Abstract
Background
Patients with COPD present a major recruitment of the inspiratory muscles, predisposing to chest incoordination, increasing the degree of dyspnea and impairing their exercise capacity. Stretching techniques could decrease the respiratory muscle activity and improve their contractile capacity; however, the systemic effects of stretching remain unknown.
Objective
The aim of this study was to evaluate the effects of aerobic training combined with respiratory muscle stretching on functional exercise capacity and thoracoabdominal kinematics in patients with COPD.
Design
This study was a randomized and controlled trial.
Participants
A total of 30 patients were allocated to a treatment group (TG) or a control group (CG; n=15, each group).
Intervention
The TG was engaged in respiratory muscle stretching and the CG in upper and lower limb muscle stretching. Both groups performed 24 sessions (twice a week, 12 weeks) of aerobic training.
Evaluations
Functional exercise capacity (6-minute walk test), thoracoabdominal kinematics (optoelectronic plethysmography), and respiratory muscle activity (surface electromyography) were evaluated during exercise. Analysis of covariance was used to compare the groups at a significance level of 5%.
Results
After the intervention, the TG showed improved abdominal (ABD) contribution, compartmental volume, mobility, and functional exercise capacity with decreased dyspnea when compared with the CG (P<0.01). The TG also showed a decreased respiratory muscle effort required to obtain the same pulmonary volume compared to the CG (P<0.001).
Conclusion
Our results suggest that aerobic training combined with respiratory muscle stretching increases the functional exercise capacity with decreased dyspnea in patients with COPD. These effects are associated with an increased efficacy of the respiratory muscles and participation of the ABD compartment.
Keywords: COPD, respiratory muscles, muscular stretching, respiratory mechanics, dyspnea
Introduction
COPD is characterized by a progressive and persistent airflow limitation and decreased parenchymal elasticity.1 Consequently, the respiratory muscles remain contracted for prolonged periods in an attempt to meet the increased ventilatory flow demand, increasing the load on the respiratory muscles.2,3 The association between both conditions (hyperinflation and increased respiratory demand) reduces the contractile range of the sarcomere of the respiratory muscles, triggering mechanoreceptors to stimulate the respiratory centers and further increase ventilation, resulting in even more severe dyspnea. This vicious cycle continues because the increase in dyspnea further stimulates increases in the ventilatory demand.3,4
Dyspnea and the shortening of respiratory muscles hamper the performance of activities that require more effort, which results in physical deconditioning in patients with COPD.5 Aerobic training can be used as an evidence-based intervention for patients with chronic respiratory diseases, which improves their physical capacity and reduces dyspnea.6 Despite these improvements, there is no evidence that aerobic training improves thoracoabdominal kinematics.7 Current evidence suggests that muscle stretching modifies the properties of tissues, increasing sarcomere size and muscle viscoelasticity.8 There is also evidence that respiratory muscle stretching increases the capacity for chest wall (CW) expansion, suggesting an improvement in ventilation in patients with COPD.2,9 However, these findings were observed in a nonrandomized study, and CW expansion was evaluated using cirtometry, a non-validated methodology.
Currently, there is no evidence to support the recommendation of respiratory muscle stretching in clinical practice for treating patients with COPD, mainly due to the methodological limitations of the previous studies that have been published in this field.10,11 Considering that aerobic training is the gold standard for the non-pharmacological treatment of COPD,6 we hypothesized that respiratory muscle stretching might potentiate the benefits of aerobic training by improving thoracoabdominal mobility, as well as lung volumes and capacities, which can be precisely quantified using validated techniques, such as optoelectronic plethysmography.12
The primary objective of the current study was to evaluate the effects of the addition of respiratory muscle stretching to an exercise program on the functional capacity, degree of dyspnea, and thoracoabdominal kinematics in patients with COPD. The secondary objective was to evaluate the respiratory muscular activity and abdominal (ABD) mobility during exercise in these patients.
Patients and methods
Study design
This study was a randomized and controlled trial with blinded assessments. After the initial evaluation, participants were randomly allocated to a treatment group (TG) or a control group (CG; n=15, each group). This study was performed at Clinics Hospital, School of Medicine, University of Sao Paulo, Sao Paulo, Brazil.
Randomization
Randomization was performed using Microsoft Excel. The procedure was designed by one investigator who was not involved in the other aspects of the protocol. The group allocations were performed using numbered, sealed, opaque envelopes that had been previously prepared for all participants.13
Subjects
Patients diagnosed with moderate-to-severe COPD (forced expiratory volume in 1 second [FEV1] ≥30% and <80% of the predicted value and FEV1/forced vital capacity [FVC] ratio <0.7) were recruited from a university hospital. COPD diagnosis was based on the Global Initiative for Chronic Obstructive Lung Disease guidelines.1 The inclusion criteria were the following: age >40 years, body mass index between 18 kg/m2 and <30 kg/m2, clinical stability (no changes in medication or symptoms during the last month), no supplemental O2 dependence, no history of cardiac or thoracic surgery or any pneumopathies, the absence of physical disabilities or functional inabilities, and no participation in pulmonary rehabilitation program within the last 3 months. The exclusion criteria were any occurrences of respiratory exacerbation (worsening of symptoms or increases in medication) prior to the randomization.
The protocol was approved by Research Ethics Committee of the Medical School of São Paulo University and was registered on Clinical Trials (NCT02036762). All patients signed a consent form agreeing to participate in this study.
Experimental design
The patients were evaluated before and after the intervention protocol by researchers blinded to the patients’ group allocation. The evaluation included an analysis of thoracoabdominal kinematics and respiratory muscle activity during exercise on a cycle ergometer. In addition, lung function (sample characterization), functional exercise capacity, and dyspnea were evaluated.
After the initial evaluation, the patients were allocated to a CG (n=15) or a TG (n=15). Both groups participated in aerobic training on a treadmill (30 minutes per session). The TG also performed a respiratory muscle-stretching program (30 minutes per session). The CG performed an upper and a lower limb muscle-stretching program (sham treatment: 30 minutes per session). The protocol included 24 sessions (twice a week for 12 weeks). Two physiotherapists supervised all interventions in both groups, according to the study protocols but did not participate in the initial or final evaluations.
Evaluations, performed by assessors blinded to the intervention received by the groups, were conducted over 1 day, 1 week before, and 1 week after the interventions. Patients were first assessed via spirometry. After 30 minutes of rest, they underwent the thoracoabdominal kinematics test and respiratory muscle activity analyses at rest and during exercise. After 1 hour of rest, they performed a 6-minute walk test (6MWT).
Interventions
Respiratory muscle stretching in the TG
Respiratory muscle stretching was performed every session before aerobic training (30 minutes). The program was based on hold–relax and passive stretching techniques9,14 and involved the scalene, sternocleidomastoid, trapezius, pectoralis major and minor, intercostal, serratus anterior, and rectus abdominis muscles (Table 1). The hold–relax stretching technique involves performing a passive stretch up to the maximum range of motion interspersed with 3 seconds of isometric contraction with three repetitions, which is called a “cycle”. Three sets of three cycles were interspersed with 1 minute of rest.9,14 Passive stretching was sustained for 1 minute, with 1 minute of rest between stretches and repeated three times, and held for the muscles (pectoralis minor, intercostal, anterior serratus, and ABDs) that showed limited performance when performing the hold–relax technique.15,16
Table 1.
Respiratory muscle stretching (treatment group)
| Muscles | Positioning | Start position | Final position |
|---|---|---|---|
| aMajor pectoralis11 | Supine decubitus | 90° shoulder abduction with external rotation, 90° elbow flexion, and sternal stabilization | Maximum horizontal extension of the shoulder |
| Minor pectoralis9 | Supine decubitus | Shoulder elevation with upper ribs stabilization | Maximum depression of third to fifth ribs |
| aUpper trapezius2 | Supine decubitus | Neck contralateral rotation and lateral inclination with shoulder stabilization | Maximum neck flexion |
| aScalene11 | Supine decubitus | Neck lateral inclination with shoulder stabilization | Maximum neck lateral inclination |
| aSternocleidomastoid11 | Supine decubitus | Neck ipsilateral rotation and extension with manubrium stabilization | Maximum manubriumdepression |
| Intercostals and anterior serratus11 | Lateral decubitus | Maximum shoulder abduction with lower ribs stabilization | Maximum lower ribs depression |
| Abdominal49 | Prone decubitus | Both hands on the stretcher with elbow extension, extension of the trunk | Maximum extension of the trunk |
Note:
Exercises using the hold–relax technique.
Upper and lower limb muscle stretching in the CG
This sham intervention was performed before aerobic training (30 minutes). The exercises were performed with active stretching in the flexors and extensors of the wrists and ankles. Every exercise was sustained for 1 minute interspersed with 1 minute of rest (Table 2).15,16
Table 2.
Upper and lower limb muscle stretching (control group)
| Muscles | Positioning | Start position | Final position |
|---|---|---|---|
| Wrist flexors50 | Standing | 90° shoulder flexion and elbow supination. The contralateral hand supporting the palmar surface of the limb to be stretched | Maximum wrist extension |
| Wrist extensors50 | Standing | 90° shoulder flexion, elbow pronation. The contralateral hand supporting the dorsal surface of the limb to be stretched | Maximum wrist flexion |
| Ankle flexors50 | Sitting | The limb to be treated crossed over the contralateral limb. One hand stabilizes the ankle and the other positioned on the dorsal surface of the foot | Maximum ankle extension |
| Ankle extensors50 | Standing | Both hands against the wall and the lower limb to be treated in knee and hip extension with flexion of the ankle | Maximum ankle flexion |
Aerobic exercises
Both groups performed aerobic training on a treadmill for 30 minutes beginning at 60% and reaching up to 85% of the average speed achieved during the 6MWT.17,18 The intensity was gradually increased and was associated with a perception effort between 4 and 7 points on the modified Borg scale.19,20
Outcome assessments
Functional exercise capacity
Functional exercise capacity was evaluated using the 6MWT performed on a 30 m long aisle according to the American Thoracic Society standardization parameters.21 Heart and respiratory rates, oxygen saturation, and dyspnea were evaluated pre- and post-testing. The 25 m difference was considered as the minimum clinically significant difference.22
Dyspnea
Dyspnea was assessed before and after each 6MWT (pre-and post-intervention) using a modified Borg scale. Each score represents the patient’s perception of the intensity of dyspnea, ranging from 0 (no dyspnea) to 10 (extremely intense dyspnea).19
Thoracoabdominal kinematics
Compartmental and total CW volumes were assessed using optoelectronic plethysmography (OEP System; BTS Bioengineering, Milan, Italy), a noninvasive system that evaluates the thoracoabdominal kinematics using 89 reflective markers at sampling rate of 100 Hz. The markers were detected by eight infrared cameras, and the CW was reconstructed using a three-compartmental model (upper ribcage [URC], lower ribcage [LRC], and abdomen).12,23
The compartmental and total CW volumes were recorded during exercise at 25% of the predicted maximal exercise level24 on a cycle ergometer at 60 rpm17 (Ergometrics 800S; SensorMedics, Yorba Linda, CA, USA). The exercise was performed with the arms kept fixed in lateral supports to prevent them from covering the reflective markers as described earlier.25 Total duration of the assessment during exercise was ~8 minutes and 30 seconds (3′30″ without a workload to allow for adaptation, 3′ with a progressive workload for recording data, and 2′ for recovery).26
For the data analysis, six consecutive and homogeneous cycles of CW volume were evaluated, which represented six breaths, and measurements were performed without a pneumotachograph to avoid changes in breathing patterns.27 The respiratory rate was calculated based on the number of respiratory cycles evaluated during 30 seconds, with the system estimating the volume in each compartment according to the three-dimensional coordinates of the markers as described earlier.26,27 OEP System measures the volumes on the CW that represent the sum of the three-compartment volume: URC, LRC, and ABD; the OEP System assessed both the expiratory flow (EF) and minute volume (VE).27,28 Both EF and VE seem to better represent ventilatory limitations in patients with COPD.29 The volume in each compartment was calculated based on CW displacement according to a methodology well established in the literature. The total volume of CW represents the sum of the three other compartments (URC + LRC + ABD), and it represents the tidal volume (VT = CW).30 The ribcage (RC)/ABD ratio between the total RC (RC = URC + LRC) and ABD volume was also calculated and the reduction in the RC/ABD ratio reflects an increase in ABD mobility.31,32
Respiratory muscle activity
The activity of the respiratory muscles (sternocleidomastoid, upper intercostals, diaphragm, and rectus abdominis) was assessed via surface electromyography (BTS OEP SYSTEM®, BTS Bioengineering) simultaneous to the thoracoabdominal kinematics analysis.33 Electrodes were placed according to the European Concerted Action Guidelines.34 The electrode on the sternocleidomastoid was placed 5 cm below the right mastoid process.35 For the upper intercostal muscles, an electrode was positioned in the right second upper intercostal space. For the diaphragm, an electrode was positioned between the left seventh and eighth left intercostal space. For the rectus abdominis, an electrode was positioned 2 cm to the left of the umbilical scar.33,36 The inspiratory muscle activity per CW volume was quantified using the total respiratory muscle activity divided by the VT (EMG/L, expressed in 10−3 mV/L), and the electromyographic data are expressed as the root mean square.33 Lower EMG/VT values reflect less respiratory muscle activity per liter of breathed air.33
Lung function
Spirometry was performed following the reproducibility and acceptability criteria adopted by the American Thoracic Society and European Respiratory Society.6 The variables measured were: FEV1 and ratio of FEV1/FVC. The FEV1 was analyzed as an absolute value and as a percentage of the predicted values for the Brazilian population (for sample characterization).28
Statistical analysis
Sample size calculation
The sample size was calculated based on the 6MWT, considering a difference of 26±23 m between groups after intervention31 and a power of 80% and an alpha of 5%. A sample size of 14 patients per group was indicated. The sample size required for assessing the thoracoabdominal kinematics was also calculated considering an average of the differences of 10%±8% in the RC/ABD ratio in the TG,31,32 with a power of 80% and alpha of 5%. A sample size of 12 patients per group was indicated.
Data analysis
The normality of the data was tested using the Shapiro–Wilks test. The data are presented as mean ± standard deviation. The treatment effect was assessed using the analysis of covariance with the pre-intervention scores as covariate. The significance level was set at 5%.
Results
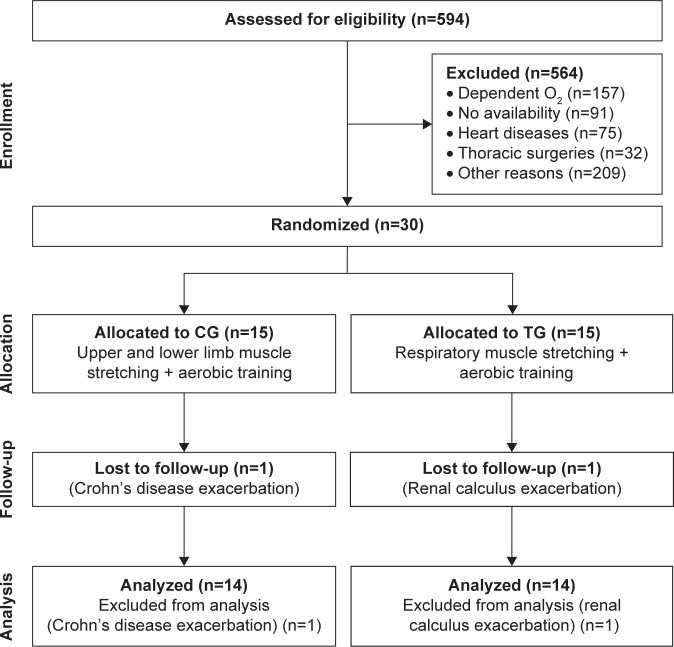
A total of 30 patients with COPD were included in the study. Of them, 28 patients (14 TG/14 CG) completed the intervention and were assessed for the primary outcome (functional exercise capacity) and thoracoabdominal kinematics. In the TG, one patient dropped out due to a renal crisis, and in the CG, one patient dropped out due to an acute exacerbation of Crohn’s disease (Figure 1). At baseline, there were no differences between the groups in anthropometric, lung function, and functional capacity characteristics (Table 3).
Figure 1.
Study flow diagram.
Abbreviations: CG, control group; TG, treatment group.
Table 3.
Baseline characteristics of the study patients
| Variables | CG | TG |
|---|---|---|
| Anthropometric data | ||
| Gender (M/F) | 7/7 | 8/6 |
| Age (years) | 64±5.6 | 61±5.4 |
| Height (m) | 1.65±0.07 | 1.65±0.08 |
| BMI (kg/m2) | 25.9±2.9 | 25.7±3.8 |
| Pulmonary function | ||
| FEV1 (% pred) | 42.6±9.5 | 45.9±15.5 |
| FEV1/FVC ratio | 0.49±0.09 | 0.56±0.13 |
| Functional exercise capacity | ||
| 6MWT (m) | 439±103 | 473±68 |
| BODE (I/II/III/IV; n) | 3/5/3/1 | 6/6/2/0 |
| Compartmental volumes (mL) | ||
| At rest | ||
| URC | 94±54 | 100±44 |
| LRC | 49±37 | 63±42 |
| ABD | 295±114 | 268±117 |
| During exercise with workload | ||
| URC | 202±93 | 161±83 |
| LRC | 97±93 | 114±112 |
| ABD | 604±253 | 556±222 |
| Inspiratory muscle activity (RMS, 10−3 mV) | ||
| At rest | ||
| Sternocleidomastoid | 0.010±0.01 | 0.011±0.01 |
| Upper intercostal | 0.008±0.01 | 0.009±0.005 |
| Diaphragm | 0.006±0.002 | 0.007±0.003 |
| Rectus abdominis | 0.007±0.001 | 0.007±0.002 |
| During exercise with workload | ||
| Sternocleidomastoid | 0.010±0.01 | 0.020±0.02 |
| Upper intercostal | 0.009±0.002 | 0.020±0.04 |
| Diaphragm | 0.013±0.01 | 0.010±0.01 |
| Rectus abdominis | 0.009±0.005 | 0.010±0.01 |
Note: Data are presented as mean ± standard deviation, except BODE which is expressed by the number of patients.
Abbreviations: ABD, abdominal; BMI, body mass index; BODE, Body-mass index, airflow Obstruction, Dyspnea, and Exercise; CG, control group; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LRC, lower ribcage; M/F, male/female; 6MWT, 6-minute walk test; pred, predicted; RMS, root mean square; TG, treatment group; URC, upper ribcage.
Thoracoabdominal kinematics
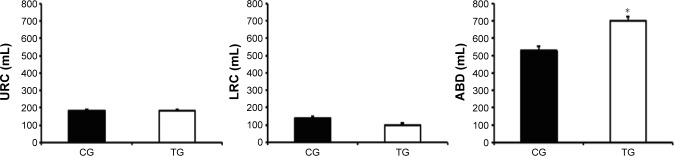
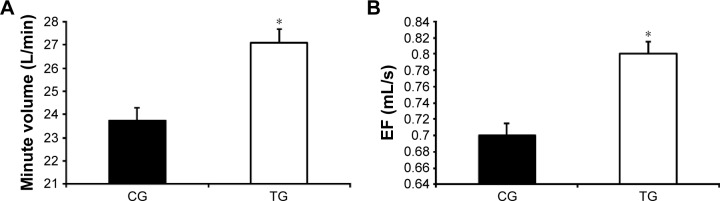
Patients from the TG presented an increase in CW volume after the intervention when compared with the CG (140±170 mL vs −86±240 mL, respectively; P<0.001), which was mainly due to an increased ABD volume (140±120 mL vs −108±210 mL, respectively; P<0.001). In contrast, no difference was observed in the URC and LRC volumes (P>0.05; Figure 2). The TG also demonstrated an increase in the VE compared with that of the CG (2.4±3.6 L/min vs −1.8±4.1 L/min, respectively; P<0.001; Figure 3A) as well as in EF (80±100 mL/s vs −30±100 mL/s, respectively; P<0.001; Figure 3B). The TG showed an increase in ABD compartment mobility (lower RC/ABD values) when compared with the CG (−0.19±0.41 mL vs 0.10±0.14 mL, respectively; P<0.05; Figure 4).
Figure 2.
Effect of accessory respiratory muscle stretching on the thoracoabdominal kinematics during exercise.
Notes: Data are presented as mean ± standard deviation. The exercise was performed with 25% of the maximal predicted load according to Jones et al.24 *P<0.05, CG vs TG (ANCOVA test).
Abbreviations: ABD, abdominal; ANCOVA, analysis of covariance; CG, control group; LRC, lower ribcage; TG, treatment group; URC, upper ribcage.
Figure 3.
Effect of accessory respiratory muscle stretching on minute volume (A) and EF (B) during exercise.
Notes: Data are presented as mean ± standard deviation. The exercise was performed with 25% of the maximal predicted load according to Jones et al.24 *P<0.05, CG vs TG (ANCOVA test).
Abbreviations: ANCOVA, analysis of covariance; CG, control group; EF, expiratory flow; TG, treatment group.
Figure 4.
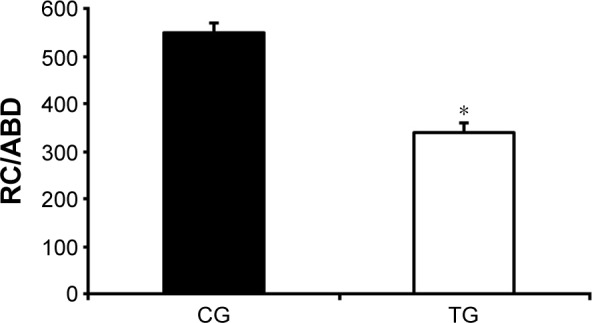
Effect of accessory respiratory muscle stretching on the rate of RC (URC and LRC) and ABD contribution during exercise.
Notes: Lower RC/ABD ratio values reflect an increase in ABD mobility.30,31 Data are presented as mean ± standard deviation. The exercise was performed with 25% of the maximal predicted load according to Jones et al.24 *P<0.05, CG vs TG (ANCOVA test).
Abbreviations: ABD, abdominal; ANCOVA, analysis of covariance; CG, control group; LRC, lower ribcage; RC, ribcage; TG, treatment group; URC, upper ribcage.
Respiratory muscle activity
After the intervention, patients from the TG, when compared with the CG, did not present with a reduction in muscle activity when analyzed individually. In addition, the TG showed a decrease in respiratory effort and improved efficiency of inspiratory muscle activity when analyzed altogether (EMG/VT; −47.9±82.8 10−3 mV/L vs 20.3±69.1 10−3 mV/L, respectively; P=0.03; Figure 5).
Figure 5.
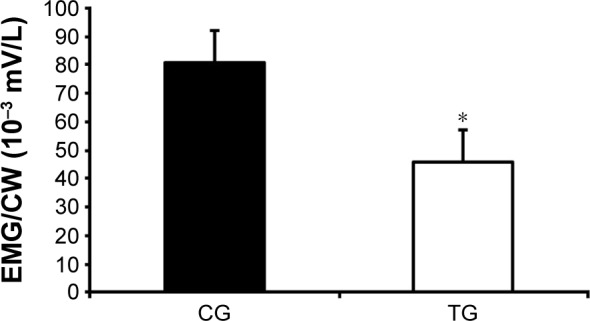
Effect of accessory respiratory muscle stretching on the total respiratory muscle activity divided by the CW volume (EMG/CW) during exercise.
Notes: Lower EMG/L reflects less respirator muscle activity per liter of breathed air.32 Data are presented as mean ± standard deviation. The exercise was performed with 25% of the maximal predicted load according to Jones et al.24 *P<0.05 CG vs TG (ANCOVA test).
Abbreviations: ANCOVA, analysis of covariance; CG, control group; CW, chest wall; EMG, respiratory muscles electromyography; TG, treatment group.
Functional exercise capacity
After the intervention, the TG had test results similar to those of the CG (488.0±17.4 m vs 454.0±17.5 m, respectively); however, the difference of 33.2 m between groups was clinically significant (Figure 6). Moreover, the TG reported a lower sensation of dyspnea after the 6MWT than was reported by the CG (1.53±0.31 points vs 2.78±0.30 points, P<0.001; Figure 7).
Figure 6.
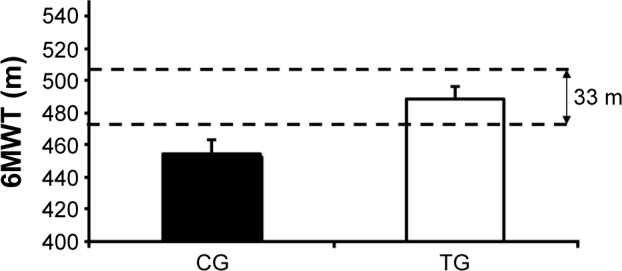
Effect of accessory respiratory muscle stretching on the 6MWT performance.
Notes: Data are presented as mean ± standard deviation. According to Holland et al,22 the clinically significant difference is 33 m.
Abbreviations: CG, control group; TG, treatment group; 6MWT, 6-minute walk test.
Figure 7.
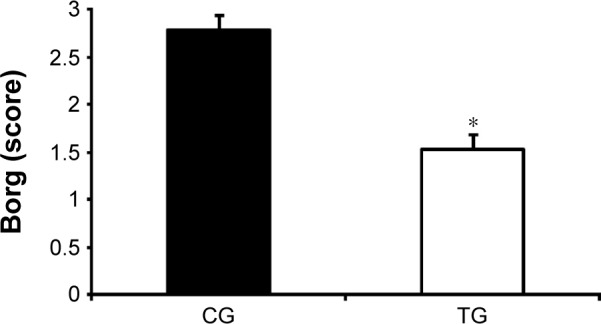
Effect of accessory respiratory muscle stretching on the modified dyspnea scale (Borg) post-6MWT.
Notes: The data are presented as mean ± standard deviation. Borg, dyspnea scale. *P<0.05, CG vs TG (ANCOVA test).
Abbreviations: ANCOVA, analysis of covariance; CG, control group; 6MWT, 6-minute walk test; TG, treatment group.
Discussion
Our results suggest that a program with respiratory muscle stretching associated with aerobic training reduces respiratory muscle activity during exercise and improves lung volumes and capacities by increasing the ABD contribution. Moreover, respiratory muscle stretching reduces dyspnea and improves the functional exercise capacity in patients with moderate-to-severe COPD.
Thoracoabdominal kinematics
The intervention program including respiratory muscle stretching improved lung volumes and capacities. Previous studies have evaluated the effect of respiratory muscle stretching during a 1-month period in patients with COPD and reported an increase in pulmonary ventilation based on spirometric parameters.4,37 In contrast, other studies have suggested that the same intervention decreases lung volume when the effect was assessed immediately after the intervention.10,38 A potential explanation for the differences between these findings may be the fact that muscle stretching can acutely activate the muscle spindle, which increases the sensorial afferent stimulus, resulting in an increase in the response of neuromotor, thereby increasing the likelihood of muscle contractions.4,39 These contractions increase muscle tension, causing an increase in the overlap between the actin and myosin filaments of the respiratory muscles and decreasing CW mobility and, consequently, reducing lung volumes.40,41 The observed effects of the respiratory muscle-stretching program may have occurred due to an improvement in muscle viscoelasticity and accommodation in the muscle spindle, consequently decreasing muscle stiffness, improving muscle performance, and increasing thoracoabdominal mobility, as suggested earlier.39,40 Although previous studies have evaluated the effect of stretching respiratory muscles, the obtained data are unreliable because CW expansion was quantified using cirtometry, a clinical assessment technique with a low accuracy.2,9 Our study is the first to evaluate the effect of a respiratory muscle stretching using OEP System, which has been widely accepted as an accurate and a reproducible technique. To the best of our knowledge, only two previous studies have used optoelectronic plethysmography but they aimed to assess the effect of aerobic training on lung volumes and capacities in patients with COPD and their results appear to be contradictory. One study did not observe an improvement in CW volume, and the other demonstrated a reduction in ABD volume.7,42 These results are consistent with those obtained in our CG, reinforcing the idea that aerobic training does not modify or reduce thoracoabdominal mobility. However, the patients in our study who engaged in respiratory muscle stretching showed an increase in CW volume, which was associated with a greater ABD contribution. The reduction in the RC/ABD ratio (representing greater ABD displacement) observed in the TG after intervention supports this hypothesis (Figure 4).
We also observed an increase in the EF in these patients, suggesting that the addition of respiratory muscle stretching to the aerobic training program changes the respiratory mechanics and improves CW ventilation.
Respiratory muscle activity
Our results also demonstrate that respiratory muscle stretching improves the efficiency of the respiratory muscles, as demonstrated by the reduction in inspiratory muscle activity per liter of air breathed (EMG/VT). A potential explanation for this finding is an increase in the sarcomere length and viscoelasticity of respiratory muscles, which would reduce neuromotor excitability, thereby accommodating muscle spindles.39,43 Muscle stretching may also stimulate Golgi tendon organs, which are located in the muscle–tendon region, causing an inhibitory effect.16,44 Consequently, muscle stretching may increase muscle fiber lengths, thereby improving muscle viscoelasticity and the overlap of actin and myosin filaments and increasing muscle contractile efficiency, resulting in less effort and a reduction in energy expenditure.16,43 It is difficult to know the duration of the effect of the stretching on respiratory muscles; however, there is evidence suggesting that passive stretching for 15–60 seconds in a 6-week stretching program reduces muscle stiffness for ~24 hours.16,39 We found one previous study in which patients with COPD had undergone a respiratory muscle-stretching program and their muscle activity was evaluated.45 In contrast to our results, the authors observed an increased activity in the sternocleidomastoid muscle; however, the study had important methodological limitations because it did not include a CG, whereas our study used a randomized, controlled, and blinded assessment. In addition, our patients underwent a longer intervention period.
Functional exercise capacity
Previous studies have shown that the effects of respiratory muscle stretching improve functional exercise capacity in patients with COPD.2,4 This improvement appears to be associated with a reduction in the sensation of dyspnea allowing patients to walk for a longer period and longer distances, as assessed via a 6MWT after the intervention.22 Previously, noncontrolled studies have showed that respiratory muscle stretching improves exercise capacity in patients with COPD;2,46 however, to the best of our knowledge, ours is the first controlled and randomized study that also demonstrated an improvement in lung volumes and capacities, as assessed using optoelectronic plethysmography. A potential explanation for such findings may be better thoracoabdominal mobility after the treatment, which enables an increase in CW volume. In addition, muscle stretching could modify the response of RC mechanoreceptors, reducing the afferent stimulation that can lead to increase the respiratory drive, muscle fatigue, and dyspnea.3,47
The current study has some limitations. First, the stretching protocol combined two techniques: passive stretching and the hold–relax technique, and it is not possible to determine which technique was more effective in leading to the observed benefits. However, the combination of both techniques could be more effective than either one alone because the hold–relax technique has a limited applicability for specific muscles,14 whereas the passive stretching technique can be applied to pectoralis minor, intercostal, anterior serratus, and ABD muscles. Second, until now, there has not been a specific technique for stretching only the diaphragm muscle. Third, instead of performing initial maximal exercise testing, we used the 25% of maximal exercise intensity,25 which might not be equally suitable for all patients. This workload was calculated based on a pilot study and was used because it was the load that triggered changes in thoracoabdominal kinematics without compromising the recording of the markers on each patient’s trunk. Fourth, the analysis was performed per protocol because two patients dropped out of the study after being hospitalized for non-respiratory diseases, which made it impossible to carry out the reassessment as an intention-to-treat analysis.
Despite these limitations, this study is the first randomized controlled trial to investigate the benefits of respiratory muscle stretching in combination with an aerobic training program. In addition, we also evaluated the thoracoabdominal kinematics using OEP System, which is considered to be a reliable technology and sensitive enough to detect differences in CW mobility, especially for the thoracoabdominal compartments.48 We hypothesize that additional effects could be obtained with the inclusion of stretches for the diaphragm or other exercises directed at increasing thoracic mobility.
Conclusion
Our results suggest that an aerobic training program combined with respiratory muscle stretching reduces dyspnea and increases functional and ventilatory capacities in patients with COPD. The program used in this study increased inspiratory muscle efficiency, especially in the ABD compartment.
Clinical implications
Therefore, respiratory muscle exercises could be useful in treating patients with severe COPD who have greater perceived levels of dyspnea and/or reduced CW mobility. Future studies should be performed to test whether lung hyperinflation influences the effect of respiratory muscle stretching, as well as to investigate how long the benefits of stretching last for patients with COPD.
Footnotes
Author contributions
JTW, EBS, and CRFC contributed significantly to writing the manuscript. DCP, DMP, AC, and ACL contributed significantly to manuscript revision. JTW, EBS, DMP, AC, ACL, and CRFC contributed to study concept and design. JTW, EBS, DCP, DMP, and ACL contributed to data acquisition. JTW, EBS, DCP, AC, ACL, and CRFC contributed to data analysis and interpretation. All authors contributed at all stages of this study, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet] Global Strategy for Diagnosis, Management, and Prevention of chronic obstructive pulmonary disease [update 2013] [Accessed September 27, 2016]. Available from: www.goldcopd.org.
- 2.Paulin E, Brunetto AF, Carvalho CRF. Effects of a physical exercises program designed to increase thoracic mobility in patients with chronic obstructive pulmonary disease. J Pneumol. 2003;29(5):287–295. [Google Scholar]
- 3.Montaldo BC, Gleeson K, Zwillich CW. The control of breathing in clinical practice. Chest. 2000;117(1):205–225. doi: 10.1378/chest.117.1.205. [DOI] [PubMed] [Google Scholar]
- 4.Minoguchi H, Shibuya M, Miyagawa T, et al. Cross-over comparison between respiratory muscle stretch gymnastics and inspiratory muscle training. Intern Med. 2002;41(10):805–812. doi: 10.2169/internalmedicine.41.805. [DOI] [PubMed] [Google Scholar]
- 5.Dodd JW, Hogg L, Nolan J, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax. 2011;66(5):425–429. doi: 10.1136/thx.2010.156372. [DOI] [PubMed] [Google Scholar]
- 6.Spruit MA, Singh SJ, Garvey C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):13–64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 7.Georgiadou O, Vogiatzis I, Stratakos G. Effects of pulmonary rehabilitation on operational chest wall volumes during exercise in COPD patients. Eur Respir J. 2007;29(2):284–291. doi: 10.1183/09031936.00121006. [DOI] [PubMed] [Google Scholar]
- 8.Riley DA, Van Dyke JM. The effects of active and passive stretching on muscle length. Phys Med Rehabil Clin N Am. 2012;23(1):51–57. doi: 10.1016/j.pmr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Putt MT, Watson M, Seale H, Paratz JD. Muscle stretching technique increases vital capacity and range of motion in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2008;89(6):1103–1107. doi: 10.1016/j.apmr.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Izumizaki M, Kakizaki F, Tanaka K, Homma I. Immediate effects of thixotropy conditioning of inspiratory muscles on chest-wall volume in chronic obstructive pulmonary disease. Respir Care. 2006;51(7):750–757. [PubMed] [Google Scholar]
- 11.Heneghan NR, Adab P, George M, Rachel E. Manual therapy for chronic obstructive airways disease: a systematic review of current evidence. Man Ther. 2012;17(6):507–518. doi: 10.1016/j.math.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Aliverti A. Lung and chest wall mechanics during exercise: effects of expiratory flow limitation. Respir Physiol Neurobiol. 2008;163(1–3):90–99. doi: 10.1016/j.resp.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20(2):187–191. doi: 10.1016/j.jcrc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Bonnar BP, Deivert RG, Gould TE. The relationship between isometric contraction durations during hold-relax stretching and improvement of hamstring flexibility. J Sports Med Phys Fitness. 2004;44(3):258–261. [PubMed] [Google Scholar]
- 15.Shrier M. Does stretching improve performance? A systematic and critical review of the literature. Clin J Sport Med. 2004;14(5):267–273. doi: 10.1097/00042752-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Siatras TA, Mittas VP, Mameletzi DN, Vamvakoudis EA. The duration of the inhibitory effects with static stretching on quadriceps peak torque production. J Strength Cond Res. 2008;22(1):40–46. doi: 10.1519/JSC.0b013e31815f970c. [DOI] [PubMed] [Google Scholar]
- 17.Zainuldin MR, Knoke D, Mackey DG, Luxton N, Alison JA. Prescribing cycle training intensity from the six-minute walk test for patients with COPD. BMC Pulm Med. 2007;7(9):1–7. doi: 10.1186/1471-2466-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ACSM. American College of Sports Medicine Position Stand The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 19.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 20.Figueiredo PHS, Guimarães FS. The average speed from six minutes walking test as a parameter to determine the training load for physical reconditioning of chronic pulmonary disease. Acta Fisiatr. 2009;16(4):156–161. [Google Scholar]
- 21.American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Holland AE. The return of the minimum clinically important difference for 6-minute-walk distance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(4):382–386. doi: 10.1164/rccm.201212-2191ED. [DOI] [PubMed] [Google Scholar]
- 23.Borges-Santos E, Wada JT, Silva CM, et al. Anxiety and depression are related to dyspnea and clinical control but not with thoracoabdominal mechanics in patients with COPD. Respir Physiol Neurobiol. 2015;210:1–6. doi: 10.1016/j.resp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Jones NL, Makrides L, Hitchock C, Chypchar T, McCartney N. Norman standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131(5):700–708. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 25.Aliverti A, Stevenson N, Dellaca R, Lo M, Pedotti A, Calverley P. Regional chest wall volumes during exercise in chronic obstructive pulmonary disease. Thorax. 2004;59(3):210–216. doi: 10.1136/thorax.2003.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aliverti A, Quaranta M, Chakrabarti B, Albuquerque AL, Calverley PM. Paradoxical movement of the lower ribcage at rest and during exercise in COPD patients. Eur Respir J. 2009;33(1):49–60. doi: 10.1183/09031936.00141607. [DOI] [PubMed] [Google Scholar]
- 27.Lunardi AC, Paisani DM, Marques da Silva CC, Cano DP, Tanaka C, Carvalho CR. Comparison of lung expansion techniques on thoracoabdominal mechanics and incidence of pulmonary complications after upper abdominal surgery: a randomized and controlled trial. Chest. 2015;148(4):100310. doi: 10.1378/chest.14-2696. [DOI] [PubMed] [Google Scholar]
- 28.Pereira CAC, Sato T, Rodrigues SC. New reference values for forced spirometry in White adults in Brazil. J Bras Pneumol. 2007;33(4):397–406. doi: 10.1590/s1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 29.Reddy MR, Guntupalli KK. Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2(4):441–452. [PMC free article] [PubMed] [Google Scholar]
- 30.Cala SJ, Kenyon CM, Ferrigno G, et al. Chest wall and lung volume estimation by optical reflectance motion analysis. J Appl Physiol. 1996;81(6):2680–2689. doi: 10.1152/jappl.1996.81.6.2680. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguti WP, Claudino RC, Neto AP, et al. Abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Arch Phys Med Rehabil. 2011;93(4):571–577. doi: 10.1016/j.apmr.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Gosselink RA, Wagenaar RC, Rijswijk H, Sargeant AJ, Decramer ML. Diaphragmatic breathing reduces efficiency of breathing in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151(4):1136–1142. doi: 10.1164/ajrccm.151.4.7697243. [DOI] [PubMed] [Google Scholar]
- 33.Lunardi AC, Porras DC, Barbosa RC, et al. Effect of volume-oriented versus flow-oriented incentive spirometry on chest wall volumes, inspiratory muscle activity, and thoracoabdominal synchrony in the elderly. Respir Care. 2014;59(3):420–426. doi: 10.4187/respcare.02665. [DOI] [PubMed] [Google Scholar]
- 34.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 35.Kallenberg LA, Preece S, Nester C, Hermens HJ. Reproducibility of MUAP properties in array surface EMG recordings of the upper trapezius and sternocleidomastoid muscle. J Electro Kinesiol. 2009;19(6):536–542. doi: 10.1016/j.jelekin.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Maarsingh EJ, van Eykern LA, Sprikkelman AB, Hoekstra MO, Van-Aalderen WM. Respiratory muscle activity measured with a noninvasive EMG technique: technical aspects and reproducibility. J Appl Physiol. 2000;88(6):1955–1961. doi: 10.1152/jappl.2000.88.6.1955. [DOI] [PubMed] [Google Scholar]
- 37.Yamada M, Shibuya M, Kanamaru A, et al. Benefits of respiratory muscle stretch gymnastics in chronic respiratory disease. Showa Univ J Med Sci. 1996;8(1):63–71. [Google Scholar]
- 38.Noll DR, Johnson JC, Baer RW, Snider EJ. The immediate effect of individual manipulation techniques on pulmonary function measures in persons with COPD. Osteopath Med Prim Care. 2009;3(9):1–12. doi: 10.1186/1750-4732-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McHugh MP, CosgraveScand CH. To stretch or not to stretch: the role of stretching in injury prevention and performance. Scand J Med Sci Sports. 2010;20(2):169–181. doi: 10.1111/j.1600-0838.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- 40.Ito M, Kakizaki F, Tsuzura Y, Yamada M. Immediate effect of respiratory muscle stretch gymnastics and diaphragmatic breathing on respiratory pattern. Intern Med. 1999;38(2):126–132. doi: 10.2169/internalmedicine.38.126. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee A, Chakravarty A. Spasticity mechanisms – for the clinician. Front Neurol. 2010;149(1):1–10. doi: 10.3389/fneur.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagliardi E, Bruni GI, Presi I, Gigliotti F, Scano G. Thoracoabdominal motion/displacement does not affect dyspnea following exercise training in COPD patients. Respir Physiol Neurobiol. 2014;190:124–130. doi: 10.1016/j.resp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Hindle KB, Whitcomb TJ, Briggs WO, Hong J. Proprioceptive neuromuscular facilitation (PNF): Its mechanisms an effects on range of motion and muscular function. J Hum Kinet. 2012;31:105–113. doi: 10.2478/v10078-012-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kokkonen J, Nelson AG, Eldredge C, Winchester J. Chronic static stretching improves exercise performance. Med Sci Sports Exerc. 2007;39(10):1825–1831. doi: 10.1249/mss.0b013e3181238a2b. [DOI] [PubMed] [Google Scholar]
- 45.Cunha NA, Marinho PEM, Silva TNS, França EET, Amorim C. Efeito do alongamento sobre a atividade dos músculos inspiratórios na DPOC [Effects of stretching on the inspiratory muscular activity in COPD] Saúde. 2005;7(17):13–19. Portuguese. [Google Scholar]
- 46.Izumizaki M, Masahiro S, Takashi H, Keiyu S, Shioya T, Homma I. Effects of inspiratory muscle thixotropy on the 6-min walk distance in COPD. Respir Med. 2008;102(7):970–977. doi: 10.1016/j.rmed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura M, Ikezoe T, Takeno Y, Ichihashi N. Effects of a 4-week static stretch training program on passive stiffness of human gastrocnemius muscle-tendon unit in vivo. Eur J Appl Physiol. 2012;112(7):2749–2755. doi: 10.1007/s00421-011-2250-3. [DOI] [PubMed] [Google Scholar]
- 48.Aliverti A, Uva B, Laviola M, et al. Concomitant ventilatory and circulatory functions of the diaphragm and abdominal muscles. J Appl Physiol. 2010;109(5):1432–1440. doi: 10.1152/japplphysiol.00576.2010. [DOI] [PubMed] [Google Scholar]
- 49.Rassier DE. Stretching human muscles makes them stronger. J Appl Physiol. 2007;102(1):5–6. doi: 10.1152/japplphysiol.00952.2006. [DOI] [PubMed] [Google Scholar]
- 50.Ylinen J. Stretching Therapy for Sport and Manual Therapies. Edinburgh: Churchill Livingstone, Elsevier; 2009. [Google Scholar]