Abstract
Background
Craniocervical junction (CCJ) anomalies and secondary syringomyelia are commonly diagnosed in Cavalier King Charles spaniel (CKCS). Familiarity with the natural history of these abnormalities is vital to understanding the disease syndrome.
Objective
To evaluate magnetic resonance imaging (MRI) predictors of worsening clinical signs, syringomyelia, and morphology in CKCS longitudinally.
Animals
Fifty‐four client‐owned CKCS, 5–13 years old; 50% currently symptomatic.
Methods
Longitudinal observational study. We enrolled CKCS with an MRI of the CCJ performed ≥3 years earlier. We used questionnaires and neurologic examinations to grade initial and current clinical status. Dogs that could be anesthetized were reimaged. Morphologic assessments included the presence and severity of: Chiari‐like malformations, medullary position, atlantooccipital overlapping (AOO), dorsal atlantoaxial bands, and syringomyelia. Cranial cavity volumes and foramen magnum height were measured.
Results
Clinical status was evaluated in 54 dogs; 36/54 were reimaged. Mean follow‐up was 71 months. Of initially asymptomatic dogs, 32% were symptomatic at re‐evaluation. Of initially symptomatic dogs, 56% had worsened; 13% had improved with medical management. The morphology of the CCJ at initial imaging did not predict development of either new or worsened signs or syringomyelia by the time of re‐evaluation.
Conclusion
Craniocervical junction anomalies assessed in this study did not appear predictive of future clinical status or syringomyelia in our cohort. The impacts of syringomyelia, AOO, and atlantoaxial bands on future clinical status merit further study in larger groups of CKCS. Clinical progression in our cohort of medically managed CKCS did not differ substantially from published reports of those treated surgically.
Keywords: Atlantoaxial band, Chiari‐like malformation, Dysesthesia, Neuropathic pain
Abbreviations
- AOO
atlantooccipital overlapping
- AUC
area under the curve
- CCJ
craniocervical junction
- CKCS
Cavalier King Charles spaniel
- CM
Chiari‐like malformations
- MRI
magnetic resonance imaging
- NPG
neck pain grade
- ROC
receiver operating characteristic
- SM
syringomyelia
Craniocervical junction anomalies frequently are diagnosed in small and toy breed dogs, particularly the Cavalier King Charles spaniel (CKCS) breed.1, 2, 3, 4, 5, 6 These can include Chiari‐like malformations (CM),3, 5, 6, 7 medullary elevation and herniation at the CCJ,1, 3, 4, 8, 9, 10 dorsal atlantoaxial bands,2, 3, 8, 11, 12, 13 atlantooccipital overlapping (AOO),7, 8, 14 and secondary syringomyelia.2, 4, 7, 15, 16, 17 Magnetic resonance imaging (MRI) of the brain and cervical spinal cord allows the diagnosis of each of these, whereas objective measurements are used to evaluate their severity (eg, cerebellar compression index, dorsal compression and medullary kinking indices, and obex position measurements).2, 4, 8 These anomalies frequently occur concurrently and may be clinically silent,2, 3, 4, 6, 15, 16 but they have been associated with a wide spectrum of clinical signs; the most common of these is neuropathic pain4, 7, 18 , 1
In CKCS, where the prevalence of CM is estimated to be as high as 99%,19 and the prevalence of asymptomatic syringomyelia as high as 70% by 6 years of age,6, 15, 16 abnormalities of the CCJ often are diagnosed during MRI screening of asymptomatic dogs before breeding, or when evaluating the brain or cervical spine for an unrelated disease (eg, intervertebral disc disease).
To guide treatment decisions and prognosis in symptomatic dogs, practitioners need an understanding of the natural history of the disease. For those in which CCJ anomalies are diagnosed incidentally, an appreciation of the long‐term clinical relevance of these incidental findings is vital to our understanding of this disease syndrome and may influence breeding guidelines. Specifically, it is necessary to develop a better understanding of the correlation between morphologic findings at the CCJ on initial imaging and future clinical status. We hypothesized that structural changes identified on MRI of the CCJ in young dogs would be predictive of the development and worsening of both clinical signs and syringomyelia over time in CKCS. In this exploratory study, we utilized longitudinal evaluations of both clinical status and morphology in affected and unaffected CKCS imaged ≥3 years previously to identify imaging predictors of clinical status (ie, the presence and severity of neurologic signs) over time. Because syringomyelia presence and severity are highly correlated with the presence and severity of clinical signs,1 predictors of syringomyelia progression also were evaluated longitudinally.
Materials and Methods
Animals
We followed a cohort of dogs imaged in a prior study3 of the morphology of the CCJ in CKCS (henceforth referred to as MRI1). The owners of these CKCS were contacted by telephone, email, online breed‐associated groups, contacts associated with CKCS clubs, and the CKCS breeder community. To account for dropout, additional CKCS (n = 17) were recruited from patients presented to the Cornell University and North Carolina State University veterinary teaching hospitals, breed‐associated clubs and online groups (Fig 1). Enrollment inclusion criteria included: age > 5 years, history of MRI of the CCJ ≥3 years before participation, access to complete (initial) MRI study, and CKCS breed. Dogs that had died or had been euthanized were enrolled in the clinical assessment portion of the study if >3 years had elapsed between the initial study and the time of death or euthanasia. Thus, 2 groups of dogs were studied: the first group had 2 MRIs performed and was used to study both predictors of the progression of clinical signs and anatomical changes on MRI over time. The second group had an initial MRI and provided clinical follow‐up, and could be used to study predictors of clinical progression only. Outcomes studied, therefore, included the development of clinical signs, changes in severity of clinical signs (ie, change in neurologic grade), development of syringomyelia, and changes in severity of syringomyelia (ie, syringomyelia grade).
Figure 1.
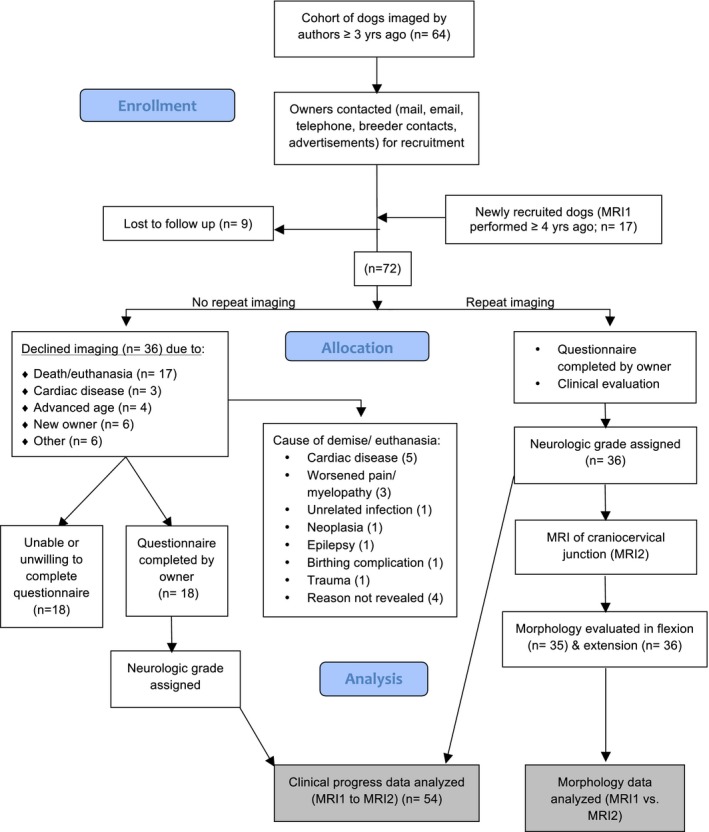
CONSORT‐style diagram. This flowchart illustrates the recruitment, loss to follow‐up, and study group assignment of initial and current study participants.
Clinical and MRI Assessment
Upon enrollment, owners completed a questionnaire evaluating their dog's current clinical status (ie, the presence and severity of neurologic signs) and treatment history (see Data S1) and underwent a telephone interview to further describe clinical status. For dogs no longer alive, we asked owners to describe the dog's clinical status just before death or euthanasia. Questionnaire responses were used to assign each participant a neurologic grade between 0 and 5 (Table 1) and a neck pain grade (NPG) between 0 and 3, as follows: NPG 1: signs of neck pain observed by owner but not evident on examination, or mild neck pain elicited on deep palpation of the neck; NPG 2: neck pain elicited on palpation but not evident in the dog's posture and movements; NPG 3: neck pain elicited with minimal neck palpation, neck guarding (eg, stops its own shaking of the head immediately after starting, neck held rigidly or straight). These grades were compared to grades assigned at the time of MRI1 to evaluate for changes in clinical status.
Table 1.
Grading of clinical signs by neurologic score. This grading system was chosen to maintain consistency with the grading system used in the dogs' initial clinical evaluation (ie, to allow comparison between initial and current neurologic grades for individual dogs)
| Neurologic Grade | Clinical Signs |
|---|---|
| 0 | Normal |
| 1 | Scratching neck/head <50% of the time observed by owner; no evidence of skin disease |
| 2 | Scratching neck/head >50% of the time and signs consistent with neck pain observed by owner (not evident on examination); no evidence of skin disease |
| 3 | Scratching observed by owner; neck pain consistently elicited on examination |
| 4 | Scratching observed owner; ataxia detected on neurologic examination |
| 5 | Scratching observed by owner; paresis detected on neurologic examination |
Dogs that could safely undergo anesthesia underwent a second MRI (MRI2). Physical and neurologic examinations were performed by investigators SCG and NJO to ensure systemic health and to evaluate for neurologic deficits and evidence of neuropathic pain (eg, dysesthesia, allodynia, and hyperesthesia). Exclusion criteria for imaging consisted of preanesthetic CBC, serum biochemistry panel, or physical examination findings contraindicating anesthesia, a heart murmur grade >4 of 6 without clearance from a veterinary cardiologist, or evidence of clinically apparent cardiac disease (eg, coughing, tachypnea).
Dogs were anesthetized and positioned in sternal recumbency for imaging, in the same manner as in MRI1.3 Two 1.5 T MRI units were used for imaging.2 Padding was used to position the CCJ in flexion to imitate a normal standing position, as described previously.3 Flexed head angles were compared with those of initial imaging at the start of the dog's second imaging study; dogs were repositioned as needed to ensure head angle did not vary >20° between studies. Sequences obtained included T1‐ and T2‐weighted sagittal and T2‐weighted transverse images of the brain, CCJ and cervical spine. The head and neck then were positioned flat on the table, with the CCJ in extension. Sequences obtained in extension included T2‐weighted sagittal and T2‐weighted transverse images of the CCJ. The latter were included for analysis in dogs that initially had (MRI1) been imaged in extension (ie, those newly recruited).
All imaging studies then were loaded onto an open‐source DICOM viewer3 for analysis. The following morphologic assessments were made: presence or absence of a CM (defined as cerebellar herniation or indentation and absence of cerebrospinal fluid at the level of the foramen magnum),3, 20 cerebellar herniation, cerebellar indentation, medullary position (ie, medullary kinking index, medullary angle, and obex to foramen magnum measurements),4, 8 presence or absence of AOO in dogs that initially had been imaged in extension,8, 14 dorsal atlantoaxial band presence or absence, and severity (ie, grade, dorsal compression index),2, 8 and syringomyelia presence, absence, grade, and height (Table 2).3 Caudal cranial fossa and total cranial fossa volumes also were determined, as previously described.3 Finally, we assessed the relative volume of the caudal fossa, in relation to total cranial cavity volume. A single observer (SCG) evaluated both initial and new imaging studies.
Table 2.
Grading criteria for determining dorsal band and syringomyelia severity (Adapted from Ref. 2)
| Grade | Atlantoaxial Band Severity | Severity of Syringomyelia |
|---|---|---|
| 0 | None | None |
| 1 | Underlying SAS reduced, but not eliminated | <33% of spinal cord |
| 2 | Underlying SAS eliminated, questionable spinal cord compression | 33–60% of spinal cord |
| 3 | Underlying spinal cord compressed/deformed | >60% of spinal cord |
SAS, subarachnoid space.
Data Analysis
Statistical analysis was performed using SAS software, using planned comparisons.4 Data type, classification, and data units are detailed in Table S1 of the Supporting information. The significance level for this study was set at .05; P‐values reflect adjustments for multiple comparisons, using the Bonferroni‐Holms step‐down adjustment method. Due to the exploratory nature of this study, raw P‐values are reported alongside adjusted P‐values in Table 3, with the aim of identifying variables that merit further investigation.21
Table 3.
Longitudinal relationships suggesting a need of further evaluation, as suggested by relationship between morphology at MRI1 and clinical status at the time of re‐evaluation (ie, MRI2) for factors showing significance prior to P‐value adjustment, suggesting a need for further evaluation
| Imaging Findings at MRI1 | Relationship to the Development of Clinical Signsa | Relationship to the Worsening of Clinical Signsa |
|---|---|---|
| Syringomyelia | ||
| Presence | P r = .02, P a = .318 | P r = .004; P a = .073 |
| Height | P r = .013, P a = .218 | P r = .032; P a = .449 |
| Grade | P r = .022, P a = .326 | P r = .042; P a = .558 |
| Length | P r = .022; P a = .330 | |
| Dorsal atlantoaxial band | P r = .041; P a = .492b | |
| Atlantooccipital overlapping | P r = .015; P a = .195b | |
P‐values are reported as “P r” for preadjustment values, and P a for adjusted P‐values).
Values reflect relationship to increases in neck pain grade, specifically.
Volume measurements were repeated at an interval of >4 months between evaluations. These were used to perform an assessment of intraobserver reliability for cranial and caudal fossa volume measurements using PROC CORR to calculate Cronbach's coefficient alpha.
Wilcoxon nonparametric tests were used to compare continuous measurements across 2 groups (eg, severity of syringomyelia) and Kruskal–Wallis tests were used for comparisons across >2 groups (eg, neurologic grades). Specific statistical tests used to compare individual morphology and clinical status between first and second evaluations are summarized in Table S1 of the Supporting information.
To examine whether morphology identified on MRI1 could predict either the development or progression of neurological signs and syringomyelia, we assessed the relationship between CCJ morphologic findings at MRI1 and changes in clinical status (ie, presence, absence, and severity of neurologic signs), syringomyelia or both at the current evaluation. This assessment was evaluated in both the cohort of dogs that had 2 MRIs and the cohort that provided clinical follow‐up only after the first MRI (MRI1). First, the relationship between morphology at MRI1 and changes in clinical status from an asymptomatic to a symptomatic status (ie, change from initial neurologic or NPG of 0 at MRI1 to a neurologic or NPG > 0 at second evaluation) was assessed in initially asymptomatic dogs using Fisher's Exact and Kruskal–Wallis tests. Next, morphologic factors on MRI1 associated with worsening of neurologic signs by second evaluation were identified in initially symptomatic dogs using Wilcoxon signed‐rank and McNemar tests. Area under the curve (AUC) was calculated using receiver operator characteristic (ROC) analyses to further evaluate the relationship between syringomyelia and worsening of neurologic signs. We then assessed the relationship between morphology at MRI1 and changes in syringomyelia status (ie, change from no syringomyelia at MRI1 to syringomyelia at MRI2, or increased SM grade or length) in the reimaged cohort. For the above tests, paired t‐tests were used to analyze data that were approximately normally distributed, and Chi‐squared tests were used for categorical data. Wilcoxon signed‐rank tests and Kruskal–Wallace tests were used for data that violated assumptions of the t‐test.
Results
Cohort Characteristics
Of the 64 CKCS initially imaged, we were able to reach the owners of 55 dogs by telephone or email (86%); the remaining 9 dogs were lost to follow‐up. We enrolled an additional group of 17 CKCS all of which underwent a second MRI, resulting in a total cohort of 72 dogs (Fig 1). Of these 72 animals, 36 were both reimaged (ie, MRI2) and provided clinical status data; the remaining 36 dog owners declined repeat imaging. Eighteen of those who declined imaging still completed a clinical status questionnaire. This resulted in a total of 54 dogs with a re‐evaluation of clinical status (36 + 18). Reasons for owners declining imaging included: cardiac disease (2), advanced age (4), or change in ownership (6); for 6 dogs the reason was not declared. Seventeen dogs had died or had been euthanized before the study (Table 4). Of these, 3 (6% overall) were euthanized before of worsened signs of neuropathic pain, cervical myelopathy or both.
Table 4.
Primary reasons given by owners for having declined repeat imaging or for imaging not having been recommended to owners (Column 4) and the primary reasons for the demise or euthanasia of prior participants (Column 2)
| Primary Reason | Cause of Death or Euthanasia (n = No. of Dogs) | Age at Death or Euthanasia (Range, Average) | Reason Imaging was Declined or Not Recommended (n = No. of Dogs) |
|---|---|---|---|
| Cardiac disease | 5 | 7–11, 9 | 3 |
| Unrelated condition | 5 | 4–11, 8.5 | – |
| Distance to imaging center | – | – | 6 |
| Worsening manifestations of neuropathic pain/cervical myelopathy | 3 | 4–10, 7 | – |
| Unknown | 4 | Unknown | – |
| Death or euthanasia | – | – | 17 |
| Advanced age | – | – | 4 |
| New owner | – | – | 6 |
| Total | 17 | Overall: 8 years | 36 |
Numbers within each of Columns 2 and 4 represent the number of dogs falling into each “Primary Reason” category. Age at the time of death or euthanasia (years of age) is also described, for dogs whose owners provided this information (Column 3).
Clinical Progression (n = 54)
See Table 5 and Figure 2 for cohort characteristics at initial and re‐evaluation. The neurologic grades of symptomatic dogs at the time of final evaluation ranged in severity as follows: grade 1, 4 dogs; grade 2, 5 dogs; grade 3, 15 dogs; grade 4, 1 dog; and grade 5, 2 dogs. Among initially symptomatic dogs, 9/16 (56%) had worsened their clinical status, 2 (13%) had improved with medical management, and 5 (31%) were unchanged. Time between evaluations did not differ significantly between those with worsened signs (mean time between evaluations, 5.9 years) and those without clinical worsening at their final evaluation (mean time between evaluations, 6.0 years; P = .9187).
Table 5.
Cohort characteristics of both entire cohort of clinically re‐evaluated dogs, and of the subset of these that were reimaged. Both groups are described at the time of initial and repeat evaluations.a
| Epidemiologic Parameters | Entire Cohort, at Initial Evaluation (n = 54) | Entire Cohort, at Re‐evaluation (n = 54) | Reimaged Dogs, Initial Evaluation (n = 36) | Reimaged Dogs, at Re‐evaluation (n = 36) |
|---|---|---|---|---|
| Age (years) | 1–7 (mean, 3) | 5–13 (mean, 9) | 1–7 (mean, 2) | 5–9 (mean, 9) |
| Male, female | N/A | 23 M, 31 F | N/A | 15 M, 21 F |
| Evaluation period (months) | N/A | 38–96 (mean, 71) | N/A | 36–96 (mean, 70) |
| Number of symptomatic dogs (%) | 16/54 (30) | 27/54 (50) | 11/36 (31) | 21/36 (58) |
| Increased neurologic grade (%) b | N/A | 9/16 (56) | N/A | 9/11 (82) |
| Mean & median neurologic grade | 1, 0 | 1, 1 | 0, 0 | 2, 2 |
Data are reported followed by mean, median values or as percentage, where applicable.
Refers to dogs that were symptomatic at first evaluation.
Figure 2.
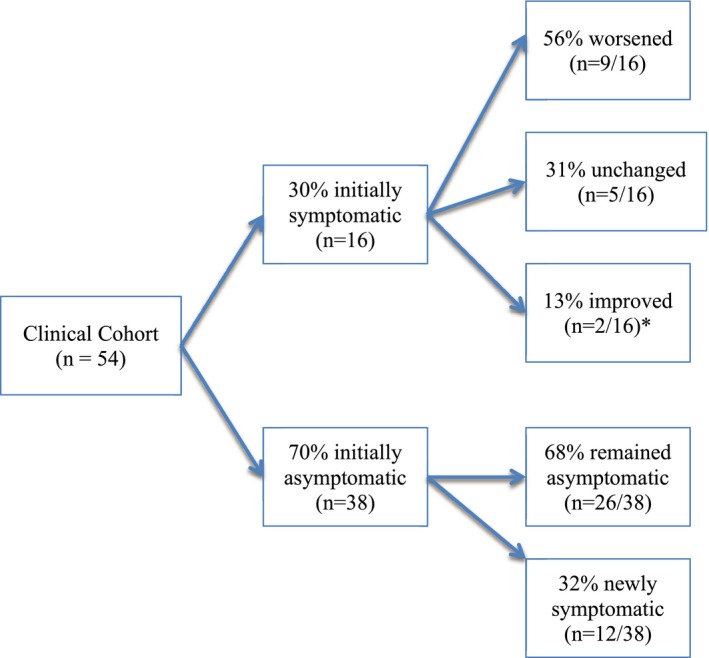
Clinical progress diagram. This flowchart demonstrates the clinical status of clinical cohort of dogs at initial and repeat evaluations.
Of the 38 initially asymptomatic dogs, 12 were symptomatic by their second evaluation (32%), with neurologic grades ranging from 1 to 3. Twelve dogs were being treated with analgesic medications at the time of enrollment, 7 of which had been receiving them since MRI1. Analgesics included gabapentin, pregabalin, and tramadol. One dog received prednisone (MRI1 group) in addition to analgesics, whereas 2 others received omeprazole regularly. Maropitant was administered intermittently to a single dog, on days in which the dog appeared acutely in pain.
Relationship between Initial Morphology and Clinical Status (n = 54)
Intraobserver reliability for caudal and cranial fossa measurements demonstrated excellent internal consistency (α = 0.89 and 0.99, respectively). Morphology at MRI1 was not associated with the development or worsening of clinical signs by the time of re‐evaluation in this cohort (see Table 3).
Relationship between Initial CCJ Morphology (MRI1) and Syringomyelia at MRI2 (n = 36)
Neither the presence nor the severity of CCJ morphologic abnormalities detected at MRI1 was associated with the development of syringomyelia by MRI2 in animals that did not previously have this finding. Morphologic abnormalities, similarly, did not predict the worsening of pre‐existing syringomyelia by MRI2. The only relationship that arose as a potential area for further evaluation on evaluation of raw P‐values was that between a larger foramen magnum height at MRI1 and increased syringomyelia grade (P raw = .015, P a = .210) and height (P raw = .037, P a = .518) by MRI2, although this relationship was not significant after adjustments for multiple comparisons.
Changes in Morphology between MRI1 and MRI2 (n = 36)
The morphologic characteristics of imaging studies are presented in Table 6. All syringomyelia grades were seen in MRI2 studies (grade 1, 6; grade 2, 10; grade 3, 10). Syringomyelia was seen in 8/15 asymptomatic dogs (53%) on MRI2, all of which were also asymptomatic on initial evaluation. These 8 asymptomatic dogs with syringomyelia at re‐evaluation included 5 dogs without evidence of syringomyelia on initial imaging, 2 dogs with previously diagnosed syringomyelia that was unchanged in its severity, and 1 dog with a 1‐grade improvement in syringomyelia severity. In the latter case, because of the dog's asymptomatic status, treatment had not been pursued between evaluations (medical or surgical). Conversely, 3 of 21 symptomatic dogs (14%) did not have syringomyelia at MRI2. Lastly, the relative volume of the caudal cranial fossa increased between MRI1 and MRI2.
Table 6.
Morphology of reimaged dogs at MRI1 and MRI2
| Morphologic Parameters | Morphology at MRI1a | N | Morphology at MRI2a | N | P‐value, If Significant b | Bonferroni‐corrected Significance Level |
|---|---|---|---|---|---|---|
| Chiari‐like malformation | 31/36 (86%) | 36 | 33/36 (92%) | 36 | – | – |
| Cerebellar herniation in extension (mm) | 1.6 (0–4) | 19 | 2 (0–3.2) | 36 | – | – |
| Cerebellar herniation in flexion (mm) | 3 (0–6.5) | 36 | 2 (0–3.8) | 34 | – | – |
| Cerebellar indentation c | 31 (86%) | 36 | 26 (72%) | 36 | – | – |
| Atlantooccipital overlapping c | 7 (37%) | 19 | 12/19 (63%) | 19 | – | – |
| Dorsal band c , d | 12 (63%) | 19 | 16/19 (84%) | 19 | – | – |
| Syringomyelia e | 15 (42%) | 36 | 26/36 (72%) | 36 | – | – |
| Maximum syringomyelia height (%) | 61 (0–76.9) | 51 | 63.5 (0–81) | 36 | .0004 | .0024 |
| Syringomyelia lengthd | 2.5 (0–7) | 51 | 3 (0–8) | 36 | .0003 | .0021 |
| Foramen magnum height (mm) | 16 (11–20.1) | 54 | 17.3 (14.3–20.4) | 35 | .0001 | .0008 |
| Cr cranial cavity volume (mm3) | 78.2 (63.4–92.2) | 35 | 74.7 (60.6–99.4) | 34 | .0638 | – |
| Cd cranial cavity volume (mm3) | 12.4 (10.1–15.7) | 36 | 12.4 (10.4–17.3) | 34 | .1780 | – |
| Cd cranial cavity proportional volume (mm3) | 13.8 (10.6–16.4) | 38 | 14.8 (13–17.7) | 34 | .0505 | .2525 |
Significantly different findings at MRI2 are specified along with associated P‐values. Bonferroni‐corrected significance levels are also specified for each significant relationship.
MRI, magnetic resonance imaging. Prevalence values are listed as the number of dogs affected, followed by the percent of the cohort in which the abnormality was seen. Measurements are listed as “median (range)” (Cd: caudal; Cr: cranial).
Listed P‐vales reflect pairwise comparisons between MRI1 and MRI2. Only significant P‐values are listed.
Indicates presence of the abnormality.
The prevalence of both dorsal bands and atlantooccipital overlapping is reported only in dogs imaged in extension due to the influence of head position on the severity of these conditions.
Measured in vertebral body lengths.
Discussion
Our study showed that CCJ morphologic abnormalities, although common in CKCS, do not predict development of clinical signs in asymptomatic dogs, nor do they predict overall clinical worsening in this breed. Thus, in our cohort, approximately two‐thirds of asymptomatic dogs remained asymptomatic throughout the study period, despite the majority (>80%) having shown CCJ morphologic abnormalities on initial imaging. Previous studies in CKCS have shown a similar absence of correlation between clinical status at the time of imaging and the presence and severity of cerebellar herniation (presence and length),3, 5, 6, 22 , 1 cerebellar indentation,3, 5, 6, 22 and foramen magnum height.3, 7, 23 One study,6 in fact, recently suggested that cerebellar indentation and impaction (ie, triangulation) should be considered a “normal” anatomic variation inherent to the CKCS breed, because of its high prevalence in asymptomatic CKCS. Similarly, cerebellar herniation associated with a Chiari type 1 malformation has a benign natural history in asymptomatic children.24, 25 in whom, cerebellar herniation does not predict the development of clinical signs over time.24, 25
In contrast, syringomyelia has been identified previously as the primary factor associated with the presence and severity of neuropathic pain at the time a dog is initially imaged,3, 26, 27 although dogs with CM also can demonstrate signs of neuropathic pain in the absence of syringomyelia.28 This is proposed to result from damage to the dorsal horn parenchyma of the cervical spinal cord, and resultant changes in neurotransmitter concentrations within the affected cord.27 In our study population, although there initially appeared to be a relationship between syringomyelia and both the development of new symptomatology and the worsening of pre‐existing signs in symptomatic dogs on raw P‐value analysis, this relationship was lost after correction for multiple comparisons. This finding suggests a need for further exploration of the relationship between syringomyelia and future symptomatology over time, employing a larger cohort of dogs with syringomyelia present at their initial evaluation.
Similarly, although the presence of atlantoaxial bands and AOO initially appeared to predict worsening of neck pain over time, this relationship was lost after adjustment for multiple comparisons, suggesting a potential need to evaluate the relationship further in a larger cohort. This finding, along with reports demonstrating a relationship between atlantoaxial bands and both the presence and severity of signs at the time of imaging,2 suggests a need to further evaluate the relationship between bands and clinical status over time. Atlantooccipital overlapping, in turn, had been suspected to contribute to chronic neuropathic pain and acute pain events, but this has not yet been demonstrated.14, 29, 30
The onset and worsening of neurologic signs over time in our cohort occurred alongside increased syringomyelia prevalence (from 42 to 72%) and worsened grade, height, and length of syringomyelia over time, although predictors of syringomyelia development and worsening were not identified. Together, these findings emphasize the need to interpret CCJ morphologic abnormalities with care, particularly in asymptomatic dogs and in those that have not yet developed syringomyelia.
Overall, although approximately half of our symptomatic dogs worsened over time (56%) throughout this evaluation period, few did so to a degree warranting euthanasia (6%), and approximately one‐third of symptomatic dogs remained unchanged. This finding corresponds with previous reports of symptomatic CKCS treated medically, in which worsening was seen in 2515 to 75%31 of CKCS, and euthanasia because of neuropathic pain was reported in 1531 to 20%15 of the overall population an average 3–5 years after their initial evaluation. Overall rates of death or euthanasia, in turn, reportedly ranged from 2531 to 65%15 in these longitudinal studies (mean ages of 7.7 and 11 years, respectively).
When these outcomes are compared to reports of postsurgical results, medical and surgical management do not appear to differ in the longer term. Although some degree of postoperative improvement is seen initially in the majority of cases after foramen magnum decompression,11, 32, 33, 34 this is followed by recurrence or worsening of signs in 25–53%11, 32, 33, 34 by 3 years postoperatively. Scratching and head rubbing also do not improve to the same extent as pain level after surgery.11, 32, 33, 34 Such prognostic data are essential to consider when owners are faced with the decision of whether to pursue medical or surgical management for symptomatic dogs, particularly if clinical signs are mild.
Lastly, our study supports the previously proposed dynamic component to cranial cavity volumetry,23 in the form of increased caudal cranial fossa volumes over time. As a preliminary hypothesis, it has been suggested that skull morphology in this breed may change in response to the pulsatile movement of the cerebellum against the supraoccipital bone, leading to increased bone resorption surrounding the foramen magnum.23 Enlargement of the caudal cranial fossa, in turn, has been proposed to cause rostral angling of the tentorium cerebelli in CKCS, in comparison to other breeds.35 Such alterations in tentorium positioning could play a role in the decrease in rostral cranial fossa volumes observed in our study.
The relationships that were significant when looking at raw P‐values, alone, highlight morphologic features that warrant further investigation into their role in the development of changes in clinical status over time, including syringomyelia, dorsal atlantoaxial bands, and AOO. Evaluating these relationships longitudinally in a larger cohort of CKCS potentially could help overcome the decreased the ability of our study to detect significant relationships because of a smaller sample size. This type of longitudinal study is difficult to perform in large numbers of dogs, but a multi‐institutional collaboration may facilitate completion of a larger case cohort study.
Additional limitations of this study include the questionnaire‐only based clinical re‐evaluation used in the majority of dogs that were not reimaged. In comparison, neurologic examination findings were used alongside questionnaire responses in reimaged dogs. Sampling bias could have been introduced as a consequence of the self‐selection of owners for participation in the study resulting in an inability to reimage all of the original participants. The motivations for an owner to take part could have varied widely from a desire to show a lack of progression to a desire to investigate clinical deterioration. However, the relationship between time to re‐evaluation and changes in clinical status did not differ significantly between those with worsened signs and those without clinical worsening (P = .9187). Lastly, not having evaluated the thoracolumbar spine could have resulted in an underestimation of syringomyelia severity.
Conclusions
Craniocervical junction morphology assessed in our study did not appear to predict the development of new and worsened clinical signs or syringomyelia over time in our cohort of CKCS. The relationships between clinical progression and syringomyelia, AOO, and dorsal bands should be evaluated further in a larger cohort of dogs. Clinical progression in our cohort of medically managed CKCS did not differ substantially from published reports of those treated surgically.
Supporting information
Data S1. Questionnaire used to evaluate clinical status. A copy of the questionnaire sent to owners is included as a Microsoft Word document. This supplemental information demonstrates the questions used to evaluate clinical status at re‐evaluation.
Table S1. Parameters evaluated statistically and their classification. Statistical tests used to compare each parameter between first and second evaluations are also specified.
Acknowledgments
The authors thank the American Cavalier King Charles Spaniel Club for their support and the owners of dogs participating in this study. We also recognize the staff of AnimalScan's Raleigh (NCSU) Imaging Center for their support of the study.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was a collaborative effort between Cornell University and North Carolina State University's Colleges of Veterinary Medicine. Imaging was performed at both institutions. This work was funded by a grant from the American Cavalier King Charles Spaniel Club Charitable Trust.
Footnotes
Bowen JE, Loderstedt S, Chandler K, et al. J Vet Intern Med 2012;26:848 (abstract)
Cornell University: Vantage Atlas, Toshiba America Medical Systems, Tustin, CA; North Carolina State University: Siemens Medical Solutions USA Inc., Malvern, PA
OsiriX Medical Imaging Software, v. 5.8.1, www.osirix-viewer.com, Geneva, Switzerland.
Software version: 9.3; SAS Institute, Cary, NC
References
- 1. Carrera I, Dennis R, Mellor DJ, et al. Use of magnetic resonance imaging for morphometric analysis of the caudal cranial fossa in Cavalier King Charles Spaniels. Am J Vet Res 2009;70:340–345. [DOI] [PubMed] [Google Scholar]
- 2. Cerda‐Gonzalez S, Olby NJ, Griffith EH. Dorsal compressive atlantoaxial bands and the craniocervical junction syndrome: Association with clinical signs and syringomyelia in mature Cavalier King Charles spaniels. J Vet Intern Med 2015;29:887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cerda‐Gonzalez S, Olby JN, McCullough S, et al. Morphology of the caudal fossa in Cavalier King Charles spaniels. Vet Radiol Ultrasound 2009;50:37–45. [DOI] [PubMed] [Google Scholar]
- 4. Cerda‐Gonzalez S, Olby NJ, Griffith EH. Medullary Position at the craniocervical junction in mature Cavalier King Charles spaniels: Relationship with neurologic signs and syringomyelia. J Vet Intern Med 2015;30:882–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Couturier J, Rault D, Cauzinille L. Chiari‐like malformation and syringomyelia in normal Cavalier King Charles spaniels: A multiple diagnostic imaging approach. J Small Anim Pract 2008;49:438–443. [DOI] [PubMed] [Google Scholar]
- 6. Harcourt‐Brown TR, Campbell J, Warren‐Smith C, et al. Prevalence of Chiari‐like malformations in clinically unaffected dogs. J Vet Intern Med 2014;15:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Driver CJ, Volk HA, Rusbridge C, Van Ham LM. An update on the pathogenesis of syringomyelia secondary to Chiari‐like malformations in dogs. Vet J 2013;198 551–559. [DOI] [PubMed] [Google Scholar]
- 8. Marino DJ, Loughin CA, Dewey CW, et al. Morphometric features of the craniocervical junction region in dogs with suspected Chiari‐like malformation determined by combined use of magnetic resonance imaging and computed tomography. Am J Vet Res 2012;73:105–111. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt MJ, Wigger A, Jawinski S, et al. Ultrasonographic appearance of the craniocervical junction in normal brachycephalic dogs and dogs with caudal occipital (Chiari‐like) malformation. Vet Radiol Ultrasound 2008;49:472–476. [DOI] [PubMed] [Google Scholar]
- 10. Rusbridge C, MacSweeny JE, Davies JV, et al. Syringohydromyelia in Cavalier King Charles spaniels. J Am Anim Hosp Assoc 2000;36:34–41. [DOI] [PubMed] [Google Scholar]
- 11. Dewey CW, Marino DJ, Bailey KS, et al. Foramen magnum decompression with cranioplasty for treatment of caudal occipital malformation syndrome in dogs. Vet Surg 2007;36:406–415. [DOI] [PubMed] [Google Scholar]
- 12. Vermeersch K, Van Ham L, Caemaert J, et al. Suboccipital craniectomy, dorsal laminectomy of C1, durotomy and dural graft placement as a treatment for syringohydromyelia with cerebellar tonsil herniation in Cavalier King Charles spaniels. Vet Surg 2004;33:355–360. [DOI] [PubMed] [Google Scholar]
- 13. Bynevelt M, Rusbridge C, Britton J. Dorsal dens angulation and a chiari type malformation in a Cavalier King Charles spaniel. Vet Radiol Ultrasound 2000;41:521–524. [DOI] [PubMed] [Google Scholar]
- 14. Cerda‐Gonzalez S, Dewey CW, Scrivani PV, Kline KL. Imaging features of atlanto‐occipital overlapping in dogs. Vet Radiol Ultrasound 2009;50:264–268. [DOI] [PubMed] [Google Scholar]
- 15. Thofner MS, Stougaard CL, Westrup U, et al. Prevalence and heritability of symptomatic syringomyelia in Cavalier King Charles spaniels and long‐term outcome in symptomatic and asymptomatic littermates. J Vet Intern Med 2014;29:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parker JE, Knowler SP, Rusbridge C, et al. Prevalence of asymptomatic syringomyelia in Cavalier King Charles spaniels. Vet Rec 2011;168:667–669. [DOI] [PubMed] [Google Scholar]
- 17. Heiss JD, Snyder K, Peterson MM, et al. Pathophysiology of primary spinal syringomyelia. J Neurosurg Spine 2012;17:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt MJ, Roth J, Ondreka N, et al. A potential role for substance P and interleukin‐6 in the cerebrospinal fluid of Cavalier King Charles spaniels with neuropathic pain. J Vet Intern Med 2013;27:530–535. [DOI] [PubMed] [Google Scholar]
- 19. Ives EJ, Doyle L, Holmes M, et al. Association between the findings on magnetic resonance imaging screening for syringomyelia in asymptomatic Cavalier King Charles spaniels and observation of clinical signs consistent with syringomyelia in later life. Vet J 2015;203:129–130. [DOI] [PubMed] [Google Scholar]
- 20. Cappello R, Rusbridge C. Report from the Chiari‐like malformation and syringomyelia working group round table. Vet Surg 2007;36:509–512. [DOI] [PubMed] [Google Scholar]
- 21. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 22. Lu D, Lamb CR, Pfeiffer DU, Targett MP. Neurological signs and results of magnetic resonance imaging in 40 Cavalier King Charles spaniels with Chiari type 1‐like malformations. Vet Rec 2003;153:260–263. [DOI] [PubMed] [Google Scholar]
- 23. Driver CJ, De Risio L, Hamilton S, et al. Changes over time in craniocerebral morphology and syringomyelia in Cavalier King Charles spaniels with Chiari‐like malformation. BMC Vet Res 2012;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strahle J, Muraszko KM, Kapurch J, et al. Natural history of Chiari malformation type I following decision for conservative treatment. J Neurosurg Pediatr 2011;8:214–221. [DOI] [PubMed] [Google Scholar]
- 25. Whitson WJ, Lane JR, Bauer DF, Durham SR. A prospective natural history study of nonoperatively managed Chiari I malformation: Does follow‐up MRI surveillance alter surgical decision making? J Neurosurg Pediatr 2015;16:159–166. [DOI] [PubMed] [Google Scholar]
- 26. Rusbridge C, Carruthers H, Dube MP, et al. Syringomyelia in Cavalier King Charles spaniels: The relationship between syrinx dimensions and pain. J Small Anim Pract 2007;48:432–436. [DOI] [PubMed] [Google Scholar]
- 27. Rusbridge C, Jeffery ND. Pathophysiology and treatment of neuropathic pain associated with syringomyelia. Vet J 2008;175:164–172. [DOI] [PubMed] [Google Scholar]
- 28. Loderstedt S, Benigni L, Chandler K, et al. Distribution of syringomyelia along the entire spinal cord in clinically affected Cavalier King Charles spaniels. Vet J 2011;190:359–363. [DOI] [PubMed] [Google Scholar]
- 29. Dewey CW, Marino DJ, Loughin CA. Craniocervical junction abnormalities in dogs. N Z Vet J 2013;61:202–211. [DOI] [PubMed] [Google Scholar]
- 30. Dewey CW, Cerda‐Gonzalez S, Scrivani PV, et al. Surgical stabilization of a craniocervical junction abnormality with atlanto‐occipital overlapping in a dog. Compend Contin Educ Vet 2009;31:E1–E6. [PubMed] [Google Scholar]
- 31. Plessas IN, Rusbridge C, Driver CJ, et al. Long‐term outcome of Cavalier King Charles spaniel dogs with clinical signs associated with Chiari‐like malformation and syringomyelia. Vet Rec 2012;171:501–505. [DOI] [PubMed] [Google Scholar]
- 32. Rusbridge C. Chiari‐like malformation with syringomyelia in the Cavalier King Charles spaniel: Long‐term outcome after surgical management. Vet Surg 2007;36:396–405. [DOI] [PubMed] [Google Scholar]
- 33. Dewey CW, Berg JM, Barone G, et al. Foramen magnum decompression for treatment of caudal occipital malformation syndrome in dogs. J Am Vet Med Assoc 2005;227:1270–1275. [DOI] [PubMed] [Google Scholar]
- 34. Ortinau N, Vitale S, Akin EY, et al. Foramen magnum decompression surgery in 23 Chiari‐like malformation patients 2007–2010: Outcomes and owner survey results. Can Vet J 2015;56:288–291. [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw TA, McGonnell IM, Driver CJ, et al. Caudal cranial fossa partitioning in Cavalier King Charles spaniels. Vet Rec 2013;172:341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Questionnaire used to evaluate clinical status. A copy of the questionnaire sent to owners is included as a Microsoft Word document. This supplemental information demonstrates the questions used to evaluate clinical status at re‐evaluation.
Table S1. Parameters evaluated statistically and their classification. Statistical tests used to compare each parameter between first and second evaluations are also specified.


