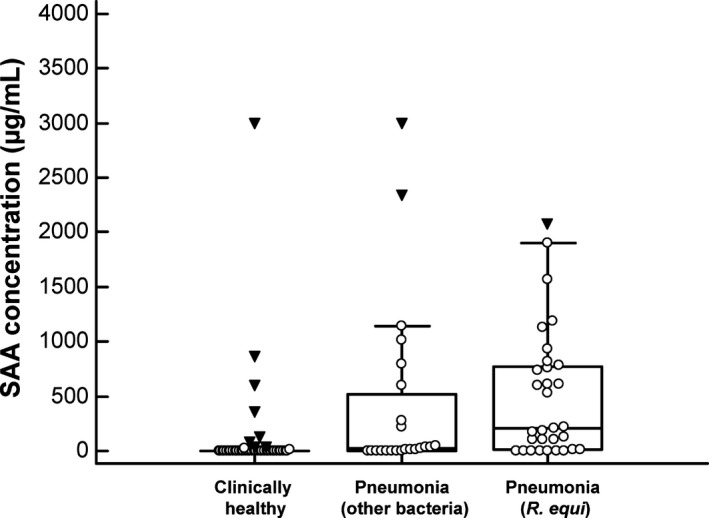
SAA concentrations in foals with R. equi pneumonia (n = 31), foals with pneumonia caused by other bacteria (n = 23), and clinically healthy foals (n = 44). Open circles represent SAA concentrations for individual foals. The central box represents the values from the lower to upper quartile (25th to 75th percentiles). The middle line represents the median. The error bars extend from the minimum to the maximum value, excluding outliers (upper or lower quartile ± 3 times the interquartile range) which are displayed as inverted solid triangles.
The AUC of SAA to correctly classify foals as being pneumonic or clinically healthy was 0.751 ± 0.048 (P < 0.0001). The best cut‐point was >5 μg/mL resulting in a sensitivity of 72.2% and a specificity of 77.3%. Increasing the cut‐point improved the specificity to the detriment of sensitivity (Table 1). Correlation between SAA concentration and severity of pneumonia as assessed by the radiographic score was not statistically significant (Fig 2; r = 0.162, P = 0.351).
Table 1.
Sensitivity and specificity of SAA concentration at selected cutoff values for the diagnosis of foals with pneumonia. Foals with pneumonia (n = 54) were compared to foals that remained clinically healthy (n = 44)
| Cut‐point (μg/mL) | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|
| ≥5 | 72 (58–83) | 77 (62–88) |
| ≥15 | 65 (51–77) | 80 (65–90) |
| ≥50 | 57 (41–69) | 86 (73–95) |
| ≥200 | 48 (35–62) | 91 (78–97) |
| ≥1,000 | 19 (9–31) | 98 (88–100) |
Figure 2.
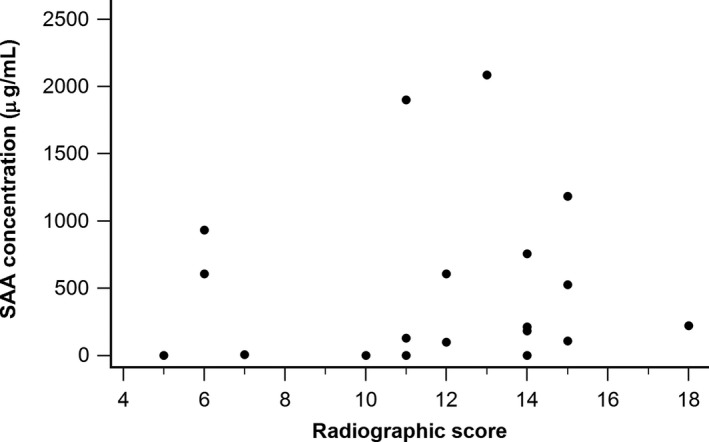
Association between SAA concentration and radiographic score in 19 foals with R. equi pneumonia.
Use of SAA for early detection of pneumonia on a farm endemic for R. equi
Of the 50 foals enrolled in this study, 25 (53.2%) developed R. equi pneumonia confirmed by culture and 22 (46.8%) remained clinically healthy until at least 6 months of age. Three foals were excluded because of illnesses unrelated to infections caused by R. equi. The median age at time of diagnosis was 36 days (34–47 days). The AUC for the ability of SAA to identify foals with R. equi pneumonia before or at the same time as detection of clinical signs was 0.676 ± 0.077 (95% confidence interval [CI] = 0.524–0.805) and significantly different (P = 0.026) from an AUC of 0.5. The optimal cut‐point was >53 μg/mL resulting in a sensitivity of 64% (95% CI = 43–82%) and a specificity of 77% (95% CI = 55–92%). Predictive values for a positive or negative test over a wide range of disease prevalence are presented in Fig 3. The AUC for the ability of SAA to identify foals with R. equi pneumonia before or at the same time as detection of clinical signs was not significantly different (P = 0.214) from that of fibrinogen (0.795 ± 0.063, 95% CI = 0.652–0.898) (Fig 4).
Figure 3.
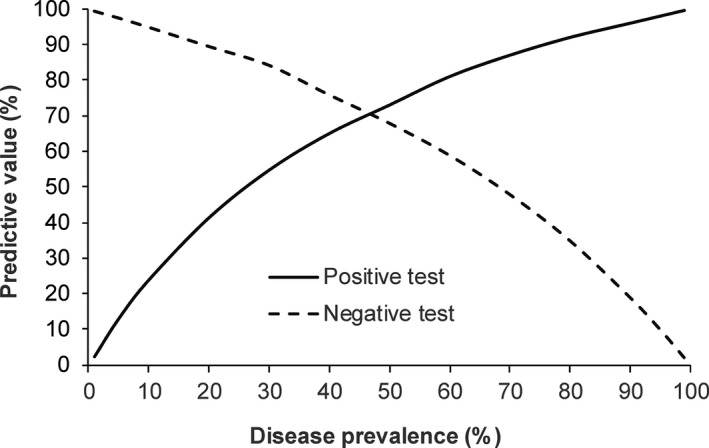
Predictive value of a positive or negative test at various disease prevalence with a cut‐point SAA concentration of >53 μg/mL. Foals with a culture‐confirmed R. equi pneumonia (n = 25) were compared to foals that remained clinically healthy during the entire breeding season (n = 22).
Figure 4.
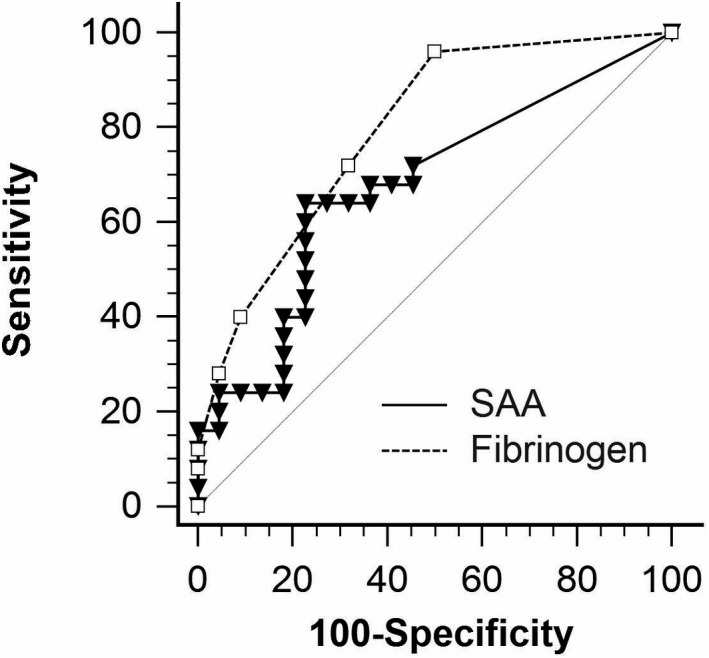
Receiver operating characteristic curves for SAA concentrations in foals relative to development of pneumonia and bacteriologic culture of R. equi from tracheobronchial aspirates. Foals with a culture‐confirmed R. equi pneumonia (n = 25) were compared to foals that remained clinically healthy during the entire breeding season (n = 22). The diagonal interrupted line indicates an AUC of 0.5.
Median peak SAA concentration before or at the time of diagnosis was significantly (P = 0.033) higher for foals that developed pneumonia caused by R. equi (114 μg/mL; 0–588 μg/mL) than for healthy controls (0 μg/mL; 0–88 μg/mL). The AUC, sensitivity, and specificity of SAA in predicting development of R. equi pneumonia at various cut‐points are presented in Table 2. At the lowest cut‐point of 5 μg/mL, disease would have been detected before development of clinical signs in 14 of 25 foals and the day of diagnosis in 4 foals. Samples collected approximately 2 weeks (median 13 days, 12–14 days) after initiation of treatment were available for 14 foals. SAA concentrations at time of diagnosis (0 μg/mL; 0–401 μg/mL) were not significantly different (P = 0.131) from those measured approximately 2 weeks after initiation of treatment (0 μg/mL; 0–4.1 μg/mL) (Fig 5). SAA concentrations at the time of diagnosis were ≥5 μg/mL in 6 foals. All 6 foals had considerably lower SAA concentrations at the follow‐up sampling (Fig 5). All foals responded to treatment with erythromycin and rifampin.
Table 2.
Sensitivity, specificity, and AUC (±SEM) of SAA to predict development of R. equi pneumonia at the same time as or before development of clinical signs at an endemic farm
| Cut‐point (μg/mL) | Sensitivity % (95% CI) | Specificity % (95% CI) | AUC | P valuea |
|---|---|---|---|---|
| ≥5 | 72 (51–88) | 45 (24–68) | 0.587 ± 0.071 | 0.300 |
| ≥15 | 72 (51–88) | 50 (28–72) | 0.610 ± 0.071 | 0.188 |
| ≥50 | 64 (43–82) | 68 (45–86) | 0.661 ± 0.071 | 0.026 |
| ≥200 | 44 (24–65) | 73 (50–89) | 0.584 ± 0.070 | 0.318 |
| ≥1,000 | 16 (5–36) | 95 (77–100) | 0.557 ± 0.044 | 0.497 |
A P value < 0.05 rejects the null hypothesis that measurement of SAA at a given cut‐off has an AUC of 0.5.
Figure 5.
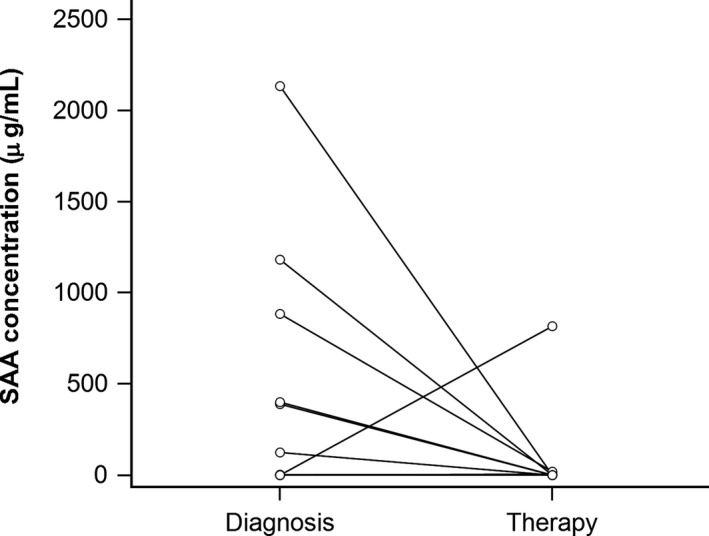
SAA concentrations in 14 foals at the time of diagnosis of pneumonia caused by R. equi and after approximately 2 weeks of treatment with oral administration of erythromycin ethylsuccinate (25 mg/kg q 8 h) and rifampin (5 mg/kg q 12 h).
Discussion
This study provides insight into SAA concentrations as measured with a commonly used point‐of‐care assay in foals with R. equi pneumonia, foals with pneumonia caused by other microorganisms, and healthy foals between 3 weeks and 5 months of age. In addition, this study sought to determine if SAA concentrations correlate with pulmonary disease severity in foals with pneumonia caused by R. equi and if the point‐of‐care SAA assay might be of benefit in early identification of foals that develop R. equi pneumonia.
As expected, foals with pneumonia had significantly higher SAA concentrations than healthy foals. Thirty‐four of 44 (77%) healthy foals had SAA concentrations ≤5 μg/mL. However, some healthy foals had SAA concentrations >200 μg/mL despite no apparent signs of disease. Because SAA concentrations were measured on archived samples, results were not available immediately and healthy foals with high SAA concentrations were not subjected to additional diagnostic testing. High SAA concentrations in a small proportion of apparently healthy foals were also reported in a prior study.11 Although it is possible that foals with high SAA concentrations had subclinical pulmonary or extrapulmonary disease, the results of these studies indicate that high SAA concentration alone is not necessarily an indication for antimicrobial therapy in foals. Even at the lowest cut‐point of ≥5, sensitivity was low at 72%, indicating that nearly 30% of foals with bacterial bronchopneumonia severe enough to necessitate referral to a veterinary hospital did not have elevated SAA concentrations. The proportion of foals with R. equi pneumonia with SAA concentrations <5 μg/mL (7 of 31 or 23%) was similar to that of foals with pneumonia caused by other microorganisms (8 of 23 or 35%). Therefore, low SAA concentrations do not necessarily rule out severe bacterial bronchopneumonia in older foals. Similarly, there was no significant correlation between SAA concentrations and severity of pulmonary lesions in foals with R. equi pneumonia. It remains unknown why some foals had low or undetectable SAA despite severe pneumonia and suppurative inflammation as assessed by radiography and airway cytology, respectively. It is possible that SAA concentrations decrease over time in chronic cases. Additional studies will be necessary to test this hypothesis because the duration of pneumonia before admission was not known in this study. In foals with pneumonia caused by R. equi, fibrinogen concentrations have been shown to be significantly higher in survivors than in non‐survivors.18, 19 Although median SAA concentration was higher in non‐survivor than in survivor in this study, the difference was not statistically significant. These results must be interpreted with caution because of the small sample size and resulting low statistical power.
Recognition of foals with R. equi pneumonia before development of clinical signs would likely reduce losses and limit costs associated with long‐term treatment of severely affected foals. In a recent study, there was no association between SAA concentrations and ultrasonographic evidence of pneumonia in 10 foals raised at a farm endemic for R. equi.20 Although thoracic ultrasonography has long been used to screen foals for R. equi pneumonia at endemic farms,21, 22 recent studies have shown that many foals with ultrasonographic lesions do not develop clinical signs of pneumonia and recover without treatment.23, 24, 25 In addition, administration of antimicrobial agents to foals with small ultrasonographic lesions does not hasten lesion resolution compared to administration of a placebo.24, 25 Therefore, this study used clinical signs of pneumonia as the outcome of interest rather than ultrasonographic detection of pulmonary lesions. In another study at a farm endemic for R. equi, there were no significant differences between SAA concentrations of foal with pneumonia and clinically unaffected foals at 7–14 days and at 21–28 days of age.11 However, most of the foals in the aforementioned study were not subjected to culture of a tracheobronchial aspirate, so it remains unknown if R. equi is the etiologic agent in all cases.11 In contrast, this study demonstrates that measurement of SAA is similar to that of measurement of fibrinogen and significantly better than flipping a coin at predicting which foals will develop clinical signs of pneumonia caused by R. equi. However, the diagnostic performance of SAA in this study was limited with low sensitivity and specificity. Improving either sensitivity or specificity of SAA by changing the cut‐point value of the test could only be done to the detriment of the other.
The choice of the most appropriate cut‐point for a diagnostic test should include consideration of the distribution of results in unaffected animals and animals with the disease, the prevalence of disease in the population to be tested, and the consequences of false‐positive and false‐negative tests results.26 On a farm with endemic R. equi infections, the consequence of failing to identify an infected foal early (false negative) might be, in the worst case scenario, death of the foal. Conversely, the consequences of false‐positive results would be wasted effort, financial losses, possible adverse effects, and potential unnecessary selection for resistant bacteria associated with treatment of healthy animals. It must be emphasized that SAA is a nonspecific indicator of infection or inflammation. High SAA concentrations in foals that remained clinically healthy in this study might be the result of subclinical pulmonary disease or infection/inflammation at other sites. Therefore, even at the highest possible cut‐point, SAA would be indicative of R. equi infection only on farms where the prevalence of the disease is high. Even at the lowest possible cut‐point of ≥5 μg/mL, sensitivity of SAA was low. Although it is possible that foals with low or undetectable SAA might have recovered without treatment, current guidelines recommend treatment of clinically affected foals.27 Therefore, it would have been unethical to withhold treatment of clinically affected foals based solely on SAA concentrations.
One of the major advantage of SAA over other APP in horses is the rapid increase during an inflammatory process followed by a rapid decrease with disease resolution. Therefore, it is possible that the interval of sample collection was too long. Whether more frequent sampling would significantly improve the diagnostic performance of each assay remains to be determined. SAA concentrations at time of initiation of treatment and again approximately 2 weeks of treatment were available for only 14 foals and SAA concentrations were ≥5 μg/mL in only 6 of the 14 foals. SAA concentrations 2 weeks after initiation of treatment were considerably lower in 5 of 6 foals and SAA was undetectable in 4 foals. One foal with undetectable SAA at the time of diagnosis had a concentration of 817 μg/mL after 2 weeks of treatment. Additional studies will be necessary to determine if measurement of SAA concentration is of value in determining the required duration of treatment.
Acknowledgment
We thank StableLab for providing the SAA test kits and handheld reader.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Funding: This work was performed with support from the Hodgson Equine Research Endowment at the University of Georgia.
Footnotes
StableLab Equine Blood Analysis kit, Sligo, Ireland
StateLab EQ‐1 Handheld Reader. Sligo, Ireland
References
- 1. Cohen ND. Causes of and farm management factors associated with disease and death in foals. J Am Vet Med Assoc 1994;204:1644–1651. [PubMed] [Google Scholar]
- 2. Anonymous . Part I: Baseline reference of 1998 equine health management. USDA‐APHIS, Veterinary Services Report, 2008.
- 3. Hoffman AM, Viel L, Prescott JF, et al. Association of microbiologic flora with clinical, endoscopic, and pulmonary cytologic findings in foals with distal respiratory tract infection. Am J Vet Res 1993;54:1615–1622. [PubMed] [Google Scholar]
- 4. Giguère S, Gaskin JM, Miller C, et al. Evaluation of a commercially available hyperimmune plasma product for prevention of naturally acquired pneumonia caused by Rhodococcus equi in foals. J Am Vet Med Assoc 2002;220:59–63. [DOI] [PubMed] [Google Scholar]
- 5. Chaffin MK, Cohen ND, Martens RJ, et al. Evaluation of the efficacy of gallium maltolate for chemoprophylaxis against pneumonia caused by Rhodococcus equi infection in foals. Am J Vet Res 2011;72:945–957. [DOI] [PubMed] [Google Scholar]
- 6. Gabay C, Kushner I. Acute‐phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–454. [DOI] [PubMed] [Google Scholar]
- 7. Crisman MV, Scarratt WK, Zimmerman KL. Blood proteins and inflammation in the horse. Vet Clin North Am Equine Pract 2008;24:285–297, vi. [DOI] [PubMed] [Google Scholar]
- 8. Reuss SM, Cohen ND. Update on bacterial pneumonia in the foal and weanling. Vet Clin North Am Equine Pract 2015;31:121–135. [DOI] [PubMed] [Google Scholar]
- 9. Leclere M, Magdesian KG, Kass PH, et al. Comparison of the clinical, microbiological, radiological and haematological features of foals with pneumonia caused by Rhodococcus equi and other bacteria. Vet J 2011;187:109–112. [DOI] [PubMed] [Google Scholar]
- 10. Belgrave RL, Dickey MM, Arheart KL, et al. Assessment of serum amyloid A testing of horses and its clinical application in a specialized equine practice. J Am Vet Med Assoc 2013;243:113–119. [DOI] [PubMed] [Google Scholar]
- 11. Cohen ND, Chaffin MK, Vandenplas ML, et al. Study of serum amyloid A concentrations as a means of achieving early diagnosis of Rhodococcus equi pneumonia. Equine Vet J 2005;37:212–216. [DOI] [PubMed] [Google Scholar]
- 12. Hulten C, Demmers S. Serum amyloid A (SAA) as an aid in the management of infectious disease in the foal: comparison with total leucocyte count, neutrophil count and fibrinogen. Equine Vet J 2002;34:693–698. [DOI] [PubMed] [Google Scholar]
- 13. Giguère S, Roberts GD. Association between radiographic pattern and outcome in foals with pneumonia caused by Rhodococcus equi . Vet Radiol Ultrasound 2012;53:601–604. [DOI] [PubMed] [Google Scholar]
- 14. Giguère S, Hernandez J, Gaskin JM, et al. Evaluation of WBC concentration, plasma fibrinogen concentration, and an agar gel immunodiffusion test for early identification of foals with Rhodococcus equi pneumonia. J Am Vet Med Assoc 2003;222:775–781. [DOI] [PubMed] [Google Scholar]
- 15. Millar HR, Simpson JG, Stalker AL. An evaluation of the heat precipitation method for plasma fibrinogen estimation. J Clin Pathol 1971;24:827–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 17. Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver‐operating characteristic analysis for diagnostic tests. Prev Vet Med 2000;45:23–41. [DOI] [PubMed] [Google Scholar]
- 18. Giguère S, Jacks S, Roberts GD, et al. Retrospective comparison of azithromycin, clarithromycin, and erythromycin for the treatment of foals with Rhodococcus equi pneumonia. J Vet Intern Med 2004;18:568–573. [DOI] [PubMed] [Google Scholar]
- 19. Falcon J, Smith BP, O'Brien TR, et al. Clinical and radiographic findings in Corynebacterium equi pneumonia of foals. J Am Vet Med Assoc 1985;186:593–599. [PubMed] [Google Scholar]
- 20. Passamonti F, Vardi DM, Stefanetti V, et al. Rhodococcus equi pneumonia in foals: an assessment of the early diagnostic value of serum amyloid A and plasma fibrinogen concentrations in equine clinical practice. Vet J 2015;203:211–218. [DOI] [PubMed] [Google Scholar]
- 21. Slovis NM, McCracken JL, Mundy G. How to use thoracic ultrasound to screen foals for Rhodococcus equi at affected farms. Proc Am Assoc Equine Pract 2005;51:274–278. [Google Scholar]
- 22. Venner M, Kerth R, Klug E. Evaluation of tulathromycin in the treatment of pulmonary abscesses in foals. Vet J 2007;174:418–421. [DOI] [PubMed] [Google Scholar]
- 23. Chaffin MK, Cohen ND, Blodgett GP, et al. Evaluation of ultrasonographic screening methods for early detection of Rhodococcus equi pneumonia in foals. J Equine Vet Sci 2012;32:S20–S21. [Google Scholar]
- 24. Venner M, Rodiger A, Laemmer M, et al. Failure of antimicrobial therapy to accelerate spontaneous healing of subclinical pulmonary abscesses on a farm with endemic infections caused by Rhodococcus equi . Vet J 2012;192:293–298. [DOI] [PubMed] [Google Scholar]
- 25. Venner M, Astheimer K, Lammer M, et al. Efficacy of mass antimicrobial treatment of foals with subclinical pulmonary abscesses associated with Rhodococcus equi . J Vet Intern Med 2013;27:171–176. [DOI] [PubMed] [Google Scholar]
- 26. Smith RD. Veterinary Clinical Epidemiology: A Problem‐Oriented Approach, 2nd ed Boca Raton, FL: CRC Press; 1995:304p. [Google Scholar]
- 27. Giguère S, Cohen ND, Keith CM, et al. Diagnosis, treatment, control, and prevention of infections caused by Rhodococcus equi in Foals. J Vet Intern Med 2011;25:1209–1220. [DOI] [PubMed] [Google Scholar]


