Abstract
Background
Major histocompatibility complex (MHC) I and II expression is not normally detected on sarcolemma, but is detected with lymphocytic infiltrates in immune‐mediated myositis (IMM) of humans and dogs and in dysferlin‐deficient muscular dystrophy.
Hypothesis/Objectives
To determine if sarcolemmal MHC is expressed in active IMM in horses, if MHC expression is associated with lymphocytic subtype, and if dysferlin is expressed in IMM.
Animals
Twenty‐one IMM horses of Quarter Horse‐related breeds, 3 healthy and 6 disease controls (3 pasture myopathy, 3 amylase‐resistant polysaccharide storage myopathy [PSSM]).
Methods
Immunohistochemical staining for MHC I, II, and CD4+, CD8+, CD20+ lymphocytes was performed on archived muscle of IMM and control horses. Scores were given for MHC I, II, and lymphocytic subtypes. Immunofluorescent staining for dysferlin, dystrophin, and a‐sarcoglycan was performed.
Results
Sarcolemmal MHC I and II expression was detected in 17/21 and 15/21 of IMM horses, respectively, and in specific fibers of PSSM horses, but not healthy or pasture myopathy controls. The CD4+, CD8+, and CD20+ cells were present in 20/21 IMM muscles with CD4+ predominance in 10/21 and CD8+ predominance in 6/21 of IMM horses. Dysferlin, dystrophin, and a‐sarcoglycan staining were similar in IMM and control muscles.
Conclusions and clinical importance
Deficiencies of dysferlin, dystrophin, and a‐sarcoglycan are not associated with IMM. Sarcolemmal MHC I and II expression in a proportion of myofibers of IMM horses in conjunction with lymphocytic infiltration supports an immune‐mediated etiology for IMM. The MHC expression also occured in specific myofibers in PSSM horses in the absence of lymphocytic infiltrates.
Keywords: Atrophy, Immunology, Inflammatory, Myopathy
Abbreviations
- AST
aspartate transaminase
- CK
creatine kinase
- CMMM
canine masticatory muscle myositis
- DM
dermatomyositis
- HE
hematoxylin and eosin
- IHC
immunohistochemical
- IMM
immune‐mediated myositis
- MHC II
major histocompatibility II
- MHC I
major histocompatibility complex I
- NMDL
University of Minnesota Neuromuscular Laboratory
- PAS
periodic acid Schiff
- PM
polymyositis
- PSSM
polysaccharide storage myopathy
Suspected immune‐mediated myositis (IMM) was first described in horses in 2007.1 Affected horses were predominantly Quarter Horses or related breeds and presented with severe atrophy of gluteal and epaxial muscles and high serum creatine kinase (CK) and aspartate transaminase (AST) activities. An immune‐mediated basis for muscle degeneration was suspected because myofibers within muscle biopsy specimens of IMM horses contained lymphocytic infiltrates, with on average more CD4+ than CD8+ lymphocytes.1 Lymphocytic infiltrates typically are found in the muscle of humans and dogs affected with various types of inflammatory and immune‐mediated myositis with specific lymphocytic subtypes predominating in some forms and not others. For example, a predominant CD4+ infiltrate is described in masticatory muscle myositis of dogs (CMMM) and dermatomyositis (DM), whereas a predominant CD8+ infiltrate is found in polymyositis (PM) in dogs.2
Lymphocytic infiltrates, however, are not restricted to immune‐mediated myositis and are also a feature of inflammatory myopathies caused by infectious agents such as Toxoplasma gondii and Neospora caninum.3 Inflammatory myopathies secondary to infectious causes, however, often have eosinophilic infiltrates in addition to lymphocytes.3 Eosinophils also have been found in inflammatory myopathies such as CMMM in dogs3 and eosinophilic polymyositis in humans.4 Muscle inflammation also may be a predominant pathological finding in dysferlin‐deficient muscular dystrophy in humans.5, 6
Expression of major histocompatibility complex class I (MHC I) and in some cases MHC II on the sarcolemma and occasionally within muscle fibers is another diagnostic tool for assessing many forms of immune‐mediated myositis in humans and dogs.7, 8 Mature muscle fibers in healthy individuals are quite unique in that MHC antigens are not normally detectable on the sarcolemma, and staining is limited to the capillary network and endothelial cells of blood vessels.9, 10 Major histocompatibility complex I, however, is detectable on the sarcolemmal surface of developing myoblasts,11 and regenerating myofibers.10 In addition to sarcolemmal expression of MHC I and II in immune‐mediated myopathies, MHC class I expression also occurs in some forms of muscular dystrophy such as limb‐girdle muscular dystrophy caused by a mutation in the dysferlin gene.6, 12
The purpose of our study was to further explore the pathogenesis of IMM in horses by determining (1) if MHC I or II expression or both is detectable on the sarcolemma of myofibers of horses with active IMM, (2) whether there is a relationship between MHC expression and the predominant subtype of lymphocytes within muscle infiltrates, and (3) whether dysferlin is expressed on the myofibers of horses with IMM.
Materials and Methods
Horses
IMM
Records of muscle biopsy specimens submitted to the University of Minnesota Neuromuscular Laboratory (NMDL) between 1995 and 2013 were reviewed to identify the breed, age, sex, and clinicopathologic abnormalities of horses with a primary diagnosis of IMM. All muscle samples had been shipped overnight on frozen gel packs to the NMDL, where they were frozen in isopentane precooled in liquid nitrogen and stored at −80°C until further processing. The diagnosis of IMM was based on identification of lymphocytic infiltration having an endomysial or perimysial distribution with invasion of nonnecrotic fibers or surrounding blood vessels in hematoxylin and eosin (HE)‐stained muscle sections.
Twenty‐one muscle biopsy specimens from 18 Quarter Horses and 3 Paint horses that had a history of muscle atrophy were selected for inclusion in the study based on the large number of lymphocytes invading myofibers, well‐preserved frozen muscle tissue with minimal freeze artifact, and the absence of evidence of polysaccharide storage myopathy (PSSM) in periodic acid Schiff's stains. The mean age was 5.0 years (range, 0.5–19 years). There were 10 mares, 5 stallions, and 6 geldings. Muscle biopsies were from the gluteal (n = 7), epaxial (n = 2), and semimembranosus (n = 12) muscles. Median reported serum CK activity was 3,165 U/L (range, 123–130,000 U/L) and median serum AST activity was 3,356 U/L (range, 460–79,700 U/L). Eighty‐nine percent (18/21) of horses with reported CK or AST results had increased enzyme activities.
Healthy and Disease Controls
Samples from the gluteus medius muscle of 3 Quarter Horses, 2 mares, and 1 gelding, with a mean age of 4.7 years (range, 4–6 years) were selected as healthy controls. The disease controls consisted of 3 horses with acute seasonal pasture myopathy and 3 horses with amylase‐resistant polysaccharide storage myopathy (PSSM). The 3 horses with pasture myopathy consisted of 1 Quarter Horse, 1 Paint horse, and 1 Appaloosa (2 geldings and 1 stallion) with a mean age of 5.5 years (range, 1–13 years). Samples were from the gluteus medius (n = 2) and semimembranosus (n = 1) muscles. The 3 geldings with PSSM consisted of 1 Quarter Horse and 2 Appaloosas with a mean age of 5 years (range, 3–7 years). All samples were from the semimembranosus muscle. Review of archived stains determined that muscle samples from horses with pasture myopathy had excessive myofiber lipid in oil Red O stains and evidence of acute myodegeneration without regeneration in HE‐stained samples. The PSSM muscle samples did not contain regenerating myofibers, based on the absence of small basophilic myofibers with prominent central nuclei in HE‐stained sections.
Muscle Analysis
Immunohistochemistry
Immunohistochemical (IHC) staining was performed on 10‐μm thick frozen sections that were fixed in acetone (75%) and ethanol (25%) at 4°C for 10 min and had nonspecific binding blocked with normal goat serum (1 : 10). Muscle sections were incubated with monoclonal antibodies for MHC class I (mouse anti‐horse CVS22), MHC class II (mouse anti‐horse CVS20), CD4+ (mouse anti‐horse HB61A), CD8+ (mouse anti‐horse CVS8), and CD20+ (rabbit anti‐horse). Secondary antibodies against MHC I, MHC II, CD4+, CD8+ (horseradish peroxidase goat anti‐mouse IgG), and CD20+ (HRP goat anti‐rabbit IgG) were from EnVision.1 Immunoreactivity was detected with 3‐amino‐9‐ethylcarbazole. Slides were counterstained with Mayer's hematoxylin. Positive control tissue consisted of normal adult horse tonsil. Negative controls used dilute negative control mouse (or rabbit for CD20+) fraction in place of the primary antibody. Because staining for the macrophage marker MAC387 cross reacts with other inflammatory cells, histochemical staining for macrophages was performed with the acid phosphatase reaction.13
Immunofluorescent staining
Immunofluorescent staining for sarcolemmal localization of dysferlin, alpha sarcoglycan, and dystrophin was performed on cryopreserved muscle samples from 5 Quarter Horses with IMM and 1 control horse using acetone/methanol fixation. Antibodies included NCL‐Hamlet and NCL Hamlet 2 monoclonal anti‐dysferlin antibodies,2 a polyclonal antibody against alpha sarcoglycan,3 , 14 and a monoclonal antibody against the rod domain of dystrophin (DYS14) using methods previously described.15 Slides were assessed for the presence or absence of dysferlin, dystrophin, and alpha sarcoglycan on the sarcolemma compared to control muscle.
Assessment of MHC I and II
A scoring system was devised to assess both the percentage of fibers that had some degree of sarcolemmal staining for MHC as well as to assess the extent of MHC staining around the periphery of a myofiber. The whole muscle section was examined at 20× magnification and 3 separate areas with the most extensive MHC staining and least cellular infiltrate were selected. These selection criteria were used to avoid confusion of MHC staining of lymphocytes with MHC staining of the sarcolemma. Each selected area was then examined at 40× magnification to estimate the number of myofibers with MHC staining on some or all of the sarcolemma and scored using: score 0 (none positive), score 1 (<25% myofibers positive), score 2 (25–50% positive), score 3 (51–75% positive), and score 4 (>75% positive). In addition, the average extent to which sarcolemmal staining for MHC extended around the sarcolemma was estimated for all fibers in the region and scored according to 0 (no positive sarcolemma staining), score 1 (<25% of sarcolemma staining positive), score 2 (25–50% of sarcolemma staining positive), score 3 (51–75% staining positive), and score 4 (>75% staining positive). The myofiber and sarcolemma scores for each objective field were multiplied, and then summed to produce an overall MHC I and MHC II score (maximum score of 48).
Assessment of Inflammatory Cells
Vascular Cuffing
Three areas with the most extensive cellular infiltration surrounding vessels were selected at 20× magnification. Each selected area was then examined at 40× magnification and the percentage of the specific lymphocyte subtype (or macrophages) that stained positively of the total number of mononuclear cell infiltrates in the area was estimated.
Myofiber Infiltrates
To compare the predominance of lymphocyte subtypes and macrophages with respect to the total mononuclear infiltrates, the whole muscle section was examined at 20× magnification and the 3 areas with the most extensive myofiber cellular infiltration were selected. Each selected area then was examined at 40× magnification and the percentage of the specific lymphocyte subtype (or macrophages) that stained positively of the total number of mononuclear cell infiltrates in the area was estimated according to: score 0 (none positive), score 1 (<25% of cells positive), score 2 (25–50% positive), score 3 (51–75% positive), and score 4 (>75% positive). To also assess the amount of inflammation in the areas examined, a score also was given for the total number of inflammatory cells in the field; score 0 (no inflammatory cells), score 1 (<25% of the field), score 2 (25–50% of the field), score 3 (51–75% of the field), and score 4 (>75% of the field). For each field, the scores for the percentage of cells staining positively and the percentage of the field containing inflammatory cells were multiplied, and the scores for the fields in each horse then were summed to produce an overall myofiber infiltrate score (maximum total score of 48).
Archived PAS stains were examined from horses with PSSM to compare the distribution of the myofibers with PAS‐positive inclusions with the distribution of MHC I‐ and II‐positive fibers.
Statistical Analysis
Based on Shapiro‐Wilk testing, data were not normally distributed. A Mann‐Whitney U‐test was used to compare scores for lymphocytic subtypes within muscle of IMM cases and scores for MHC, lymphocytes, and macrophages between IMM cases and disease controls. A Spearman Correlation was used to assess the relationship between the cell type and MHC class. All statistical analyses were performed using commercial software.5 Significance was set at P = .05.
Results
MHC Immunohistochemistry
Healthy Control Horses
Vascular endothelium stained positively for MHC I and MHC II, whereas MHC I or II staining was not observed on the sarcolemma of myofibers of healthy control horses (Fig 1A).
Figure 1.

Control and immune‐mediated myositis (IMM) horses: (A) Major histocompatibility complex (MHC) I staining of a healthy control muscle showing endothelial staining of capillaries but no staining of the sarcolemma. (B) MHC I staining of a pasture myopathy disease control muscle showing endothelial staining of capillaries but no staining of the sarcolemma of degenerate myofibers. (C) Focal sarcolemmal MHC I staining of an individual myofiber in a horse with IMM (IMM horse 1). (D) Generalized MHC I staining of the sarcolemma in a horse with IMM (IMM horse 2). (E) A lack of sarcolemma MHC II staining in a serial section of muscle from the IMM horse 2. (F) MHC II staining of an individual degenerating myofiber containing mononuclear infiltrates (IMM horse 3).
IMM Horses
Vascular endothelium and mononuclear cells were darkly stained for MHC I and MHC II in IMM horses (Fig 2A,B). There was a wide range of MHC I and II staining of the sarcolemma within the same muscle biopsy and among different horses with IMM (Figs 1C–F, 3A,B). Both nondegenerate and degenerate myofibers had MHC I or II sarcolemmal staining in IMM horses. Fibers with MHC I or II sarcolemmal staining were scattered throughout the biopsy specimens in areas without mononuclear infiltrates (Fig 1C–F) and concentrated in areas with infiltrates (Fig 3A,B). Fibers with MHC I sarcolemmal staining did not consistently have MHC II staining (Fig 1D,E). In regions with minimal lymphocytic infiltrates, some degree of MHC I sarcolemmal staining was present in 81% (17/21) of IMM horses and MHC II staining was present in 71% (15/21) of IMM horses. The MHC I scores were higher than MHC II scores in 82% (14/17) of IMM horses that had myofibers staining for MHC (Table 1).
Figure 2.
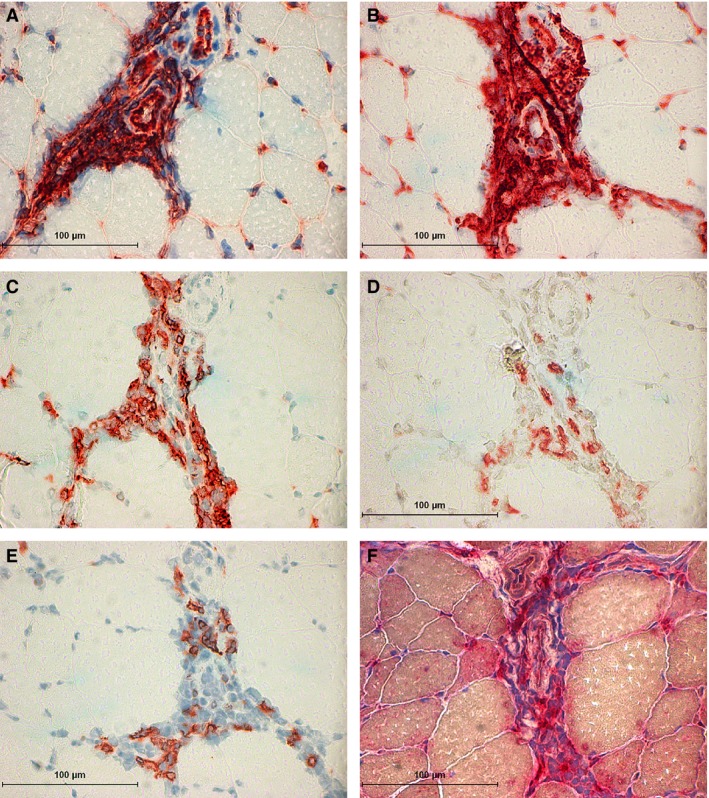
Immune‐mediated myositis (IMM) horse 3. (A) Major histocompatibility complex (MHC) I staining of the endothelium and the mononuclear infiltrates that surround two arterioles. (B) MHC II staining of the endothelium and the mononuclear infiltrates that surround two arterioles. (C) Immunohistochemical (IHC) staining for many CD4+ lymphocytes that are surrounding the arterioles. (D) IHC staining of a small number of CD8+ lymphocytes surrounding the arterioles. (E) IHC staining of a small number of CD20+ lymphocytes found scattered around arterioles. (F) Acid phosphatase staining of macrophages (aggregates of deep red stain) around an arteriole.
Figure 3.
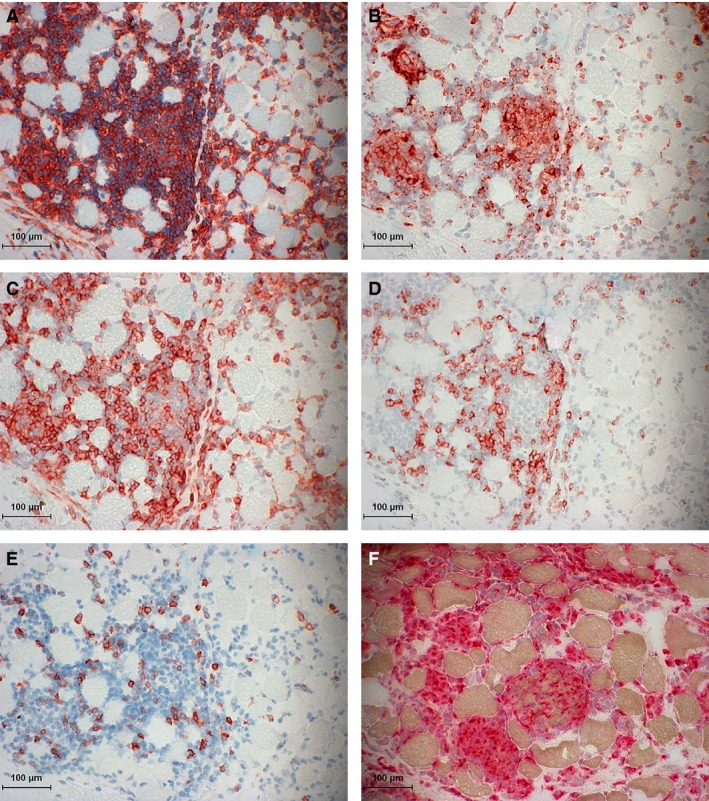
Immune‐mediated myositis (IMM) horse 2. (A) Major histocompatibility complex (MHC) I staining of mononuclear infiltrates that is so dense it obscures potential sarcolemmal MHC I staining. (B) MHC II staining of mononuclear infiltrates without evident MHC II sarcolemmal staining. (C) Immunohistochemical (IHC) staining of numerous CD4+ lymphocytes in the endomysium and within myofibers. (D) IHC staining of CD8+ lymphocytes in the endomysium. (E) IHC staining of a few CD20+ lymphocytes scattered in the endomysium. (F) Acid phosphatase staining of numerous macrophages (red aggregates) infiltrating myofibers.
Table 1.
Major histocompatibility complex (MHC) I and II, lymphocyte and macrophage scores of myofiber infiltrates in immune‐mediated myositis (IMM) horses. The higher the MHC scores the more fibers with MHC sarcolemmal staining. The higher the lymphocyte or macrophage score, the higher the proportion of mononuclear cells of that specific subtype (CD4+, CD8+, CD20+, or macrophages) was relative to the total number of inflammatory cells. There was no significant difference between scores for MHC I versus II staining or among lymphocyte subpopulations in IMM muscle indicating most IMM muscle had a mixture of inflammatory cell infiltrates
| IMM horses | MHC I | MHC II | CD4+ | CD8+ | CD20+ | Macrophages |
|---|---|---|---|---|---|---|
| Maximum score | 48 | 48 | 48 | 48 | 48 | 48 |
| Median ± SD | 9 ± 12 | 7 ± 10 | 14 ± 9 | 8 ± 9 | 7 ± 7 | 13 ± 13 |
| Range | 0–44 | 0–40 | 0–41 | 0–32 | 0–24 | 0–41 |
Disease Control Horses
The MHC I staining was not present in muscle samples from horses with seasonal pasture myopathy (Fig 1B), but MHC II staining was observed in a few degenerating myofibers of 2 horses with seasonal pasture myopathy (Table 1). Sarcolemmal and aggregates of cytoplasmic MHC I and II staining were observed in the same myofibers of all 3 PSSM horses (Fig 4B,C; Table 1). Unlike IMM cases, myofibers with MHC staining were often clustered along the edge of muscle fascicles. In regions containing fibers with abnormal polysaccharide (Fig 4A), the cytoplasm had stippled MHC staining and the fibers with MHC staining did not appear to be regenerating based on the review of HE sections.
Figure 4.
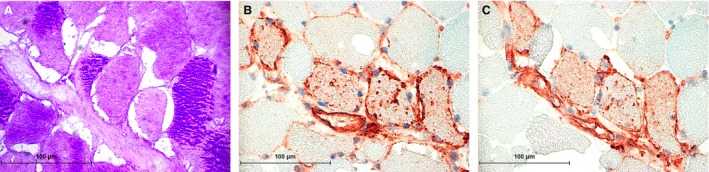
Polysaccharide storage myopathy (PSSM) horse. (A) Abnormal Periodic acid Schiff (PAS) positive inclusions in myofibers. (B) Major histocompatibility complex (MHC) I staining of the sarcolemma and granular material within myofibers. (C) MHC II staining of the sarcolemma and granular material in the same fibers.
Mononuclear Cell Histochemistry and Immunohistochemistry
Healthy Control Horses
Macrophages and CD4+ and CD8+ lymphocytes were not observed near blood vessels or among myofibers in healthy control horses. A muscle sample from 1 control horse had a few CD20+ lymphocytes between myofibers.
IMM Horses
Vascular Cuffing
Mononuclear cells, predominantly lymphocytes, were present around at least 1 blood vessel in the muscle biopsy specimens of all IMM horses. Only a few macrophages were present around blood vessels in IMM horses (Fig 2F). The CD4+ cells were present around blood vessels in 76% (16/21) of IMM horses, CD8+ cells in 71% (15/21), and CD20+ cells in 62% (13/21) of IMM horses (Fig 2C,D,E).
Myofiber Infiltrates
Mononuclear cell infiltrates were present around or in myofibers in 95% (20/21) of muscle samples from IMM horses. One IMM case only had vascular cuffing. Five horses had >50% of the biopsy specimen consisting of inflammatory infiltrates comprised of CD4+, CD8+, CD20+, and macrophages (Fig 3C,D,E,F). In 48% (10/21) of IMM horses, CD4+ lymphocytes predominated over CD8+ and CD20+ cells and in 28% (6/21) of IMM horses CD8+ lymphocytes predominated (assessed by comparison of scores within individuals). The median score for CD4+ lymphocytes in IMM horses was not significantly different from CD8+ scores (P = .35), CD20+ lymphocytes (P = .07), or macrophages (P = .90) in IMM horses (Table 1). Scores for CD8+ and CD20+ lymphocytes (P = .22), CD8+ lymphocytes and macrophages (P = .36), and CD20+ lymphocytes and macrophages (P = .07) in IMM horses were similar (Table 1). The CD4+ lymphocyte score was positively correlated with CD8+ lymphocyte score (r = 0.53, P = .01; Fig 5) and macrophage score (r = 0.46, P = .037). No significant correlation between CD4+ and CD20+ scores was identified (r = 0.38, P = .09). The CD8+ lymphocyte score was positively correlated with CD20+ lymphocyte score (r = 0.70, P = <.001) and macrophage score (r = 0.64, P = .002).
Figure 5.
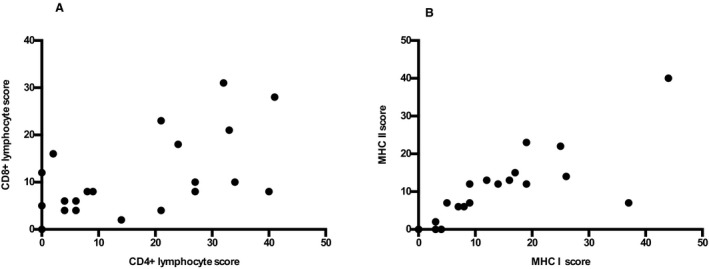
Correlation between CD4+ and CD8+ lymphocyte and major histocompatibility complex (MHC) class I and II scores. MHC I scores were positively correlated with MHC II scores (r = 0.89, P < .001). CD4+ scores were positively correlated with CD8+ scores (r = 0.53, P = .01).
The MHC I scores were positively correlated with MHC II scores (r = 0.89, P = <.001; Fig 5) and CD8+ (r = 0.64, P = .002) and CD20+ (r = 0.66, P = .001) lymphocyte and macrophage scores (r = 0.70, P = <.001). The MHC I scores were not significantly correlated with CD4+ (r = 0.22, P = .34) lymphocyte scores. The MHC II scores were positively correlated with CD8+ (r = 0.59, P = .005), CD20+ (r = 0.61, P = .004) lymphocyte and macrophage (r = 0.70, P = <.001) scores. The MHC II scores were not significantly correlated with CD4+ (r = 0.11, P = .63) lymphocyte scores.
Disease Control Horses
In seasonal pasture myopathy horses, CD4+ lymphocytes were not observed in any horses. No CD8+ or CD20+ lymphocytes were observed in the muscles of 2 horses, whereas 1 horse had a few CD8+ and CD20+ lymphocytes in the muscle sample. A few macrophages were identified in necrotic myofibers in all horses.
In PSSM horses, a few CD4+ and CD8+ lymphocytes were present in muscle samples of 1 of the 3 PSSM horses. Two of 3 PSSM horses had a few CD20+ lymphocytes. All 3 PSSM horses had a few scattered macrophages in myofibers.
Dysferlin, Dystrophin, and alpha Sarcoglycan
Compared to control muscle, muscle samples from all IMM horses tested showed a normal pattern of uniform sarcolemmal staining for dysferlin, dystrophin, and alpha sarcoglycan (Fig 6).
Figure 6.
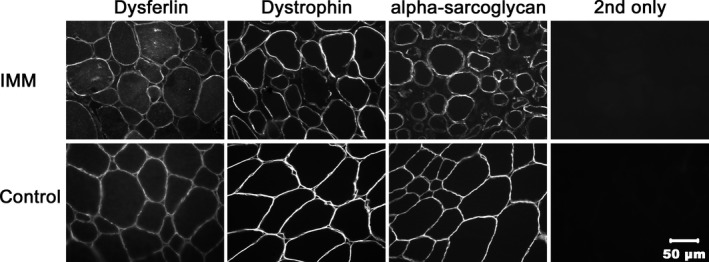
Immunofluorescent staining of immune‐mediated myositis (IMM) (horse 2) and healthy control muscle for localization of dysferlin, dystrophin, and alpha sarcoglycan. Cryosections stained with antibodies against dysferlin, dystrophin, and alpha sarcoglycan show comparable staining of the sarcolemma in IMM and control horse muscle.
Discussion
Our results expand upon the previous study of suspected IMM in horses1 and support an immune‐mediated basis for this disorder in Quarter Horses. Similar to cases of immune‐mediated myopathies in humans16 and dogs,3 infiltrates of CD8+ and CD4+ T cells are observed in myofibers of IMM horses and, when inflammatory infiltrates are present, the majority of Quarter Horses with IMM express detectable MHC I or II on the sarcolemma of some myofibers. This is important because MHC expression normally is not detectable on mature skeletal muscle fibers,7, 9, 11 but is detectable on myofibers of humans and dogs with immune‐mediated myopathies.8, 17, 18 Investigations into immune‐mediated myopathies in humans and dogs have found antigen processing and presentation by myofibers expressing MHC.19, 20 Thus, MHC expression in myofibers of horses with IMM suggests that these fibers have become immunologically activated and could serve as antigen‐presenting cells, presenting autoantigens to inflammatory cells.8, 20
Overexpression of MHC I is not specific to inflammatory myopathies, however, and may also occur in dystrophin‐deficient muscular dystrophy,9 some human limb‐girdle muscular dystrophies,4 and in limb‐girdle muscular dystrophy caused by a dysferlin deficiency.6 The histopathology of dysferlinopathy bears a resemblance to IMM in horses in that infiltrates of CD4+ lymphocytes can be present together with MHC I and rarely with MHC II expression on myofibers.6, 12 A lack of dysferlin on the sarcolemma, a characteristic of dysferlinopathies, was not identified in immunofluorescent stains of IMM horse muscle. Neither do our results support dystrophin‐ or sarcoglycan‐deficient muscular dystrophy as a cause of IMM because dystrophin and alpha sarcoglycan were clearly evident on the sarcolemma of IMM‐affected horses.
The MHC II antigens typically are restricted to antigen‐presenting cells of the immune system and interact with CD4+ lymphocytes. The MHC II staining of the sarcolemma was apparent in nondegenerate myofibers of some IMM horses as well as in the degenerate myofibers of IMM and some disease control horses. In other species, MHC II sarcolemmal staining does not appear to be as consistent a feature of inflammatory myopathies as MHC I sarcolemmal staining. When present, however, MHC II is highly specific for idiopathic inflammatory myopathies.21 Although scores for MHC II expression correlated with scores for MHC I expression, MHC I and II expression did not necessarily colocalize to the same myofibers in IMM horses.
The MHC expression on the sarcolemma of myofibers of IMM horses was not uniform and often only involved scattered myofibers or a portion of the sarcolemma. Variability in MHC expression with immune‐mediated myopathies may relate to the degree of cytokine expression in myofibers, type of fibers affected, severity of inflammation or stage of disease.21 Cytokines can up‐regulate MHC I and may play a role in nonspecific MHC I expression.20 In dogs with acute, severe CMMM, MHC I and II staining commonly occurs in >70% of myofibers, whereas in dogs with more chronic disease, MHC I and II staining is much less extensive.8 Unfortunately, the onset of clinical signs relative to the time of muscle biopsy was not recorded for many of the cases in our study, and it was not possible to determine if there was a temporal relationship between time of biopsy, the amount of MHC staining on the sarcolemma, and time course of infiltration of myofibers with lymphocytes. Because the presence of lymphocytic infiltrates was an inclusion criterion for the study, it is not possible to know whether MHC I or II expression occurred without active inflammation, but subjectively it appeared that those biopsy specimen with the most inflammation usually had the highest MHC expression and those with the least infiltrates usually had the least MHC expression. In addition, sarcolemmal membrane integrity is important for reliable MHC antibody binding, and variable MHC staining may have been related to the degradation of some of the muscle samples before being frozen or during shipping. To manage this possibility, IMM samples selected for inclusion in the present study were chosen such that they had minimal shipping artifacts, but delay in freezing could have impacted the results in some biopsy specimens.
Most horses with IMM have increased muscle enzyme activities and it was important to rule out rhabdomyolysis alone as a cause for upregulation of MHC on myofibers. Horses with PSSM and pasture myopathy have rhabdomyolysis without marked myofiber infiltration by lymphocytes. Neither MHC class I or II were detected in normal‐appearing muscle fibers of horses with PSSM or pasture myopathy. Interestingly, clusters of mature myofibers from horses with PSSM expressed MHC class I and II in the absence of any lymphocytic infiltrates and in the absence of regenerative features such as prominent centrally displaced nuclei or basophilic cytoplasm in HE‐stained sections. The distribution of MHC‐positive myofibers in PSSM horses was very similar to the distribution of fibers with aggregates of abnormal polysaccharide. A number of protein aggregates have been found in myofibers of horses with type 1 PSSM including myoglobin and desmin, suggesting that the cytoplasmic aggregates of MHC protein could be a nonspecific finding.22 Another possibility is that sarcolemmal MHC expression in fibers with abnormal polysaccharide results from inflammatory cytokines produced because of the presence of the abnormal polysaccharide and leading to MHC upregulation. The lack of cellular inflammatory response in the face of MHC I or II expression, however, suggests that the sarcolemmal MHC:peptide complexes are recognized as ‘self’ peptides in PSSM horses and therefore no additional immune response is targeted toward these myofibers. A further explanation for a lack of inflammatory response in the face of MHC expression could be a lack of upregulation of those proinflammatory cytokines that directly incite an immune response.20, 23 Major histocompatibility complex expression has been found to occur in other myopathies that do not have an immune‐mediated etiology, and the reason for this expression is not fully understood.21
Scores for MHC I expression were significantly correlated with scores for predominance of CD8+ lymphocytes, and considering that CD8+ lymphocytes require MHC I expression for activation this observation is not surprising. However, scores for MHC II expression were not correlated with scores for predominance of CD4+ lymphocytes in IMM muscle biopsy specimens although CD4+ lymphocytes require MHC II expression for activation. This could be because of the fact that both CD8+ and CD4+ lymphocytes were present in significant numbers in IMM muscle compared with controls, with 48% of IMM horses having a predominance of CD4+ cells compared to CD8+ and CD20+ lymphocytes. The mixed population of CD4+ and CD8+ lymphocytes in horses with IMM suggests that multiple immune‐mediated events lead to myofiber destruction. The CD8+ lymphocytes lead to direct cell‐mediated cytotoxicity and the CD4+ cells typically mediate disease by interaction with B cells and the secondary antibody response. In many of the immune‐mediated myopathies of humans and dogs, there is a characteristic predominance of either CD4+ or CD8+ lymphocytes.24 For example, PM is characterized by a predominantly CD8+ infiltrate25 and DM and CMMM are characterized by predominantly CD4+ lymphocytic infiltrates.3, 26 Although CD4+ cells predominate in CMMM, affected masticatory muscle are reported to have higher MHC class I than II scores suggesting that there may not be a strong correlation between MHC I and II expression and scores for CD8+ and CD4+ lymphocytes in muscle biopsy specimens.8 Dermatomyositis is relatively unique in that it typically presents with characteristic skin lesions in addition to muscle weakness.16
In conclusion, MHC I and II expression occur in mature nondegenerate myofibers of horses with IMM in conjunction with a phase of active lymphocytic infiltration, findings that together support an immune‐mediated etiology for the disease. Unlike IMM, MHC expression in specific myofibers of PSSM horses occurs in the absence of lymphocytic infiltrates. Because the degree of MHC sarcolemmal expression varies in IMM‐affected horses and also is present in horses with PSSM, MHC staining alone is not diagnostic for IMM in horses. Muscular dystrophies associated with dysferlin, dystrophin, and sarcoglycan deficiencies were ruled out based on normal IF staining of IMM muscle samples.
Acknowledgments
The study was supported by the American Quarter Horse Association.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The histochemical and immunohistochemical work was performed at the Neuromuscular Diagnostic Laboratory at the College of Veterinary Medicine University of Minnesota. The immunofluorescent work was performed at the Comparative Neuromuscular Laboratory, Department of Pathology, University of California, San Diego, La Jolla CA 92093.
An abstract was published and the paper was presented at the 2015 American College of Veterinary Internal Medicine Forum, Indianapolis, IN.
Footnotes
EnVision Systems, Dako North America, Inc. 6392 Via Real, Carpinteria, CA 93013
Novocastra, Leica Biosystems, Buffalo Grove, Illinois
Gift from Eva Engvall
Novocastra, Leica Biosystems, Buffalo Grove, Illinois
GraphpadPrism version 6.00 for Mac OS X, Graphpad Software, La Jolla, California
References
- 1. Lewis SS, Valberg SJ, Nielsen IL. Suspected immune‐mediated myositis in horses. J Vet Intern Med 2007;21:495–503. [DOI] [PubMed] [Google Scholar]
- 2. Pumarola M, Moore PF, Shelton GD. Canine inflammatory myopathy: Analysis of cellular infiltrates. Muscle Nerve 2004;29:782–789. [DOI] [PubMed] [Google Scholar]
- 3. Evans J, Levesque D, Shelton GD. Canine inflammatory myopathies: A clinicopathologic review of 200 cases. J Vet Intern Med 2004;18:679–691. [DOI] [PubMed] [Google Scholar]
- 4. Hewer E, Goebel HH. Myopathology of non‐infectious inflammatory myopathies – the current status. Pathol Res Pract 2008;204:609–623. [DOI] [PubMed] [Google Scholar]
- 5. Gallardo E, Rojas‐Garcia R, de Luna N, et al. Inflammation in dysferlin myopathy: Immunohistochemical characterization of 13 patients. Neurology 2001;57:2136–8213. [DOI] [PubMed] [Google Scholar]
- 6. Confalonieri P, Oliva L, Andreetta F, et al. Muscle inflammation and MHC class I up‐regulation in muscular dystrophy with lack of dysferlin: An immunopathological study. J Neuroimmunol 2003;142:130–136. [DOI] [PubMed] [Google Scholar]
- 7. Karpati G, Pouliot Y, Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol 1988;23:64–72. [DOI] [PubMed] [Google Scholar]
- 8. Paciello O, Shelton GD, Papparella S. Expression of major histocompatibility complex class I and class II antigens in canine masticatory muscle myositis. Neuromuscul Disord 2007;17:313–320. [DOI] [PubMed] [Google Scholar]
- 9. Appleyard ST, Dunn MJ, Dubowitz V, Rose ML. Increased expression of HLA ABC class I antigens by muscle fibres in duchenne muscular dystrophy, inflammatory myopathy, and other neuromuscular disorders. Lancet 1985;1:361–363. [DOI] [PubMed] [Google Scholar]
- 10. McDouall RM, Dunn MJ, Dubowitz V. Expression of class I and class II MHC antigens in neuromuscular diseases. J Neurol Sci 1989;89:213–226. [DOI] [PubMed] [Google Scholar]
- 11. Hohlfield R. Immune mechanisms in muscle diseases In: Engel A, Franzini‐Armstrong C, eds. Myology: Basic and Clinical. 3rd ed New York: McGraw‐Hill, Medical Publishing Division; 2004:889–914. [Google Scholar]
- 12. Yin X, Wang Q, Chen T, et al. CD4+ Cells, macrophages, MHC‐I and C5b‐9 involve the pathogenesis of dysferlinopathy. Int J Clin Exp Pathol 2015;8:3069–3075. [PMC free article] [PubMed] [Google Scholar]
- 13. Cumming WJK. Color Atlas of Muscle Pathology, 1st ed London; Baltimore: Mosby‐Wolfe; 1994. [Google Scholar]
- 14. Liu LA, Engvall E. Sarcoglycan isoforms in skeletal muscle. J Biol Chem 1999;274:38171–38176. [DOI] [PubMed] [Google Scholar]
- 15. Guo LT, Moore SA, Forcales S, et al. Evaluation of commercial dysferlin antibodies on canine, mouse and human skeletal muscle. Neuromuscul Disord 2010;20:820–825. [DOI] [PubMed] [Google Scholar]
- 16. Shelton GD. From dog to man: The broad spectrum of inflammatory myopathies. Neuromuscul Disord 2007;17:663–670. [DOI] [PubMed] [Google Scholar]
- 17. Morita T, Shimada A, Yashiro S, et al. Myofiber expression of class I major histocompatibility complex accompanied by CD8+ T‐cell‐associated myofiber injury in a case of canine polymyositis. Vet Pathol 2002;39:512–515. [DOI] [PubMed] [Google Scholar]
- 18. Bartoccioni E, Gallucci S, Scuderi F, et al. MHC class I, MHC class II and intercellular adhesion molecule‐1 (ICAM‐1) expression in inflammatory myopathies. Clin Exp Immunol 1994;95:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shelton GD, Hoffman EP, Ghimbovschi S, et al. Immunopathogenic pathways in canine inflammatory myopathies resemble human myositis. Vet Immunol Immunopathol 2006;113:200–214. [DOI] [PubMed] [Google Scholar]
- 20. Wiendl H, Hohlfeld R, Kieseier BC. Immunobiology of muscle: Advances in understanding an immunological microenvironment. Trends Immunol 2005;26:373–380. [DOI] [PubMed] [Google Scholar]
- 21. Jain A, Sharma MC, Sarkar C, et al. Major histocompatibility complex class I and II detection as a diagnostic tool in idiopathic inflammatory myopathies. Arch Pathol Lab Med 2007;131:1070. [DOI] [PubMed] [Google Scholar]
- 22. McCue ME, Armien AG, Lucio M, et al. Comparative skeletal muscle histopathologic and ultrastructural features in two forms of polysaccharide storage myopathy in horses. Vet Pathol 2009;46:1281–1291. [DOI] [PubMed] [Google Scholar]
- 23. Tews DS, Goebel HH. Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol 1996;55:342–347. [DOI] [PubMed] [Google Scholar]
- 24. Dalakas MC. Immunopathogenesis of inflammatory myopathies. Ann Neurol 1995;37:S74–S86. [DOI] [PubMed] [Google Scholar]
- 25. Dalakas MC. Review: An update on inflammatory and autoimmune myopathies. Neuropathol Appl Neurobiol 2011;37:226–242. [DOI] [PubMed] [Google Scholar]
- 26. Shinjo SK, Sallum AM, Silva CA, Marie SK. Skeletal muscle major histocompatibility complex class I and II expression differences in adult and juvenile dermatomyositis. Clinics (Sao Paulo) 2012;67:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]


