Abstract
The purpose of the present study was to evaluate the validity and reliability of a Japanese version of an electromagnetic hypersensitivity (EHS) questionnaire, originally developed by Eltiti et al. in the United Kingdom. Using this Japanese EHS questionnaire, surveys were conducted on 1306 controls and 127 self‐selected EHS subjects in Japan. Principal component analysis of controls revealed eight principal symptom groups, namely, nervous, skin‐related, head‐related, auditory and vestibular, musculoskeletal, allergy‐related, sensory, and heart/chest‐related. The reliability of the Japanese EHS questionnaire was confirmed by high to moderate intraclass correlation coefficients in a test–retest analysis, and high Cronbach's α coefficients (0.853–0.953) from each subscale. A comparison of scores of each subscale between self‐selected EHS subjects and age‐ and sex‐matched controls using bivariate logistic regression analysis, Mann–Whitney U‐ and χ 2 tests, verified the validity of the questionnaire. This study demonstrated that the Japanese EHS questionnaire is reliable and valid, and can be used for surveillance of EHS individuals in Japan. Furthermore, based on multiple logistic regression and receiver operating characteristic analyses, we propose specific preliminary criteria for screening EHS individuals in Japan. Bioelectromagnetics. 37:353–372, 2016. © 2016 The Authors. Bioelectromagnetics Published by Wiley Periodicals, Inc.
Keywords: idiopathic environmental intolerance, electromagnetic fields, EHS screening tool, multiple chemical sensitivities, allergy symptoms
INTRODUCTION
Sensitivity to electromagnetic fields (EMFs) has generally been referred to as “electromagnetic hypersensitivity (EHS),” whereas the scientific term for this phenomenon is “idiopathic environmental intolerances attributed to electromagnetic fields (IEI‐EMFs)” [WHO, 2005; COST, 2011]. We have used the term “EHS” throughout this paper because it was used by Eltiti et al. [2007] in the originally developed questionnaire. The questionnaire was subsequently translated to Japanese and has been used as the Japanese version of Eltiti's questionnaire in the present study.
EHS is characterized by a variety of non‐specific symptoms, which affected individuals have attributed to exposure to EMFs [WHO, 2005]. Health effects of exposure to strong EMFs are well‐documented, and are generally controlled by regulations and guidelines [ICNIRP, 2009]. In addition, numerous reports exist on health effects of low‐level EMF exposure [Hillert et al., 2002; Mohler et al., 2010; Röösli et al., 2010; Rubin et al., 2010; Baliatsas et al., 2012]. However, there has not been, until now, conclusive evidence linking the pathophysiology of EHS to any previous exposure to EMFs [WHO, 2005; COST, 2011]. There is also evidence of a “nocebo effect” in triggering acute health reactions [Rubin et al., 2011; Witthöft and Rubin, 2013]. Moreover, other factors, including poor indoor air quality or stress in the workplace/living environment, may also play significant roles [WHO, 2005].
A very wide range (1.2–13.3%) of estimates exists regarding the prevalence of EHS in the general population [Meg Tseng et al., 2001; Hillert et al., 2002; Levallois et al., 2002; Leitgeb and Schröttner, 2003; Schreier et al., 2006; Preece et al., 2007; Landgrebe et al., 2008; Schröttner and Leitgeb, 2008; Mohler et al., 2010; Röösli et al., 2010; Rubin et al., 2010, 2011; Baliatsas et al., 2012; Hojo and Tokiya, 2012; Nordin et al., 2013]. In Japan, Furubayashi et al. [2009] reported that 1.2% of females showed mobile phone‐related and other unusual symptoms around telecommunication masts; however, further reports concerning EHS among the Japanese population are lacking. As well, the number of people with an EHS condition varies between countries. This may be attributed to differences in the definitions of EHS, the methods of assessment used, and media coverage during the survey [COST, 2011].
The lack of a general case definition for EHS, and the absence of a standardized approach for measuring concrete aspects of EHS that would permit a cross‐comparison by different investigators, have delayed further studies in this area. Surveys using conventional psychological tests or questions with “yes” or “no” answers have been the most commonly used measures for the evaluation of effects of EMFs on health [Abdel‐Rassoul et al., 2007, Berg‐Beckhoff et al., 2009; Blettner et al., 2009]. However, investigations using conventional questionnaires failed to reveal any specific symptoms of EHS, nor clarified the severity of symptoms associated with exposure to specific EMF objects.
In the United Kingdom (UK), Eltiti et al. [2007] developed the EHS questionnaire, which evaluates effects of EMF exposure, particularly on EMF‐related health conditions.
The main advantage of this EHS questionnaire is that it not only takes into account the individual's belief as to the cause of their symptom(s), but also includes the degree of symptom severity. The study reported that 145 (4.0%) out of 3,633 respondents from a randomly selected general population of 20,000 people met the “screening criteria for EHS.” Subsequently, we translated “Eltiti's questionnaire” into Japanese by modifying and adding several questions unique to the Oita version of the Japanese EHS questionnaire, which was confirmed for its validity and reliability, and was used for investigating health effects of EMFs from mobile phone base stations on 520 members of a randomly selected general public (230 males, 20–89 years old; and 290 females, 18–87 years old) in Oita Prefecture, Japan in 2010.
In the past, we [Hojo, 2002; Hojo et al., 2003, 2004, 2005, 2007, 2008a, 2008b, 2009; Hojo and Tokiya, 2012] conducted several investigations in order to elucidate the actual status of multiple chemical sensitivity (MCS) and sick building syndrome or sick house syndrome (SHS) patients in Japan using the quick environmental exposure and sensitivity inventory (QEESI) developed by Miller and Prihoda [1999]. Our findings revealed that a number of MCS/SHS patients complained of hypersensitive reactions to various EMF sources. We have, therefore, been on the lookout for a questionnaire that can evaluate hypersensitive reactions to sources of EMFs in order to assess the genuineness or spuriousness of patients’ complaints. However, the above‐mentioned “Oita version of Japanese EHS questionnaire” was not used, because it had been significantly modified from the original one [Eltiti et al., 2007]. Therefore, in this study, we accurately re‐translated Eltiti's questionnaire, and subsequently developed a new Japanese version (Japanese EHS questionnaire), courtesy of Dr. S. Eltiti.
The aims of the present study were as follows: (1) to evaluate the reliability and validity of the Japanese EHS questionnaire; (2) to reveal characteristics of symptoms in Japanese self‐selected EHS subjects; (3) to reveal characteristics of EMF objects that Japanese self‐selected EHS subjects believed were the cause of their EHS‐related symptoms and reactions; (4) to reveal characteristics of chronic illnesses (past and present) in Japanese self‐selected EHS subjects; and (5) to propose preliminary criteria for the screening of EHS individuals in Japan on the basis of the findings of this study.
METHODS
Structure of the Japanese EHS Questionnaire
The Japanese EHS questionnaire consisted of four sections: (I) biographical information; (II) symptoms and causes; (III) general health data; and (IV) additional questions unique to the Japanese questionnaire. Sections I, II, and III were almost identical to those in the original EHS questionnaire [Eltiti et al., 2007] in terms of the content of questions and the manner in which participants were required to answer them. Some parts of questions were slightly modified after consultation with MCS/EHS specialists, based on specific needs of the Japanese population.
Biographical information
In this section, the participants were asked six questions regarding their age, gender, address, occupation, final academic background, and mean working hours per day.
Symptoms and causes (q1–71)
Symptoms (q1–57)
In this section, participants were questioned about the frequency of occurrence of 57 symptoms (Supplementary Table). They were required to indicate their responses using a 5‐point scale: 1 (not at all), 2 (a little bit), 3 (moderately), 4 (quite a bit), and 5 (a great deal). It should be noted that symptoms were presented as single words or phrases in the EHS questionnaire used by Eltiti et al. [2007], whereas in the Japanese version, symptoms were presented in the form of questions (Supplementary Table).
EMF‐producing objects (q58–66)
In this section, nine questions were asked regarding the perception of a link between EMF‐producing objects and 57 symptoms. Participants needed to indicate their responses using the 5‐point scale described earlier. Furthermore, each participant was asked to write the name of an object and the severity of the symptom, in order to ascertain the presence of any additional EMF objects that were not mentioned in the EHS questionnaire used by Eltiti et al. [2007].
Reactions to EMF exposure (q67–71)
In this section, participants were required to answer three questions regarding EMF sensitivity (q67), occurrence of static shocks (q70), and negative health changes around EMFs (q71) using the 5‐point scale. Moreover, in order to determine if there were any additional EMF‐producing objects that were not mentioned in the original EHS questionnaire [Eltiti et al., 2007], participants were required to state specific EMF object(s) affecting their health, along with a detailed description of symptom(s) experienced (q68, a descriptive‐type question). Participants were also asked to indicate if they had ever experienced severe electric shock (q69, a “yes” or “no” question).
General health (1–4)
In this section, questions 1 to 3–1 and 4 were identical to those in the EHS questionnaire [Eltiti et al., 2007]. However, in order to evaluate the quality of sleep, participants were questioned about average sleeping hours (3–2), and quality of sleep (3–3, sleep disorder; Japanese modification; Supplementary Materials).
Additional questions unique to the Japanese questionnaire
The todai health index‐depression (THI‐D) scale (10 items)
The THI‐D scale is frequently used to assess symptoms of depression in Japan [Takeuchi et al., 1994; Kawada et al., 1999]. Results of the THI‐D scale in the present study were compared with those of existing health surveys that have used this method in Japan because most self‐selected EHS subjects generally develop symptoms of depression.
Physician‐diagnosed chronic illness (present and past)
In order to determine the relationship between EHS and other chronic illnesses, including MCS and SHS, participants were provided with a list of chronic illnesses and asked to mark their current illness with a circle within a circle (⊚), and their past chronic illnesses with a circle only (○).
Sequence of EHS development
Participants who believed that they had developed EHS or MCS were asked to answer questions regarding the time and sequence of onset of EHS, MCS, and SHS (Supplementary Materials).
STUDY PERIOD AND PARTICIPANTS
Surveys were conducted between 2009 and 2015.
Controls
The control group was comprised of ordinary residents living in cities across Japan. Participants exhibited a wide age range, and were recruited via mailing lists and information magazines using universities and local non‐profit organizations (NPOs) as contacts. The questionnaire was mailed to 2,000 selected persons, with 1,320 questionnaires returned (participation rate, 66%). However, valid data (data concerning age, sex, and at least 90% other entries) were obtained from only 1,306 of the 1,320 returned questionnaires.
Self‐Selected EHS Subjects
Self‐selected EHS subjects were recruited via two self‐help EHS groups in Japan. Questionnaires were mailed to 165 people who cooperated with our research, and were returned from 128 patients (participation rate, 77.6%). However, valid data were obtained from only 127 patients as one patient complained of MCS symptoms and was, therefore, excluded from the study.
Statistical Analyses
Statistical analyses were performed using SPSS for Windows (version 22; IBM, Armonk, NY).
Reliability
Test–retest measures were applied to 52 NPO members (males: n = 27, average age: 48.93 ± 16.70 years; females: n = 25, average age: 42.76 ± 14.75 years) and 121 students from three universities (males: n = 38, average age: 20.00 ± 0.62 years; females: n = 83, average age: 20.83 ± 0.91 years). Thus, a total of 173 subjects from the general public (males: n = 65, average age: 32.02 ± 17.89 years, age range: 19–76; females: n = 108, average age: 25.91 ±11.65 years, age range: 17–66) were included for test–retest measures. The same test was performed twice in 1–2‐week intervals by the same subjects. The score obtained for each question, using the 5‐point scale, and the total score from each subscale were determined by intraclass correlation coefficients (ICC). The Kappa (κ) coefficient was calculated for q68 and q69 due to dichotomous response options (“yes” or “no”) for these questions. Internal consistency was calculated using Cronbach's α coefficient [Cronbach, 1951] for each subscale in questionnaires obtained from controls and self‐selected EHS subjects.
Validity
Discrimination validity of the Japanese questionnaire was determined by comparing scores of each question and total scores for each subscale between self‐selected EHS subjects (n = 127), and sex‐ and age‐matched (±5 years) controls (n = 127), using bivariate logistic regression analyses, Mann–Whitney U‐ and χ 2 tests, and with Bonferroni correction for multiple tests (100 tests; P < 0.0005).
Furthermore, the distribution pattern of total symptom scores concerning 57 symptoms in both groups is depicted using a histogram (Fig. 1a) and box‐and‐whisker plots (Fig. 1b). The 25th, 50th, and 75th percentiles were estimated for scores of total symptoms in the control group. The horizontal line in each box represents the median value, whereas those at the bottom and top of the box represent 25th and 75th percentile values, respectively. The median score of total symptoms in self‐selected EHS subjects was compared with that of controls using a Mann–Whitney U‐test (Fig. 1a and b) with Bonferroni correction for multiple tests (100 tests; P < 0.0005). Median values of symptoms (eight principal components) are shown in a radar chart (Fig. 1c).
Figure 1.
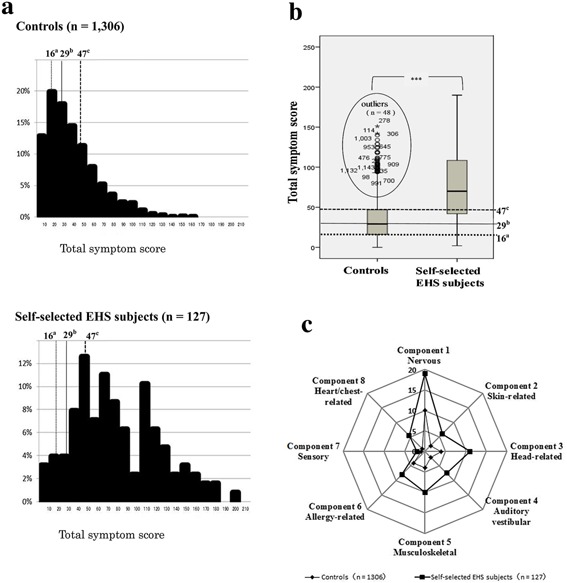
Comparison of total symptom scores between controls and self‐selected EHS subjects. 1a. Histogram showing total symptom scores. EHS: electromagnetic hypersensitivity. 1b. Box‐and‐whisker plots showing total symptom scores. Plots inside ellipse represent outliers that are not within normal range. Note: differences in median scores of total symptoms between self‐selected EHS subjects (n = 127) and controls (n = 127) were compared using a Mann–Whitney U‐test with Bonferroni correction for multiple tests (100 tests; P < 0.0005); ***P ≤ 0.00001. For 1a and 1b: The a25th percentile (16 points), b50th percentile (29 points), and c75th percentile value of controls (47 points). 1c. Radar chart showing median values of symptoms for eight principal components (factors 1–8). Differences in median values of symptoms for all eight principal components between self‐selected EHS subjects (n = 127) and controls (n = 127) were analyzed using a Mann–Whitney U‐test as above. There were significant differences (P ≤ 0.00001) in median values of symptoms for all eight principal components.
Preliminary screening criteria for EHS individuals
Multiple logistic regression and receiver operating characteristic (ROC) analyses were implemented between the self‐selected EHS subject group (n = 127) and the sex‐ and age‐matched control group (n = 127); preliminary screening criteria for EHS individuals from the general population were calculated using results of these analyses.
Ethical Considerations
The present study was approved by the ethics committees of the following institutes in Japan: Oita University (approved on June 4, 2009); National Hospital Organization (NHO), Morioka National Hospital, Morioka (approved on June 6, 2012); and NHO, Sagamihara National Hospital, Sagamihara (approved on July 9, 2013), in accordance with the Declaration of Helsinki [WMA, 2013].
RESULTS
Sample Characteristics
Table 1 shows characteristics of controls and self‐selected EHS subjects. Mean working hours of EHS patients (6.52 h) were significantly lower than those of controls (8.42 h). There were no significant differences in resident area and final academic background between self‐selected EHS subjects, and age‐ and sex‐matched controls. On the other hand, there were significantly fewer full‐time workers and more unemployed persons among self‐selected EHS subjects when compared with controls.
Table 1.
Characteristics of Controls and Self‐Selected EHS Subjects
| Controls (total) (n = 1306) | Controls (met set screening criteria a ) (n = 60) | Controls (age and sex matched) (n = 127) | Self‐selected EHS subjects (n = 127) | χ 2 test P value | |
|---|---|---|---|---|---|
| Biographical information | |||||
| Gender | |||||
| Male | 373 (28.6%) | 10 (16.7%) | 26 (20.5%) | 26 (20.5%) | 1.000 n.s. |
| Female | 933 (71.4%) | 50 (83.3%) | 101 (79.5%) | 101 (79.5%) | |
| Total | 1306 (100%) | 60 (100%) | 127 (100%) | 127 (100%) | |
| Age (years) | |||||
| 19≧ | 144 (11.0%) | 4 (6.7%) | 3 (2.4%) | 4 (3.1%) | 0.977 n.s. |
| 20–39 | 491 (37.6%) | 22 (36.7%) | 9 (7.1%) | 8 (6.3%) | |
| 40–50 | 424 (32.5%) | 20 (33.3%) | 68 (53.5%) | 68 (53.5%) | |
| 60≦ | 247 (18.9%) | 14 (23.2%) | 47 (37.0%) | 47 (37.0%) | |
| Total | 1306 (100%) | 60 (100%) | 127 (100%) | 127 (100%) | |
| Mean ± SD | 40.49 ± 18.00 | 43.10 ± 17.03 | 54.28 ± 13.87 | 54.35 ± 14.34 | |
| Area | |||||
| Hokkaido/Tohoku b | 602 (46.6%) | 31 (51.7%) | 6 (4.7%) | 6 (4.7%) | |
| Kantou/Koussin/Hokuriku c | 232 (18.0%) | 9 (15.0%) | 51 (40.2%) | 54 (42.5%) | 0.941 n.s. |
| Tokai/Kinki/Chugoku d | 286 (22.1%) | 11 (18.3%) | 54 (42.5%) | 54 (42.5%) | |
| Kyushu/Shikoku/Okinawa e | 172 (13.3%) | 9. (15.0%) | 16 (12.6%) | 13 (10.2%) | |
| Education | |||||
| Primary school | 17 (1.4%) | 2 (3.4%) | 5 (4.1%) | 3 (2.4%) | 0.113 n.s. |
| High school | 610 (50.1%) | 21(35.6%) | 47 (38.5%) | 32 (25.6%) | |
| College/University | 530 (43.5%) | 30 (50.8%) | 65 (53.3%) | 82 (65.6%) | |
| Graduate | 60 (4.9%) | 6 (10.2%) | 5 (4.1%) | 8 (6.4%) | |
| Total | 1217 (100%) | 59 (100%) | 122 (100%) | 125 (100%) | |
| Occupation | |||||
| Unemployed | 127 (11.1%) | 4 (6.8%) | 16 (13.1%) | 42 (33.9%) | |
| Student | 171 (14.9%) | 16 (29.1%) | 6 (4.9%) | 4 (3.2%) | |
| Homeworker | 184 (16.0%) | 7 (11.9%) | 22 (16.4%) | 37 (29.8%) | 4.59 × 10−6 * |
| Part‐time worker | 178 (15.5%) | 7 (11.9%) | 26 (21.3%) | 16 (12.9%) | |
| Full‐time worker | 489 (42.5%) | 25 (42.4%) | 54 (49.6%) | 25 (20.2%) | |
| Total | 1149 (100%) | 59 (100%) | 122 (100%) | 124 (100%) | |
| Mean working hours per day f ± SD | 8.21 ± 3.57 | 9.06 ± 3.16 | 8.42 ± 3.57 | 6.52 ± 3.38 | 6.25 × 10−5 * |
Note: Differences in scores between groups were analyzed by χ 2 tests with Bonferroni correction for multiple tests (100 tests; P < 0.0005).
EHS, electromagnetic hypersensitivity; SD, standard deviation; n.s., not significant.
Controls met set screening criteria for EHS: controls who met set screening criteria for EHS individuals.
Hokkaido/Tohoku: seven prefectures (e.g., Hokkaido, Aomori, Iwate, Miyagi, Akita, Yamagata, Fukushima).
Kantou/Koussin/Hokuriku: thirteen prefectures (e.g., Ibaraki, Tochigi, Gunma, Saitama, Chiba, Tokyo, Kanagawa, Niigata, Toyama, Ishikawa, Fukui, Yamanashi, Nagano).
Tokai/Kinki/Chugoku: thirteen prefectures (e.g., Gifu, Shizuoka, Aichi, Mie, Shiga, Kyoto, Osaka, Hyogo, Nara, Wakayama, Tottori, Shimane, Okayama, Hiroshima, Yamaguchi).
Kyushu/Shikoku/Okinawa: twelve prefectures (e.g., Tokushima, Kagawa, Ehime, Kochi, Fukuoka, Saga, Nagasaki, Kumamoto, Oita, Miyazaki, Kagoshima, Okinawa).
Mean working hours per day: included housework and school hours.
P ≤ 0.00001.
Principal Component Analysis
Firstly, an exploratory principal component analysis (PCA) of 57 symptoms was carried out on controls (n = 1,306), using direct oblimin rotation, in order to examine the underlying pattern of symptoms and to condense their total number. Direct oblimin rotation was chosen over the usual varimax rotation owing to the viewpoint that this allows for components to be correlated to one another. Components with a factor loading over 0.4 were chosen, resulting in 12 components with eigenvalues greater than one, and accounting for 59.3% of the variance. Next, evaluation of the scree plot revealed only one component above the marked elbow, indicating that the first component accounted for 28.79% of the variance. However, owing to the complex nature of EHS and the lack of information regarding the pattern of EHS symptoms, we deemed it more appropriate to examine several multivariate solutions. The present study was aimed at developing a measure that could be used to explore specific aspects of EHS; therefore, we looked for several forced factor solutions (10‐, 9‐, 8‐, 7‐, and 6‐factor) with direct oblimin rotation. A forced eight‐factor solution was chosen for the following five reasons: (1) it contained the least number of cross‐loaded items; (2) it also had the highest number of items loaded onto each component; (3) the items that loaded onto each component resulted in cohesive symptom categories; (4) it was able to account for 51.9% of the variance; and (5) the same analysis was used in the study by Eltiti et al. [2007]. Table 2 illustrates eight categories that were revealed following further inspection of items within each component: nervous (10 items), skin‐related (8 items), head‐related (7 items), auditory and vestibular (8 items), musculoskeletal (8 items), allergy‐related (6 items), sensory (4 items), and heart/chest‐related (6 items). Factor loadings resulted in a high value of over 0.4 (0.406–0.881) with the exception of two symptoms, blisters on the skin (0.379) and high blood pressure (0.387). An eight‐factor principal component analysis was implemented in 246 people who presented with “q67 ≥ 1 point” in the general population (EHS group), in the same way as reported by Eltiti et al. [2007]. Finally, eight‐factor principal component analysis was attempted in 127 self‐selected EHS subjects. As a result, principal components were almost consistent with those of the entire general population (Table 3).
Table 2.
Factor Loadings From Forced Eight‐Factor Principal Component Analysis of Controls (n = 1306)
| Principal components | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Nervous | Skin‐related | Head‐related | Auditory vestibular | Musculo‐skeletal | Allergy‐related | Sensory | Heart/chest‐related | |
| Eigenvalue | 16.41 | 2.77 | 2.22 | 2.08 | 1.58 | 1.53 | 1.52 | 1.32 | |
| Proportion (%) | 28.79 | 4.85 | 3.89 | 3.64 | 2.76 | 2.68 | 2.67 | 2.31 | |
| Cumulative proportion (%) | 28.79 | 33.64 | 37.53 | 41.17 | 43.93 | 46.61 | 49.28 | 51.59 | |
| q12 | Depression | 0.811 | 0.190 | ‐0.363 | 0.302 | ‐0.245 | 0.241 | 0.263 | 0.371 |
| q13 | Difficulty in concentrating | 0.806 | 0.219 | ‐0.347 | 0.282 | ‐0.169 | 0.242 | 0.408 | 0.352 |
| q14 | Difficulty in focusing attention | 0.767 | 0.230 | −0.332 | 0.275 | −0.188 | 0.234 | 0.426 | 0.354 |
| q24 | Fatigue | 0.752 | 0.195 | −0.432 | 0.262 | −0.461 | 0.296 | 0.241 | 0.277 |
| q55 | Stress | 0.721 | 0.199 | −0.373 | 0.203 | −0.377 | 0.232 | 0.248 | 0.260 |
| q25 | Foggy thinking | 0.702 | 0.184 | −0.503 | 0.375 | −0.323 | 0.244 | 0.292 | 0.338 |
| q21 | Exhaustion | 0.669 | 0.161 | −0.468 | 0.231 | −0.426 | 0.314 | 0.234 | 0.266 |
| q2 | Anxiety | 0.633 | 0.194 | −0.184 | 0.165 | −0.229 | 0.381 | 0.099 | 0.320 |
| q34 | Memory difficulties | 0.472 | 0.120 | −0.206 | 0.197 | −0.440 | 0.214 | 0.448 | 0.300 |
| q54 | Sleep disturbances | 0.439 | 0.212 | −0.334 | 0.298 | −0.344 | 0.117 | 0.348 | 0.313 |
| q49 | Skin irritation | 0.232 | 0.757 | −0.212 | 0.270 | −0.248 | 0.307 | 0.166 | 0.168 |
| q52 | Skin redness | 0.235 | 0.741 | −0.236 | 0.187 | −0.071 | 0.342 | 0.165 | 0.228 |
| q53 | Skin swelling | 0.137 | 0.720 | −0.186 | 0.149 | −0.121 | 0.225 | 0.221 | 0.269 |
| q56 | Tingling sensations | 0.193 | 0.689 | −0.203 | 0.284 | −0.270 | 0.105 | 0.290 | 0.192 |
| q41 | Pain/Soreness of the skin | 0.126 | 0.611 | −0.183 | 0.360 | −0.272 | 0.250 | 0.219 | 0.217 |
| q48 | Skin burning sensations | 0.073 | 0.548 | −0.164 | 0.240 | −0.270 | −0.056 | 0.273 | 0.231 |
| q51 | Skin rash | 0.319 | 0.427 | −0.306 | 0.238 | 0.051 | 0.255 | 0.242 | 0.190 |
| q6 | Blisters on the skin | 0.129 | 0.397 | −0.141 | 0.247 | −0.019 | 0.388 | −0.012 | 0.264 |
| q26 | Headaches | 0.349 | 0.162 | −0.881 | 0.277 | −0.224 | 0.206 | 0.196 | 0.266 |
| q20 | Dull headaches | 0.294 | 0.195 | −0.871 | 0.282 | −0.293 | 0.260 | 0.232 | 0.305 |
| q35 | Migraines | 0.198 | 0.115 | −0.851 | 0.187 | −0.157 | 0.125 | 0.217 | 0.193 |
| q28 | Heaviness in the head | 0.436 | 0.195 | −0.803 | 0.301 | −0.339 | 0.196 | 0.208 | 0.356 |
| q46 | Sharp pain in the head | 0.209 | 0.290 | −0.790 | 0.314 | −0.254 | 0.183 | 0.263 | 0.303 |
| q22 | Eye problems | 0.365 | 0.227 | −0.442 | 0.364 | −0.348 | 0.307 | 0.222 | 0.189 |
| q15 | Digestive problems | 0.351 | 0.217 | −0.406 | 0.374 | −0.087 | 0.277 | 0.394 | 0.327 |
| q43 | Pressure in the ear | 0.198 | 0.272 | −0.323 | 0.716 | −0.251 | 0.122 | 0.308 | 0.305 |
| q39 | Pain in the ear | 0.174 | 0.190 | −0.324 | 0.713 | −0.153 | 0.181 | 0.356 | 0.320 |
| q44 | Ringing in the ear | 0.266 | 0.181 | −.0284 | 0.648 | −0.230 | 0.217 | 0.163 | 0.340 |
| q38 | Nausea | 0.331 | 0.196 | −0.420 | 0.561 | −0.182 | 0.327 | 0.355 | 0.382 |
| q23 | Facial prickling | 0.146 | 0.401 | −0.291 | 0.559 | −0.141 | 0.130 | 0.378 | 0.229 |
| q57 | Warmth in the ear | 0.143 | 0.365 | −0.094 | 0.533 | −0.221 | −0.079 | 0.177 | 0.278 |
| q33 | Loss of appetite | 0.390 | 0.078 | −0.365 | 0.437 | −0.065 | 0.209 | 0.414 | 0.267 |
| q42 | Pain/Warmth in the head | 0.264 | 0.259 | −0.257 | 0.436 | −0.275 | 0.225 | 0.205 | 0.370 |
| q36 | Muscle tension | 0.235 | 0.212 | −0.291 | 0.278 | −0.661 | 0.178 | 0.330 | 0.168 |
| q37 | Muscle weakness | 0.270 | 0.190 | −0.225 | 0.477 | −0.591 | 0.153 | 0.335 | 0.268 |
| q40 | Pain in joints | 0.210 | 0.192 | −0.341 | 0.461 | −0.587 | 0.327 | 0.293 | 0.233 |
| q50 | Numbness | 0.255 | 0.339 | −0.238 | 0.421 | −0.568 | 0.125 | 0.253 | 0.285 |
| q47 | Sickness | 0.401 | 0.330 | −0.359 | 0.332 | −0.558 | 0.236 | 0.268 | 0.373 |
| q4 | Back pain | 0.344 | 0.208 | −0.332 | 0.198 | −0.550 | 0.338 | 0.095 | 0.356 |
| q7 | Blurry vision | 0.351 | 0.090 | −0.311 | 0.229 | −0.460 | 0.295 | 0.211 | 0.358 |
| q29 | High blood pressure | 0.097 | 0.099 | −0.151 | −0.041 | −0.387 | 0.052 | 0.235 | 0.310 |
| q1 | Allergies | 0.226 | 0.265 | −0.173 | 0.155 | −0.056 | 0.707 | 0.105 | 0.075 |
| q45 | Runny or stuffy nose | 0.264 | 0.208 | −0.248 | 0.278 | −0.142 | 0.612 | 0.333 | 0.073 |
| q19 | Dry skin | 0.313 | 0.455 | −0.305 | 0.211 | −0.167 | 0.546 | 0.142 | 0.177 |
| q3 | Asthma | 0.054 | 0.063 | −0.037 | −0.046 | −0.130 | 0.525 | 0.106 | 0.197 |
| q18 | Dry cough | 0.164 | 0.089 | −0.231 | 0.199 | −0.252 | 0.497 | 0.320 | 0.320 |
| q5 | Bad taste in the mouth | 0.305 | 0.208 | −0.287 | 0.208 | −0.346 | 0.418 | 0.388 | 0.397 |
| q31 | Impaired sense of smell | 0.105 | 0.153 | −0.134 | 0.179 | −0.132 | 0.193 | 0.789 | 0.173 |
| q32 | Impaired sense of taste | 0.135 | 0.195 | −0.193 | 0.203 | −0.241 | 0.135 | 0.770 | 0.184 |
| q30 | Hoarse dry throat | 0.333 | 0.177 | −0.244 | 0.314 | −0.339 | 0.394 | 0.462 | 0.309 |
| q16 | Disorientation | 0.223 | 0.127 | −0.080 | 0.013 | −0.086 | −0.024 | 0.450 | 0.431 |
| q9 | Cardiac(Heart) pains | 0.178 | 0.161 | −0.268 | 0.268 | −0.168 | 0.124 | 0.231 | 0.824 |
| q10 | Chest pains | 0.254 | 0.228 | −0.318 | 0.312 | −0.279 | 0.226 | 0.298 | 0.782 |
| q8 | Breathing difficulties | 0.318 | 0.214 | −0.260 | 0.360 | −0.383 | 0.374 | 0.292 | 0.644 |
| q27 | Heart palpitations | 0.232 | 0.225 | −0.358 | 0.367 | −0.448 | 0.225 | 0.240 | 0.620 |
| q17 | Dizziness | 0.353 | 0.151 | −0.450 | 0.451 | −0.180 | 0.273 | 0.086 | 0.490 |
| q11 | Cold sweat | 0.385 | 0.265 | −0.194 | 0.299 | −0.056 | 0.229 | 0.268 | 0.473 |
Fifty‐seven symptoms in control group (n = 1,306) were analyzed by forced eight‐factor principal component analysis with oblimin rotation and Kaiser's normalization as described by Eltiti et al. [2007].
Table 3.
Factor Loadings for Controls, EHS Group a and Self‐Selected EHS Subjects in a Forced Eight‐Factor Principal Component Analysis
| Group | ||||
|---|---|---|---|---|
| Symptom | Controls (n = 1306) | EHS group a (n = 246) | Self‐selected EHS subjects (n = 127) | |
| Nervous | ||||
| q12 | Depression | 0.811 | 0.792 | 0.736 |
| q13 | Difficulty in concentrating | 0.806 | 0.656 | 0.790 |
| q14 | Difficulty in focusing attention | 0.767 | 0.639 | 0.755 |
| q24 | Fatigue | 0.752 | 0.786 | 0.584 |
| q55 | Stress | 0.721 | 0.776 | 0.651 |
| q25 | Foggy thinking | 0.702 | 0.560 | 0.770 |
| q21 | Exhaustion | 0.669 | 0.677 | 0.521 |
| q2 | Anxiety | 0.633 | 0.678 | 0.699 |
| q34 | Memory difficulties | 0.472 | 0.489 | −0.607 |
| q54 | Sleep disturbances | 0.439 | 0.486 | 0.657 |
| Skin‐related | ||||
| q49 | Skin irritation | 0.757 | 0.742 | 0.678 |
| q52 | Skin redness | 0.741 | 0.695 | 0.631 |
| q53 | Skin swelling | 0.720 | 0.722 | 0.705 |
| q56 | Tingling sensations | 0.689 | 0.698 | 0.772 |
| q41 | Pain/soreness of the skin | 0.611 | 0.603 | 0.700 |
| q48 | Skin burning sensations | 0.548 | 0.744 | −0.533 |
| q51 | Skin rash | 0.427 | 0.462 | 0.457 |
| q6 | Blisters on the skin | 0.397 | 0.516 | 0.350 |
| Head‐related | ||||
| q26 | Headache | −0.881 | −0.831 | 0.827 |
| q20 | Dull headache | −0.871 | −0.779 | 0.794 |
| q35 | Migraines | −0.851 | −0.807 | 0.751 |
| q28 | Heaviness in the head | −0.803 | −0.672 | 0.803 |
| q46 | Sharp pain in the head | −0.790 | −0.728 | 0.689 |
| q22 | Eye problems | −0.442 | 0.509 | 0.554 |
| q15 | Digestive problems | −0.406 | −0.551 | 0.603 |
| Auditory vestibular | ||||
| q43 | Pressure in the ear | 0.716 | 0.795 | −0.724 |
| q39 | Pain in the ear | 0.713 | 0.738 | −0.707 |
| q44 | Ringing in the ear | 0.648 | 0.583 | −0.497 |
| q38 | Nausea | 0.561 | 0.591 | 0.574 |
| q23 | Facial prickling | 0.559 | 0.570 | 0.672 |
| q57 | Warmth in the ear | 0.533 | 0.625 | 0.455 |
| q33 | Loss of appetite | 0.437 | −0.432 | 0.720 |
| q42 | Pain/warmth in the head | 0.436 | 0.507 | 0.531 |
| Musculo/Skeletal | ||||
| q36 | Muscle tension | −0.661 | 0.598 | 0.748 |
| q37 | Muscle weakness | −0.591 | 0.671 | 0.661 |
| q40 | Pain in the joints | −0.587 | 0.625 | 0.688 |
| q50 | Numbness | −0.568 | 0.529 | −0.650 |
| q47 | Sick feeling | −0.558 | 0.497 | 0.572 |
| q4 | Back pain | −0.550 | 0.458 | 0.666 |
| q7 | Blurry vision | −0.460 | 0.577 | −0.624 |
| q29 | High blood pressure | −0.387 | 0.329 | 0.751 |
| Allergy‐related | ||||
| q1 | Allergies | 0.707 | 0.694 | 0.498 |
| q45 | Runny or stuffy nose | 0.612 | 0.544 | 0.543 |
| q19 | Dry skin | 0.546 | 0.689 | 0.568 |
| q3 | Asthma | 0.525 | 0.531 | 0.714 |
| q18 | Dry cough | 0.497 | 0.485 | 0.655 |
| q5 | Bad taste in the mouth | 0.418 | 0.502 | 0.568 |
| Sensory | ||||
| q31 | Impaired sense of smell | 0.789 | 0.760 | 0.675 |
| q32 | Impaired sense of taste | 0.770 | 0.734 | 0.461 |
| q30 | Hoarse dry throat | 0.462 | 0.516 | 0.581 |
| q16 | Disorientation | 0.450 | 0.493 | −0.753 |
| Heart/Chest‐related | ||||
| q9 | Cardiac/heart pains | 0.824 | 0.812 | 0.684 |
| q10 | Chest pains | 0.782 | 0.816 | 0.654 |
| q8 | Breathing difficulties | 0.644 | 0.516 | 0.700 |
| q27 | Heart palpitations | 0.620 | 0.568 | 0.542 |
| q17 | Dizziness | 0.490 | 0.553 | 0.589 |
| q11 | Cold sweat | 0.473 | 0.445 | 0.510 |
EHS group Controls who had a score higher than 1 point for q67 (“Are you sensitive to electromagnetic fields, e.g., radio frequencies and magnetic fields produced by electrical objects such as televisions, computers, and mobile phones?”).
Reliability
The test–retest method revealed significant correlations for ICCs of all 5‐point scale questions and total scores of each subscale (Table 4). A high to moderate value of over 0.6 (0.623–0.863) was noted with the exception of component 4, q64, q65, and q71 (Table 4). In addition, κ coefficients of q68 and q69 were 0.442 and 0.484, respectively. Although significant, reproducibility of these values was not high. Taken together, these findings indicate high reliability of the Japanese EHS questionnaire. High Cronbach's a coefficients (0.853–0.953) from all subscales, with the exception of “Reaction to EMFs (0.528),” indicated good internal consistency for each question (Table 5).
Table 4.
Intraclass Correlation Coefficients of Test–Retest Data
| Subscales | Questionnaire items | Intraclass correlation coefficient (ICC) | P‐value |
|---|---|---|---|
| II‐1 Symptoms | Component 1_Nervous score (10 items) | 0.791 | <0.0001 |
| Component 2_Skin‐related score (8 items) | 0.863 | <0.0001 | |
| Component 3_Head‐related score (7 items) | 0.705 | <0.0001 | |
| Component 4_ Auditory vestibular score (8 items) | 0.585 | <0.0001 | |
| Component 5_Musculoskeletal score (8 items) | 0.716 | <0.0001 | |
| Component 6_Allergy‐related score (6 items) | 0.815 | <0.0001 | |
| Component 7_Sensory score (4 items) | 0.641 | <0.0001 | |
| Component 8_ Heart/chest‐related score (6 items) | 0.708 | <0.0001 | |
| Total score of Symptoms (57 items) | 0.773 | <0.0001 | |
| II‐2 EMF‐producing objects | q58_Computers | 0.573 | <0.0001 |
| q59_Electric appliances | 0.623 | <0.0001 | |
| q60_Fluorescent lighting | 0.789 | <0.0001 | |
| q61_Microwave ovens | 0.816 | <0.0001 | |
| q62_Mobile phones | 0.645 | <0.0001 | |
| q63_Power lines | 0.658 | <0.0001 | |
| q64_Radio/Television transmitters | 0.389 | 0.0012 | |
| q65_Telecommunication masts | 0.421 | 0.0003 | |
| q66_Televisions | 0.620 | <0.0001 | |
| Total score of EMF‐producing objects (9 items) | 0.709 | <0.0001 | |
| II‐3 Reaction to EMFs | q67_Sensitive to EMFs | 0.656 | <0.0001 |
| q70_ Occurrences of static electric shock | 0.719 | <0.0001 | |
| q71_Negative health change around EMFs | 0.417 | 0.0003 | |
| Total score of Reaction to EMFs (5 items) a | 0.655 | <0.0001 | |
| III General health | 1_ Well‐being | 0.829 | <0.0001 |
| 2_ Good health | 0.670 | <0.0001 | |
| 3_1 Sleep | 0.703 | <0.0001 | |
| 3_2 Sleeping hours per day | 0.746 | <0.0001 | |
| 3_3 Sleep disorder | 0.715 | <0.0001 | |
| IV‐1 THI‐D | Total score of THI‐D (10 items) | 0.783 | <0.0001 |
Test–retest reliability was estimated by calculating intraclass correlation coefficients (ICC); significance of ICC was determined using an F‐test. A total of 173 people from the general population participated in this study (see text).
EMF, electromagnetic field; THI‐D, todai health index‐depression scale.
Total score of reaction to EMF score: calculated by adding scores of q67–q71.
Table 5.
Cronbach's α Coefficient from Each Subclass in Controls and Self‐Selected EHS Subjects
| Cronbach's alpha coefficient | ||
|---|---|---|
| Questionnaire items | Controls (n = 1306) | Self‐selected EHS subjects (n = 127) |
| II‐1 Symptoms (57 items) | 0.953 | 0.968 |
| Eight factor principal components (8 items) | 0.872 | 0.921 |
| Component 1 (Nervous; 10 items) | 0.905 | 0.928 |
| Component 2 (Skin‐related; 8 items) | 0.794 | 0.865 |
| Component 3 (Head‐related; 7 items) | 0.873 | 0.904 |
| Component 4 (Auditory vestibular; 8 items) | 0.780 | 0.832 |
| Component 5 (Musculo‐skeletal; 8 items) | 0.785 | 0.837 |
| Component 6 (Allergy‐related; 6 items) | 0.678 | 0.657 |
| Component 7 (Sensory; 4 items) | 0.574 | 0.616 |
| Component 8 (Heart/chest‐related; 6 items) | 0.797 | 0.837 |
| II‐2 EMF‐producing objects (9 items) | 0.900 | 0.953 |
| II‐3 Reaction to EMFs (5 items) | 0.500 | 0.582 |
| IV‐1 THI‐D (10 items) | 0.893 | 0.912 |
EMF, electromagnetic field; THI‐D, todai health index‐depression scale.
Total Symptom Score and Eight Symptoms Component
Total symptom scores for self‐selected EHS subjects were widely distributed, as seen in Figure 1a. The 25th, 50th, and 75th percentiles for total symptom scores in controls were 16, 29, and 47 points, respectively. The median score of self‐selected EHS subjects was significantly higher than that of controls (P < 0.0005), as shown in Figure 1b. An outlier is defined as a point that exceeds 1.5 times the interquartile range from the 75th percentile line, and it should be noted that there were 48 (3.7%) outliers for the controls. With regard to the median score of eight principal components, shapes of the radar chart in both groups were quite similar (Fig. 1c). The median score of each component for self‐selected EHS subjects was significantly higher (P < 0.0005) than that of controls.
Validity
Firstly, validity was evaluated in the same way as reported by Eltiti et al. [2007], that is, by comparing EHS responses of self‐selected EHS subjects with those of the control group; the Mann–Whitney U‐test with Bonferroni correction for multiple tests (100 tests; P < 0.0005) was used, revealing significant differences between both groups (Table 6). Furthermore, the χ 2 test revealed that a significantly higher (P < 0.0005) proportion of self‐selected EHS subjects (87.4%) had identified EMF‐producing object(s) and described, in detail, specific symptoms they believed were caused by the object(s) (q68, χ 2 = 150.20, P < 0.0005) when compared with controls (12.6%). A significantly higher number of self‐selected EHS subjects (66.4%) experienced severe electric shock (q69, χ 2 = 10.0, P = 0.003) when compared with controls (49.6%). In summary, Mann–Whitney U‐ and χ 2 tests revealed that when compared with age‐ and sex‐matched controls, self‐selected EHS subjects described a greater severity of symptoms, poorer levels of general health and well‐being, and the belief that their symptoms were caused by exposure to objects that emitted EMFs.
Table 6.
Answer Distribution for Reactions to Nine EMF‐Producing Objects in Self‐Selected EHS Subjects and in Age‐ and Sex‐Matched Controls
| Objects | Not at all n (%) | A little bit n (%) | Moderately n (%) | Quite a bit n (%) | A great deal n (%) | Z a P‐value |
|---|---|---|---|---|---|---|
| q58_Computers | ||||||
| Controls | 82 (65.1) | 24 (19.0) | 13 (10.3) | 3 (2.4) | 4 (3.2) | −9.23 |
| Self‐selected EHS subjects | 16 (14.4) | 14 (12.6) | 27 (24.3) | 18 (16.2) | 36 (32.4) | 2.72 × 10−20 * |
| q59_Electrical appliances | ||||||
| Controls | 98 (79.7) | 16 (13.0) | 5 (4.1) | 3 (2.4) | 1 (0.8) | −10.724 |
| Self‐selected EHS subjects | 13 (12.4) | 19 (18.1) | 20 (19.1) | 18 (17.1) | 35 (33.3) | 7.85 × 10−27 * |
| q60_Fluorescent lighting | ||||||
| Controls | 110 (88.7) | 8 (6.5) | 3 (2.4) | 2 (1.6) | 1 (0.8) | −9.772 |
| Self‐selected EHS subjects | 33 (28.5) | 14 (12.1) | 15 (12.9) | 21 (18.1) | 33 (28.4) | 1.49 × 10−22 * |
| q61_Microwave ovens | ||||||
| Controls | 108 (86.4) | 9 (7.2) | 4 (3.2) | 2 (1.6) | 2 (1.6) | −9.005 |
| Self‐selected EHS subjects | 31 (31.0) | 9 (9.0) | 8 (8.0) | 16 (16.0) | 36 (36.0) | 2.16 × 10−19 * |
| q62_Mobile phones | ||||||
| Controls | 95 (75.4) | 14 (11.1) | 9 (7.1) | 5 (4.0) | 3 (2.4) | −10.38 |
| Self‐selected EHS subjects | 16 (14.3) | 12 (10.7) | 14 (12.5) | 16 (14.3) | 54 (48.2) | 3.06 × 10−25 * |
| q63_Power lines | ||||||
| Controls | 105 (85.4) | 7 (5.7) | 7 (5.7) | 1 (0.8) | 3 (2.4) | −8.499 |
| Self‐selected EHS subjects | 31 (32.0) | 10 (10.3) | 9 (9.3) | 14 (14.4) | 33 (34.0) | 1.92 × 10−17 * |
| q64_Radio/Television transmitters | ||||||
| Controls | 108 (87.8) | 8 (6.5) | 5 (4.1) | 1 (0.8) | 1 (0.8) | −8.045 |
| Self‐selected EHS subjects | 31 (37.8) | 6 (7.3) | 9 (11.0) | 9 (11.0) | 27 (32.9) | 8.64 × 10−16 * |
| q65_Telecommunication masts | ||||||
| Controls | 108 (87.8) | 4 (3.3) | 8 (6.5) | 2 (1.6) | 1 (0.8) | −11.288 |
| Self‐selected EHS subjects | 15 (14.7) | 10 (9.8) | 12 (11.8) | 12 (11.8) | 53 (52.0) | 1.51 × 10−29 * |
| q66_Televisions | ||||||
| Controls | 95 (74.8) | 20 (15.7) | 8 (6.3) | 3 (2.4) | 1 (0.8) | −8.493 |
| Self‐selected EHS subjects | 27 (23.9) | 27 (23.9) | 20 (17.7) | 16 (14.2) | 23 (20.4) | 2.02 × 10−17 * |
| Total score of EMF‐producing objects | 0 | 1–9 | 10–18 | 19–27 | 28–36 | Z P‐value |
| Controls | 72 (56.7) | 42 (33.1) | 9 (7.1) | 3 (2.4) | 1 (0.8) | −10.783 |
| Self‐selected EHS subjects | 8 (6.3) | 29 (22.8) | 27 (21.3) | 39 (30.7) | 24 (18.9) | 4.16 × 10−27 * |
| q67 b _Sensitive to EMFs | ||||||
| Controls | 106 (86.2) | 14 (11.4) | 3 (2.4) | 0 (0.0) | 0 (0.0) | −12.58 |
| Self‐selected EHS subjects | 11 (9.0) | 19 (15.6) | 17 (13.9) | 14 (11.5) | 61 (50.0) | 2.71 × 10−36 * |
| q70 c _Occurrences of static electric shock | ||||||
| Controls | 32 (25.2) | 51 (40.2) | 17 (13.4) | 16 (12.6) | 11 (8.7) | −3.445 |
| Self‐selected EHS subjects | 12 (9.7) | 48 (38.7) | 24 (19.4) | 17 (13.7) | 23 (18.5) | 5.71 × 10−4 n.s. |
Note: Differences in scores between groups were analyzed using a Mann–Whitney U‐test with Bonferroni correction for multiple tests (100 tests; P < 0.0005).
EMF electromagnetic field; n.s: not significant.
Z: Mann–Whitney U‐test; α level: 0.0005.
q67: “Are you sensitive to electromagnetic fields (e.g., radio frequency and magnetic fields produced by electrical objects such as television, computers, and mobile phones)?”
q70: “How frequently do you experience static shocks (e.g., from metals and car doors)?”
P ≤ 0.00005.
Furthermore, in the present study, logistic regression analysis was also performed to compare the discriminatory power of each question in the Japanese EHS questionnaire (Table 7). Results of the logistic regression analysis revealed that the odds ratio (OR; 0.85) for mean working hours per day was significantly lower in self‐selected EHS subjects than in controls (I. Biographical Information). Furthermore, ORs for total symptoms score and for each of the eight principal components (components 1–8; 1.05–1.60) were significantly higher (II–1. Symptoms). Similarly, ORs for total score (1.22) and for each of the nine EMF‐producing objects (2.61–4.29), especially electrical appliances (q59, 4.29), telecommunication masts (q65, 3.87), and fluorescent lighting (q60, 3.60), were also very high (II–2. EMF‐producing Objects). The ORs for all questions, particularly q68 (90.96) and q67 (9.96), were very high (II–3. Reactions to EMFs), whereas ORs for well‐being (0.59), good health (0.34), and sleep (0.53) were significantly lower (III. General Health). The OR for average sleeping hours per day was 1.22, indicating that self‐selected EHS subjects slept longer than controls. However, the OR for sleep disorders was 2.22, implying that self‐selected EHS subjects experienced more sleep disorders than controls. Furthermore, the OR for the total score of THI‐D was 1.21, suggestive of an increased tendency for depression amongst self‐selected EHS subjects when compared with controls.
Table 7.
Estimates of the Relative Risks of EHS for Each Potential Predictor Tested Separately in the Japanese EHS Questionnaire
| Questionnaire items | Odds ratio | 95%CI (min–max) | P‐value | |
|---|---|---|---|---|
| I Biographical | Final academic background | 1.63 | (1.08–2.46) | 0.021 |
| Mean working hours per day a | 0.85 | (0.79–0.93) | 1.27 × 10−4 | |
| II‐1 Symptoms | Component 1_Nervous score (10 items) | 1.15 | (1.11–1.19) | 2.83 × 10−12 |
| Component 2_Skin‐related score (8 items) | 1.26 | (1.17–1.35) | 4.37 × 10−10 | |
| Component 3_Head‐related score (7 items) | 1.18 | (1.12–1.24) | 7.25 × 10−11 | |
| Component 4_Auditory vestibular score (8 items) | 1.44 | (1.30–1.59) | 1.87 × 10−12 | |
| Component 5_Musculoskeletal score (8 items) | 1.21 | (1.14–1.28) | 3.85 × 10−11 | |
| Component 6_Allergy‐related score (6 items) | 1.28 | (1.19–1.38) | 4.12 × 10−10 | |
| Component 7_Sensory score (4 items) | 1.60 | (1.35–1.90) | 6.33 × 10−8 | |
| Component 8_ Heart/chest‐related score (6 items) | 1.47 | (1.33–1.64) | 8.78 × 10−13 | |
| Total score of Symptoms (57 items) | 1.05 | (1.03–1.06) | 6.78 × 10−14 | |
| II‐2 EMFc‐producing objects | q58_Computers | 2.85 | (2.20–3.71) | 4.77 × 10−15 |
| q59_Electrical appliances | 4.29 | (2.97–6.19) | 8.33 × 10−15 | |
| q60_Fluorescent lighting | 3.60 | (2.52–5.14) | 2.03 × 10−12 | |
| q61_Microwave ovens | 2.87 | (2.15–3.83) | 9.45 × 10−13 | |
| q62_Mobile phones | 2.98 | (2.32–3.83) | 1.48 × 10−17 | |
| q63_Power lines | 2.61 | (1.99–3.42) | 3.74 × 10−12 | |
| q64_Radio/Television transmitters | 2.92 | (2.10–4.05) | 1.75 × 10−10 | |
| q65_Telecommunication masts | 3.87 | (2.79–5.38) | 5.94 × 10−16 | |
| q66_Televisions | 2.95 | (2.17–4.00) | 4.94 × 10−12 | |
| Total score of EMF‐producing objects (9 items) | 1.22 | (1.16–1.28) | 6.90 × 10−15 | |
| II‐3 Reactions to EMFs | q67_Sensitive to EMFs | 9.66 | (5.16–18.09) | 1.40 × 10−12 |
| q68_Detailed description | 90.96 | (38.61–214.26) | 5.85 × 10−25 | |
| q69_Experience a severe electric shock | 2.40 | (1.44–4.01) | 8.19 × 10−4 | |
| q70_Occurrences of static electric shock | 1.40 | (1.14–1.71) | 0.001 | |
| Total score of Reactions to EMFs (4 items) | 2.20 | (1.80–2.68) | 7.56 × 10−15 | |
| III General health | 1_ Well‐being | 0.59 | (0.45–0.78) | 2.05 × 10−4 |
| 2_ Good health | 0.34 | (0.24–0.48) | 3.74 × 10−10 | |
| 3_1 Sleep | 0.53 | (0.40–0.70) | 9.44 × 10−6 | |
| 3_2 Sleeping hours per a day | 1.22 | (0.99–1.50) | 0.058 | |
| 3_3 Sleep disorder | 2.22 | (1.73–2.84) | 3.37 × 10−10 | |
| 4_ Chronic illnesses | 1.83 | (1.10–3.02) | 0.019 | |
| IV‐1 THI‐D | Total score of THI‐D (10 items) | 1.21 | (1.14–1.28) | 2.20 × 10−11 |
Note: Differences in scores between groups were analyzed using bivariate logistic regression analysis with Bonferroni correction for multiple tests (100 tests; P < 0.0005).
CI, confidence interval; EMF, electromagnetic field; THI‐D, todai health index‐depression scale.
Mean working hours per day: including housework and school hours.
Multiple Logistic Regression and ROC Analyses
Multiple logistic regression and ROC analyses were performed in self‐selected EHS subjects, and in sex‐ and age‐matched control groups to narrow down items that can aid in discrimination of EHS individuals from the general population during screening. Multiple logistic regression analysis revealed the following three items: “Total symptom score;” “q67, Sensitive to EMFs;” and “q68, Detailed description” (Table 8). The area under the ROC curve for predicted values was high (0.976). Sensitivity and specificity of predicted values using the three items were 94.3% and 94.3%, respectively, higher than analysis results obtained from individual items (Table 9). Based on these findings, we suggested the following preliminary screening criteria for EHS individuals from the general population: (1) the total symptom score should be greater than or equal to 47 points (the 75th percentile of the controls, Fig. 1a); (2) the score for q67 (“Are you sensitive to electromagnetic fields?”) should be greater than or equal to 1; and (3) individuals should be able to describe, in detail, the EMF source and the kind of symptoms developed in response to q68. A total of 82 (64.6%) self‐selected EHS subjects and 60 (4.59%) controls met the set screening criteria for EHS. Significant differences in resident areas, final academic background, and occupation were not noted between individual characteristics of these 60 controls and those of the remaining 1,246 control subjects (Table 1). However, the number of females was significantly higher among the 60 controls who met the set screening criteria (P < 0.0005) when compared to others (Table 1).
Table 8.
Discrimination Power of EHS Scales When Used Alone (Univariate) or When Combined in a Multiple Logistic Regression Model
| Scale | P‐value | Odds ratios (95%CI) for one point increase | Area under ROC curve |
|---|---|---|---|
| Individual scale | |||
| Total symptom score | 1.77 × 10−4 | 1.044 (1.021–1.067) | 0.851 (0.803–0.900) |
| q67_Sensitive to EMFs | 3.73 × 10−4 | 3.119 (1.667–5.835) | 0.934 (0.901–0.968) |
| q68_Detailed description | 7.18 × 10−6 | 21.252 (5.594–80.737) | 0.906 (0.864–0.948) |
| Multiple scales a | |||
| Total symptom score | 1.17 × 10−4 | 1.041 (1.020–1.063) | |
| q67_Sensitive to EMFsd | 1.41 × 10−5 | 3.503 (1.989–6.169) | 0.976 (0.959–0.994) |
| q68_Detailed description | 2.64 × 10−7 | 22.755 (6.924–74.784) | |
Note: Significant differences in scores between groups were evaluated by setting an α value of 0.0005 in consideration of multiplicity, according to a Bonferroni correction.
CI, confidence interval; ROC, receiver operating characteristic; EMFs, electromagnetic fields.
Multiple scales: each of three items listed below is a factor in one multiple logistic prediction (see text).
Table 9.
Sensitivity and Specificity Resulting From the Application of High Cut‐Off Points for Total Symptoms, q67 and q68 scales, and for All Three Scales Taken Together
| Scale | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Individual scale | ||
| Total symptom cut‐off | ||
| 45 | 73.8 | 79.7 |
| 47 a | 73.8 | 81.3 |
| 48 | 72.1 | 81.3 |
| q67_Sensitive to EMFs cut‐off | ||
| 1 | 91.0 | 86.2 |
| 2 | 75.4 | 97.6 |
| 3 | 61.5 | 100.0 |
| q68_Detailed description cut‐off | ||
| 1 | 88.5 | 92.7 |
| Multiple scale scores b | ||
| Total symptom score | ||
| q67_Sensitive to EMFs | 94.3 | 94.3 |
| q68_Detailed description | ||
Note: Significant differences in scores between groups were evaluated by setting an α value of 0.0005 in consideration of multiplicity according to a Bonferroni correction.
47: The 75th percentile of controls.
Multiple scale scores: Each of three items listed is a factor in one multiple logistic prediction (see text).
EMF Objects That Self‐Selected EHS Subjects Believed Were the Cause of Their Symptoms
Almost none of the controls responded to q68 (“Provide a detailed description of EMF‐producing objects and the specific symptoms caused by these.”), whereas 111 (87.4%) of the 127 self‐selected EHS subjects responded to the same question in detail. Thus, we analyzed contents described by the 111 subjects in response to q68.
A summary of nine EMF‐producing objects (q58–66), which Japanese self‐selected EHS subjects believed were the cause of their symptoms, is presented in Figure 2a. Furthermore, details of electrical appliances listed in this study are presented in Figure 2b.
Figure 2.
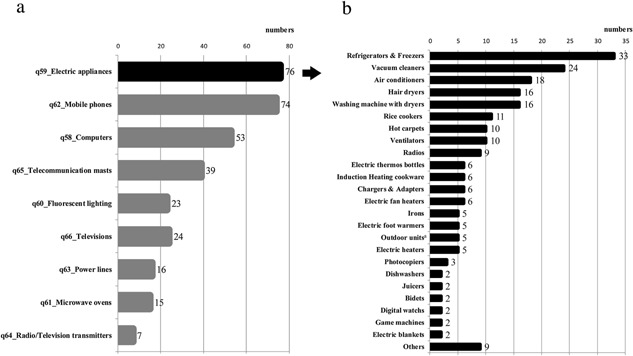
EMF sources. 2a. Electromagnetic field objects that Japanese self‐selected EHS subjects (n = 165) believed as cause of symptoms (multiple answers). 2b. Details of electrical appliances from Fig. 2a. Outdoor unitsa included air conditioner compressors and heat pump‐type water heater systems, among others (multiple answers).
The following three additional types of EMF‐producing objects, other than the nine (q58‐q66) included in the questionnaire, were also reported to cause symptoms in Japanese self‐selected EHS subjects: vehicles (75 respondents, 63.6%) including cars and buses (28.8%), trains (21.2%), Shinkansen high speed bullet trains (3.4%), and subways (3.4%); telecommunications equipment other than mobile phones (61 respondents, 53.4%) including wireless LANs (22.9%), landline phones (15.3%), security sensors (12.7%), and equipment using Wi‐Fi (7.6%); and medical equipment (nine respondents, 7.6%) including magnetic resonance imaging (MRI; 2.5%), medical measuring instruments emitting low‐frequency EMFs (1.7%), X‐ray (0.8%), dental equipment (0.8%), and apparatus measuring bone density (0.8%).
Physician‐Diagnosed Chronic Illness (Past and Present)
Results from 116 of the 127 self‐selected EHS subjects and 681 of the 1,306 subjects among the controls, who agreed to provide valid responses for a list of “physician‐diagnosed chronic illnesses,” are shown in Table 10. At the time of answering, none of the controls were undergoing treatment for environmental hypersensitivity (SHS, MSC, and EHS) as depicted in Table 10. However, in 46 (39.66%) of the 116 self‐selected EHS subjects, their symptoms had been diagnosed as EHS, which was further complicated by MCS. A χ 2 test revealed that proportions of participants who claimed to be undergoing treatment for chronic illnesses entitled “Autonomic imbalance” and “Other allergy symptoms” at the time of the survey were highly significant among the self‐selected EHS subjects compared with controls (Table 10).
Table 10.
Comparison of the Prevalence of Chronic Illnesses Between Controls and Self‐Selected EHS Subjects
| Chronic illnesses at present | Chronic illnesses in the past | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls (n = 681) | Self‐selected EHS subjects (n = 116) | χ 2 test | Controls (n = 681) | Self‐selected EHS subjects (n = 116) | χ 2 test | |||
| Chronic illness | n (%) | n (%) | P‐value | n (%) | n (%) | P value | ||
| Diabetes mellitus | 7 (1.03) | 1 (0.86) | 0.868 | n.s. | 11 (1.62) | 2 (1.72) | 0.932 | n.s. |
| High blood pressure | 49 (7.20) | 5 (4.31) | 0.253 | n.s. | 37 (5.43) | 7 (6.03) | 0.793 | n.s. |
| Heart diseases | 10 (1.47) | 2 (1.72) | 0.834 | n.s. | 9 (1.32) | 6 (5.17) | 0.005 | n.s. |
| Autonomic imbalance | 5 (0.73) | 6 (5.17) | 1.52 × 10−4 | ** | 23 (3.38) | 20 (17.24) | 1.00 × 10−9 | *** |
| Migraines | 8 (1.17) | 2 (1.72) | 0.623 | n.s. | 33 (4.85) | 9 (7.76) | 0.194 | n.s. |
| Allergy symptoms a | 84 (12.33) | 16 (13.79) | 0.661 | n.s. | 285 (41.85) | 75 (64.66) | 5.06 × 10−6 | *** |
| Atopic dermatitis | 11 (1.62) | 3 (2.59) | 0.462 | n.s. | 60 (8.81) | 16 (13.79) | 0.091 | n.s. |
| Bronchial asthma | 9 (1.32) | 4 (3.45) | 0.095 | n.s. | 52 (7.64) | 15 (12.93) | 0.057 | n.s. |
| Allergic nasal catarrh | 31 (4.55) | 2 (1.72) | 0.158 | n.s. | 109 (16.01) | 36 (31.03) | 1.05 × 10−4 | ** |
| Allergic conjunctivitis | 12 (1.76) | 2 (1.72) | 0.977 | n.s. | 59 (8.66) | 28 (24.14) | 7.80 × 10−7 | *** |
| Rash | 8 (1.17) | 0 (0.00) | 0.241 | n.s. | 69 (10.13) | 25 (21.55) | 4.24 × 10−4 | *** |
| Hey fever | 44 (6.46) | 4 (3.45) | 0.207 | n.s. | 123 (18.06) | 39 (33.62) | 1.19 × 10−4 | *** |
| Food allergies | 5 (0.73) | 5 (4.31) | 0.001 | n.s. | 46 (6.75) | 24 (20.69) | 9.52 × 10−7 | *** |
| Other allergy symptom b | 0 (0.00) | 2 (1.72) | 6.02 × 10−4 | * | 33 (4.85) | 9 (7.76) | 0.194 | n.s. |
| Sick house syndrome | 0 (0.00) | 4 (3.45) | 1.19 × 10−6 | *** | 8 (1.17) | 23 (19.83) | 7.64 × 10−22 | *** |
| Multiple chemical sensitivity | 0 (0.00) | 46 (39.66) | 2.75 × 10−64 | *** | 7 (1.03) | 19 (16.38) | 7.74 × 10−18 | *** |
| Electromagnetic hypersensitivity | 0 (0.00) | 46 (39.66) | 2.75 × 10−64 | *** | 1 (0.15) | 19 (16.38) | 5.04 × 10−25 | *** |
| Others | 7 (1.03) | 9 (7.76) | 1.77 × 10−6 | *** | 42 (6.17) | 41 (35.34) | 1.90 × 10−21 | *** |
Note: Differences in prevalence of chronic illnesses between self‐selected EHS subjects (n = 116) and controls (n = 681) were analyzed using χ 2 test with Bonferroni correction for multiple tests (40 tests; P < 0.000125). n.s.: not significant.
Allergy symptoms: percentage for “Allergy symptoms” does not represent the sum of percentages from “Atopic dermatitis” to “Other allergy symptoms” because some participants described more than one allergy symptom.
Other allergy symptoms: allergy diseases other than those described above.
P ≤ 0.0005.
P ≤ 0.0001.
P ≤ 0.00001.
It is notable that the proportion of self‐selected EHS subjects who suffered from several allergy symptoms (64.66%) in the past was significantly higher (P < 0.00001) than corresponding proportion of controls (41.85%), as seen in Table 10. The proportion of patients whose symptoms were diagnosed as autonomic imbalance, allergy nasal catarrh, allergy conjunctivitis, rash, hay fever, food allergies, and other allergy symptoms in the past was also significantly higher in self‐selected EHS subjects than in controls. However, there was no significant difference in the proportion of patients diagnosed with diabetes mellitus, high blood pressure, heart disease, migraine, atopic dermatitis, and bronchial asthma between the two groups (Table 10).
Sequence of EHS Development Estimated by Self‐Selected EHS Subjects
A summary of the estimated progression of symptoms by self‐selected EHS subjects is shown in Figure 3. Our study revealed that EHS was present in only 18.52% of self‐selected EHS subjects, with a majority (81.52%) of self‐selected EHS subjects having presented with both MCS and EHS symptoms. In half of such self‐selected EHS subjects SHS or MCS symptoms occurred before EHS symptoms, whereas a relatively small proportion of self‐selected EHS subjects (14.81%) presented with EHS symptoms were subsequently followed by MCS symptoms.
Figure 3.
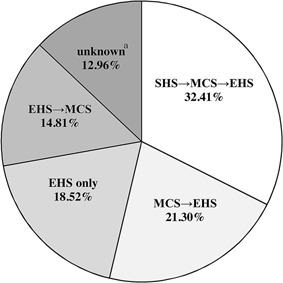
Speculated sequence of onset of SHS, MCS, and EHS by Japanese self‐selected EHS subjects. SHS: sick house syndrome; MCS: multiple chemical sensitivity; EHS: electromagnetic hypersensitivity. aBoth MCS and EHS were associated; however, it is unknown which occurred first.
Responses to Open Question of How the Individual Has Been Suffering From EHS Syndrome
Most self‐selected EHS subjects described their symptoms in detail. Furthermore, many of them expressed facing financial difficulties following resignations due to sickness, and their concern regarding a lack of understanding about EHS by general physicians and the public. Firstly, self‐selected EHS subjects stated there were few physicians who had profound knowledge of both MCS and EHS. Secondly, they claimed that they were treated for diseases with various names based on their symptoms but to no avail; moreover, they were even forced to change hospitals several times. Thirdly, their symptoms were diagnosed as mental disorders, resulting in strained family relationships.
DISCUSSION
The reliability of the Japanese EHS questionnaire was confirmed by high to moderate ICC values (0.623–0.863) obtained for all 5‐point scale questions and total scores of each subscale, with the exception of four items (component 4, q64, q65, and q71) in the test–retest analysis (Table 4). In addition, high Cronbach's α coefficients (0.853–0.953) from all subscales, with the exception of “Reaction to EMFs (0.528),” indicated good internal consistency for each question (Table 5).
The validity of the Japanese EHS Questionnaire was confirmed by observing significant differences (P < 0.0005) in all scorers, with the exception of two items (q69 and q70) between self‐selected EHS subjects, and age‐ and sex‐matched controls, using simple logistic regression analysis (Table 7). ORs for q68 (90.96) and q67 (9.66) were extremely high, indicating the high discriminatory power of these two questions in the screening of EHS individuals from the general public. Similarly, the OR of the total symptom score, which was used as the main criterion, was also found to be significantly high (OR; 1.05, P = 6.78 × 10−4). In addition, significantly high ORs were observed for all nine EMF‐producing objects, especially electrical appliances (OR: 4.29), telecommunication masts (OR: 3.87), and fluorescent lighting (OR: 3.60), and for the total score for EMF‐producing objects in this study (OR: 2.61–4.29). Thus, these three items may be considered to be the primary EMF‐producing objects believed by self‐selected EHS subjects in Japan to be the cause of symptom onset; further studies exploring this are warranted.
Results of Mann–Whitney U‐ and χ 2 tests also indicated that, compared to controls, self‐selected EHS subjects reported a greater severity of symptoms, poorer levels of general health and well‐being, and a belief that their symptoms were due to exposure to objects that emit EMFs (Table 6). These results were consistent with those reported by Eltiti et al. [2007], who used the same tests (Mann–Whitney U‐ and χ 2 tests) in their UK‐based study. The extreme statistical significances shown in Table 6 arose from the selection process: the EHS subjects already shared these beliefs, while the controls did not even understand the questions.
Characteristics of Symptoms in Japanese Self‐Selected EHS Subjects
Principal component analysis of 57 symptoms in controls (n = 1,306) revealed the following eight main symptom subscales: nervous system, skin, head, auditory and vestibular, musculoskeletal system, allergy, sensory system, and heart/chest (Tables 2 and 3). Findings of the present study are similar to those reported by Kato and Johansson [2012], who used a different questionnaire and observed that major subjective symptoms developed by Japanese self‐selected EHS subjects included fatigue/tiredness, headache, and difficulty in concentrating, remembering, and thinking. In the study by Eltiti et al. [2007], an eight‐factor principal components analysis of symptoms in the English general population resulted in eight symptom subscales: neurovegetative, skin, auditory, headache, cardiorespiratory, cold‐related, locomotor, and allergy‐related symptoms; these findings are similar to those observed in the present study. Nordin et al. [2013] conducted an exploratory principal component analysis and reported that symptoms of EHS individuals in Sweden could be divided into five significant groups: airway symptoms, skin and eye symptoms, cardiac, dizziness and nausea, and cognitive and affective symptoms. As described in WHO fact sheet 296 [WHO, 2005], EHS is characterized by a variety of non‐specific symptoms, which are attributed to EMF exposure by afflicted individuals. Symptoms most commonly experienced included dermatological symptoms (redness, tingling, and burning sensation) as well as neurasthenia and vegetative symptoms (fatigue, tiredness, concentration difficulties, dizziness, nausea, heart palpitations, and digestive disturbances). Hence, taking these factors into consideration, we assumed that the main symptoms of self‐selected EHS subjects in Japan could be evaluated using eight symptom categories described in previous studies conducted in Europe.
Relationship Between EHS and Other Chronic Illnesses (IV–3 Additional Questions)
Close relationships between allergy symptoms and MCS/EHS are often observed in clinical practice [Rea et al., 1991]. However, so far, few reports exist describing the relationship between MCS/EHS and chronic illness, including allergy symptoms. Findings of the present study (Table 9) are new and are suggestive of a close relationship between MCS/EHS and allergy symptoms. Nevertheless, further studies are required to investigate underlying mechanisms responsible for this close relationship. The Japanese EHS questionnaire can be used as an effective tool to analyze the relationship between MCS/EHS and allergy symptoms, and to determine effective, future treatment modalities for MCS/EHS patients.
EMF Sources Believed by Japanese Self‐Selected EHS Subjects as Cause of Their Symptoms
Similar to the study conducted in the UK, we found nine types of EMF sources (computers, electrical appliances, fluorescent lighting, microwave ovens, mobile phones, power lines, radio/television transmitters, telecommunication masts, and televisions), which Japanese self‐selected EHS subjects considered as the cause of their symptoms in the present study. Interestingly, the following EMF sources were selected by the majority of Japanese self‐selected EHS subjects: electric appliances, followed by mobile phones, computers, and telecommunication masts (Fig. 2). Furthermore, besides the aforementioned nine sources in the questionnaire, several new types of EMF sources, including those used for transportation (e.g., cars, buses, Shinkansen), communication devices other than mobile phones (e.g., wireless LAN, Wi‐Fi, security equipment), and medical devices (MRI, X‐rays, dental therapeutic instrument) were also nominated by self‐selected EHS subjects. Hence, we believe that these new EMF sources should be added to the Japanese version of EHS questionnaires in future surveys.
Work Style and Relationship With MCS
Consistent with results reported by Kato and Johansson [2012] in Japan and Hillert et al. [2002] in Sweden, the number of full‐time workers was significantly lower, whereas the number of unemployed persons and part‐time workers was significantly higher among self‐selected EHS subjects when compared with controls in the present study (Table 1).
EHS resembles MCS, another disorder associated with low‐level environmental exposure to chemicals, because both EHS and MCS are characterized by a range of non‐specific symptoms that lack an apparent toxicological and physiological basis and/or independent verification [WHO, 2005]. Rea et al. [1991] reported that more than 80% of EHS patients presented with MCS. The results of the present study (Fig. 3) are consistent with those of Rea et al. [1991].
Proposal of Preliminary Criteria for Screening Japanese EHS Individuals
Based on the results of multiple logistic regression and ROC analyses in the present study, we propose the following preliminary criteria for the screening of Japanese EHS individuals: (1) the total symptom score should be greater than, or equal to, 47 points (75th percentile of controls, Fig 1a); (2) the score for q67 (“Are you sensitive to electromagnetic fields?”) should be greater than, or equal to, 1; and (3) individuals should be able to describe the EMF source and the kind of symptoms developed in detail in response to q68.
Preliminary criteria suggested by the present study for the screening of EHS individuals in the general population are similar to those proposed by Eltiti et al. [2007]. However, the 75th percentile for controls was 26 points in the UK, and 47 points in Japan. Eighty‐two (64.6%) of the 127 self‐selected EHS subjects met with set screening criteria for EHS individuals. Furthermore, 60 subjects from the controls (1,306 respondents out of 2,000) also met with these preliminary screening criteria, suggesting that 3.0–4.6% of the general public in Japan may be EHS individuals, even though none are currently diagnosed with SHS or MCS /EHS. In addition, significant differences were not observed in all scores between 60 subjects from the control group and self‐selected EHS subjects (data not shown).
Based on the fact that only 1% of the population of Japan is aware of EHS, determined by a preliminary survey [Hojo and Tokiya, 2012], some of these 60 subjects may have some knowledge of EHS; however, the majority of them most probably have no knowledge of this condition. Thus, it is very important that these unsuspecting individuals, who may have developed EHS symptoms, visit a qualified medical specialist. However, it is worthy to note that in response to the open question on the last page of the questionnaire (“Please provide a detailed description of how you have been suffering from EHS syndromes,” Supplementary Materials), many self‐selected EHS subjects described how only a few medical doctors had professional knowledge of EHS/MCS, and most were generally incompetent with regards to the treatment of this condition in Japan. Consequently, Japanese self‐selected EHS subjects have suffered greatly in their day‐to‐day life [Ito et al., 2012], making it imperative to have specialists who are familiar with EHS, as well as MCS and SHS.
Future Directions
As stated in the COST fact sheet [COST, 2011], individuals who think they are sensitive to EMF actually feel symptoms; therefore, it is important to endeavor to improve, and to understand the mechanisms and causal relations associated with their condition. The limitation of this study is that this is one of the descriptive survey findings for perceptions of causation in a self‐selected group who believes EMF is a cause of their symptoms. Thus, systematic approaches, including the provision of information, support for patients with symptoms of earlier stages, and treatment for persons with prolonged and severe symptoms, are required. We believe that it is time to develop and initiate a systematic approach, wherein the provision of information about EHS to general physicians and the public is mandatory in Japan. The Japanese EHS questionnaire can be used as an effective tool for providing such information.
The COST fact sheet also states that “the choice of treatment should be based on a broad evaluation of the patient's symptoms and situation (including medical, psychosocial, and environmental aspects) and taking the patient's motivation for different interventions into account. Cognitive therapy has been reported to improve well‐being and the ability to cope with persisting symptoms in some patients.” We agree that multi‐faceted therapies are useful for the treatment of EHS individuals with severe and long‐lasting symptoms, and believe the Japanese EHS Questionnaire will be useful in evaluating such therapies.
CONCLUSION
The Japanese EHS questionnaire is highly reliable and valid, and can be used to screen EHS individuals from the general population in Japan. This questionnaire can be used to elucidate the actual status of EHS individuals in Japan, to evaluate the effects of therapies or lifestyle changes in people presenting with EHS‐related symptoms, and as a cross‐comparison of groups studied by different investigators.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's website.
Table S1. Comparison of question style between original (left) and Japanese (right) EHS questionnaires in II‐I symptoms.
ACKNOWLEDGEMENTS
We would like to thank all participants who volunteered for the present study. Special appreciation is extended to the late Dr. K. Akiyama (former director of the National Hospital Organization, Sagamihara National Hospital), Emeritus Prof. S. Ishikawa (Kitasato University), Prof. K. Sakabe (Tokai University), Profs. T. Yamaguchi and T. Koseki (Tohoku University), Associate Profs. H. Miyata and T. Hondo (Tohoku University), Associate Prof. A. Mizukoshi (Kindai University), Drs. K. Igarashi, H. Inoue, and T. Yazaki (Miyagi Medical and Dental Practitioners for the Improvement of Medical Care), and Ms. E. Itahashi and Mr. T. Furomoto (self‐help groups for EHS).
Conflict of interest: None.
REFERENCES
- Abdel‐Rassoul G, El‐Fateh OA, Salem AM, Michael A, Farahat F, El‐Batanouny M, Salem E. 2007. Neurobehavioral effects among inhabitants around mobile phone base stations. Neuro Toxicology 28:434–440. [DOI] [PubMed] [Google Scholar]
- Baliatsas C, Kamp I, Van Bolte J, Schipper M, Yzermans J, Lebret E. 2012. Non‐specific physical symptoms and electromagnetic field exposure in the general population: Can we get more specific? A systematic review. Environ Internatl 41:15–28. [DOI] [PubMed] [Google Scholar]
- Berg‐Beckhoff G, Blettner M, Kowall B, Breckenlamp J, Schlehofer B, Bronkessel C, Reis U, Potthoff P, Schüz J. 2009. Mobile phone base stations and adverse health effects: Phase 2 of a cross‐sectional study with measured radio frequency electromagnetic fields. Occup Environ Med 66:124–130. [DOI] [PubMed] [Google Scholar]
- Blettner M, Schlehofer B, Breckenkamp J, Kowall B, Schmiedel S, Reis U, Potthoff P, Schüz J, Berg‐Beckhoff G. 2009. Mobile phone base stations and adverse health effects: Phase 1 of a population‐based, cross‐sectional study in Germany. Occup Environ Med 66:118–123. [DOI] [PubMed] [Google Scholar]
- COST: European Cooperation in Science and Technology: Factsheet. 2011. Idiopathic environmental intolerance attributed to electromagnetic fields (IEI‐EMF) or ‘electromagnetic hypersensitivity’. Available from: http://www.mobileresearch.ethz.ch/fileadmin/redaktion/public/downloads/projekte/IEI-factsheet301111.pdf [last accessed 18 January 2013].
- Cronbach LJ. 1951. Coefficient alpha and the internal structure of tests. Psychometrika 16:297–334. [Google Scholar]
- Eltiti S, Wallace D, Zougkou K, Russo R, Joseph S, Rasor P, Fox E. 2007. Development and evaluation of the electromagnetic hypersensitivity questionnaire. Bioelectromagenetics 28:137–151. [DOI] [PubMed] [Google Scholar]
- Furubayashi T, Ushiyama A, Terao Y, Mizuno Y, Shirasawa K, Pongpaibool P, Shirasawa K, Pongpaibool P, Simba AY, Wake K, Nishikawa M, Yamashita HK, Masuda H, Hirota S, Takahashi M, Okano T, Inomata‐Terada S, Sekijima S, Maruyama A, Watanabe S, Taki M, Ohkubo C, Ugawa Y. 2009. Effects of short‐term W‐CDMA mobile phone base station exposure on women with or without mobile phone related symptoms. Bioelectromagnetics 30:100–113. [DOI] [PubMed] [Google Scholar]
- Hillert L, Berglin N, Arnetz BB, Bellander T. 2002. Prevalence of self‐selected hypersensitivity to electric or magnetic fields in a population‐based questionnaire survey. Scand J Work Environ Health 28:33–41. [DOI] [PubMed] [Google Scholar]
- Hojo S. 2002. A questionnaire survey of multiple chemical sensitivity in Japan by using QEESI. Neuro‐Ophthalmol 19:169–175 (In Japanese). [Google Scholar]
- Hojo S, Kumano H, Yoshino H, Kakuta K, Ishikawa S. 2003. Application of Quick Environment Exposure Sensitivity Inventory (QEESI©) for Japanese population: Study of reliability and validity of the questionnaire. Toxicol Ind Health 19:41–49. [DOI] [PubMed] [Google Scholar]
- Hojo S, Yoshino H, Kumano H, Kakuta K, Miyata M, Sakabe K, Matsui T, Ikeda K, Nozaki A, Ishikawa A. 2004. A case study on use of QEESI© as a questionnaire for screening MCS and/or sick building syndrome patients. Jpn J Clin Ecol 13:110–119 (In Japanese). [Google Scholar]
- Hojo S, Yoshino H, Kumano H, Kakuta K, Miyata M, Sakabe K, Matsui T, Ikeda K, Nozaki A, Ishikawa S. 2005. Use of QEESI© questionnaire for a screening study in Japan. Toxicol Ind Health 21:113–124. [Google Scholar]
- Hojo S, Ishikawa S, Kumano H, Miyata M, Matsui T, Sakabe K. 2007. Subjective and objective characteristics of patients with multiple chemical sensitivity in Japan. Jpn J Clin Ecol 16:104–116 (In Japanese). [Google Scholar]
- Hojo S, Ishikawa S, Kumano H, Miyata M, Sakabe K. 2008a. Clinical characteristics of physician‐diagnosed patients with multiple chemical sensitivity in Japan. Int J Hyg Environ Health 211:682–689. [DOI] [PubMed] [Google Scholar]
- Hojo S, Kumano H, Ishikawa S, Miyata M, Matsui T, Sakabe K. 2008b. Analysis of cut off‐point and ongoing exposure to chemicals on the onset for Japanese multiple chemical sensitivity patients using QEESI©. Jpn J Clin Ecol 17:118–132 (In Japanese). [Google Scholar]
- Hojo S, Sakabe K, Ishikaw S, Miyata M, Kumano K. 2009. Evaluation of subjective symptoms of Japanese patients with multiple chemical sensitivity using QEESI©. Environ Health Prev Med 14:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo S, Tokiya M. 2012. An overview of the current scientific knowledge of electromagnetic hypersensitivity and issues in the future. Jpn J Clin Ecol 21:131–151 (In Japanese). [Google Scholar]
- ICNIRP: International commission on non‐ionizing radiation protection 2009. ICNIRP statement on the ‘Guidelines for limiting exposure to time‐varying electric, magnetic, and electromagnetic fields (up to 300 GHz)’. Health Physics 97:257–258. [DOI] [PubMed] [Google Scholar]
- Ito Y, Suzuki K, Tsujiuchi T. 2012. What is the boundary between illness and disability? Medical anthropological study for electromagnetic hypersensitivity. Waseda J Human Sci 25:205–220. [Google Scholar]
- Kato Y, Johansson O. 2012. Reported functional impairments of electrohypersensitive Japanese: A questionnaire survey. Pathophysiol 19:95–100. [DOI] [PubMed] [Google Scholar]
- Kawada T, Suzuki S, Kubota F, Ohnishi N, Satoh K. 1999. Content and cross validity of the Todai health index depression scale in relation to the center for epidemiologic studies depression scale and the Zung self‐rating depression scale. J Occup Health 41:154–159. [Google Scholar]
- Landgrebe M, Barta W, Rosengarth K, Frick U, Hauser S, Langguth B, Rutschmann R, Greenlee MW, Hajak G, Eichhammer P. 2008. Neuronal correlates of symptom formation in functional somatic syndromes: A f‐MRI study. NeuroImage 41:1336–1344. [DOI] [PubMed] [Google Scholar]
- Leitgeb N, Schröttner J. 2003. Electrosensibility and electromagnetic hypersensitivity. Bioelectromagnetics 24:387–394. [DOI] [PubMed] [Google Scholar]
- Levallois P, Neutra R, Lee G, Hristova L. 2002. Study of self‐reported hypersensitivity to electromagnetic fields in California. Environ Health Perspect 110:619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meg Tseng MC, Lin YP, Cheng TJ. 2001. Prevalence and psychiatric comorbidity of self‐reported electromagnetic field sensitivity in Taiwan: a population‐based study. J Formosan Med Assoc 110:634–641. [DOI] [PubMed] [Google Scholar]
- Miller CS, Prihoda TJ. 1999. The Environmental Exposure and Sensitivity Inventory (EESI): a standardized approach for measuring chemical intolerances for research and clinical applications. Toxicol Indust Health 15:370–385. [DOI] [PubMed] [Google Scholar]
- Mohler E, Frei P, Braun‐Fahrländer C, Fröhlich J, Neubauer G, Röösli M, Team Q. 2010. Effects of everyday radiofrequency electromagnetic‐field exposure on sleep quality: A cross‐sectional study. Radiat Res 174:347–356. [DOI] [PubMed] [Google Scholar]
- Nordin S, Palmquist E, Claeson AS, Stenberg B. 2013. The environmental hypersensitivity symptom inventory: Metric properties and normative data from a population‐based study. Arch Public Health 71:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece AW, Georgiou AG, Dunn EJ, Farrow SC. 2007. Health response of two communities to military antennae in Cyprus. Occup Environ Med 64:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea WJ, Pan Y, Fenyves EJ, Sijisawa I, Samadi N, Ross GH. 1991. Electromagnetic field sensitivity. J Bioelectricity 10:241–256. [Google Scholar]
- Röösli M, Frei P, Mohler E, Hug K. 2010. Systematic review on the health effects of exposure to radiofrequency electromagnetic fields from mobile phone base stations. Bull World Health Organ 88:887G–896G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GJ, Nieto‐Hernandez R, Wessely S. 2010. Idiopathic environmental intolerance attributed to electromagnetic fields (formerly ‘electromagnetic hypersensitivity’): An undated systematic review of provocation studied. Bioelectromagnetics 31:1–11. [DOI] [PubMed] [Google Scholar]
- Rubin GJ, Hillert L, Nieto‐Hernandez R, van Rongen E, Oftedal G. 2011. Do people with idiopathic environmental intolerance attributed to electromagnetic fields display physiological effects when exposed to electromagnetic fields? A systematic review of provocation studies. Bioelectromagnetics 32:593–609. [DOI] [PubMed] [Google Scholar]
- Schreier N, Huss A, Löösli M. 2006. The prevalence of symptoms attributed to electromagnetic field exposure: A cross‐sectional representative survey in Switzerland. Soz Präventiv Med 51:202–209. [DOI] [PubMed] [Google Scholar]
- Schröttner J, Leitgeb N. 2008. Sensitivity to electricity – Temporal changes in Austria. BMC Public Health 8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Roberts RE, Suzuki S. 1994. Depressive symptoms among Japanese and American adolescents. Psychiatry Res 53:259–274. [DOI] [PubMed] [Google Scholar]
- WHO: World Health Organization: Factsheets 296. 2005. Electromagnetic fields and public health; Electromagnetic hypersensitivity. Available from: http://www.who.int/peh-emf/publications/facts/fs296/en/index [last accessed 30 November 2015].
- Witthöft M, Rubin GJ. 2013. Are media warnings about the adverse health effects of modern life self‐fulfilling? An experimental study on idiopathic environmental intolerance attributed to electromagnetic fields (IEI‐EMF). J Psychoses Res 74:206–212. [DOI] [PubMed] [Google Scholar]
- WMA: World Medical Association. Declaration of Helsinki. 2013. Available from: http://neurocirurgiabr.com/2013-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [last accessed 30 November 2013].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's website.
Table S1. Comparison of question style between original (left) and Japanese (right) EHS questionnaires in II‐I symptoms.


