Abstract
The Jumonji-containing domain protein, KDM4C, is a histone demethylase associated with the development of several forms of human cancer. However, its specific function in the viability of tumoral lineages is yet to be determined. This work investigates the importance of KDM4C activity in cell proliferation and chromosome segregation of three triple-negative breast cancer cell lines using a specific demethylase inhibitor. Immunofluorescence assays show that KDM4C is recruited to mitotic chromosomes and that the modulation of its activity increases the number of mitotic segregation errors. However, 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) cell proliferation assays demonstrate that the demethylase activity is required for cell viability. These results suggest that the histone demethylase activity of KDM4C is essential for breast cancer progression given its role in the maintenance of chromosomal stability and cell growth, thus highlighting it as a potential therapeutic target.
Keywords: KDM4C, breast cancer, chromosome segregation, histone demethylation
Introduction
The development of human cancer is strongly related to alterations in the cellular genetic expression profile and defects in the distribution of chromosomal information during mitosis, both factors associated with macromolecules in charge of the epigenetic regulation of cell cycle.1,2 Of the several types of cancers, breast cancer is a heterogeneous neoplasm that is further classified depending on its biological expression profile into subtypes, which include basal like (closely related to the triple-negative breast cancer subtype), normal like, Luminal A, Luminal B, and HER2/ERBB2 enriched.3 Of these, the basal-like/triple-negative tumors (which do not present the expression of estrogens, progesterones, and HER2 receptors) exhibit the most aggressive phenotype with a high rate of metastasis and poor prognosis.4,5 Additionally, triple-negative breast cancer accounts for 15%–20% of the 500,000 breast cancer-associated female deaths worldwide each year and is considered as an international public health issue, thus supporting the continuous search for novel therapeutic targets.4,6–8
The preservation of the phenotype of eukaryotic cells depends closely on the structural conservation and the dynamic regulation of chromatin, the main nuclear DNA packaging structure.9,10 Chromosomal information of mother cells is duplicated and segregated to its daughter cells in a highly regulated and complex process known as mitosis.11,12 Defects in the distribution of this genetic information are associated with the development of cancer.13–15 The transcription of genetic information is regulated by different mechanisms, including RNA interference, DNA methylation, and posttranscriptional modification (PTM) of histones.1,16,17 Among these, histones can undergo various covalent modifications such as phosphorylation, ubiquitylation, sumoylation, ADP-ribosylation, acetylation, deamination, proline isomerization, and methylation.18,19
Histone methylation/demethylation is one of the most important PTMs that is directly associated with the activation or repression of genes and is involved in DNA recombination, repair, imprinting, and mitosis.20–22 Histone methylation was considered as a stable chromatin mark until the relatively recent discovery of histone demethylases that have provided a new perspective, as alterations in these chromatin remodelers are associated with the development of several diseases, including cancer.23–27
Demethylation of histones at lysine is associated with two families of macromolecules known as histone lysine demethylases (KDMs). The lysine-specific demethylases (LSD/KDM1), which are flavin-dependent enzymes, can act on the di- and mono-methylated forms of histone lysines, and the JMJs present the unique ability to modify a trimethylated residue of lysine in an Fe2+- and 2-oxoglutarate-dependent mechanism.27–29 The JMJ proteins have raised great interest due to their significance in the development of a wide number of cancer cell types.30–32 As part of this family, the members of the subset JMJD2/KDM4 are associated with breast cancer development, tumor cell development and proliferation, as well as progression of abnormal cells to an invasive and highly metastatic form by regulating their migratory and invasive capacities.28,33–36
An important member of this protein family, the lysine demethylase KDM4C, is a crucial oncogene that is amplified and overexpressed in several neoplasms,37–42 including the most aggressive forms of basal-like breast cancers,43 and is recognized as a potential prognostic marker for the invasive phenotype of this disease.44,45 On the other hand, KDM4C is also the only member of the KDM4/JMJD2 subfamily associated with the segregation of chromosomes during mitosis in the U2OS osteosarcoma cell line,46 underscoring its potential relevance in the evolution of cancer associated with chromosomal instability and mitotic defects.14,47 However, the specific mechanism and pathways associated with KDM4C activity in tumor progression remain to be determined.
This article presents the importance of KDM4C activity for cell proliferation and chromosome segregation of three triple-negative breast cancer cell lines by using a specific KDM4 inhibitor, NCDM-32b.48 Our results show that KDM4C is important for cell proliferation and for maintaining a correct distribution of the genetic information during mitosis for these aggressive and highly invasive breast neoplasms, highlighting the importance of this histone demethylase as a potential anti-breast cancer therapeutic target.
Methods
Cell culture and activity inhibition
The HCC38, MDA-MB-436, and MDA-MB-453 triple-negative breast cancer cell lines were obtained from American Tissue Culture Collection (ATCC). HCC38 was grown in Roswell Park Memorial Institute-1640 media (ATCC modification, Gibco), while MDA-MB-436 and MDA-MB-453 were grown in Dulbecco’s Modified Eagle medium media (Gibco) in the presence of 0.01 mg/mL insulin. All media were supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C under 5% CO2. To inhibit KDM4C activity for subsequent experiments, cells were cultured up to 30%–40% confluence and synchronized by serum starvation in a serum-free medium overnight (mean efficiency 82%, data not shown), followed by release from cell cycle arrest by phosphate-buffered saline (PBS) washing and the addition of fresh medium supplied with 10% fetal bovine serum.49 Cells were then subcultured in six-well plates (1 × 106 cells/well) up to 70% confluence and incubated with the specific inhibitor NCDM-32b (Wako Chemicals) at 50 and 100 μM/well. KDM4C inhibition was evaluated by immunofluorescence assay (IFA) or MTT methodology.50
Immunofluorescence assays
Triple-negative breast cancer cells were grown on coverslips and treated with NCDM-32b as previously described. After 48 hours, the samples were pre-extracted with 0.2% PBS/Triton X-100 and fixed in 3.7% formaldehyde for 15 minutes. The fixed cells were rendered permeable with 0.5% Triton X-100 in PBS and then incubated with 3% bovine serum albumin in PBS for one hour. Next, the cells were treated with anti-KDM4A (Abcam ab24545), anti-KDM4C (Abcam ab85454), or anti-trimethyl H3K9 (Abcam ab10812) antibody overnight at 4°C at 1:250 dilution, followed by three washes with PBS containing 0.01% Triton X-100 and the secondary antibody incubation (Alexa Fluor 488, 1:500 dilution, Abcam 150077) for one hour. Finally, the cells were washed twice and mounted in ProLong Diamond Antifade Mountant with 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies). All images were obtained on an Eclipse Ni-E microscope (Nikon) and analyzed with ImageJ software (National Institutes of Health).51
Proliferation assays
Treated cells were tested for cancer cell proliferation by an MTT assay.36 Briefly, 1500 cells were seeded and cultured in 96-well plates according to previously described conditions. One day later (day 0), the cells were treated with NCDM-32b (100 μL/well) under conditions similar to that of corresponding controls without the inhibitor. Each experiment included blanks without cells, without MTT, and without treatment to overcome any background interference. The samples were then treated with 20 μL MTT (Life Technologies M6494) for four hours at 24 and 48 hours post inhibition. Next, the supernatant was removed, and 150 μL of dimethyl sulfoxide was added. Absorbance was measured at 550 nm using a microplate reader (TriStar2 LB 942 Multimode Reader, Berthold Technologies).
Statistical analysis
All the experiments were performed in duplicate, and the results obtained were from at least two independent experiments. The statistical comparison of results was performed by applying analysis of variance (one way) and two means evaluation (Student’s t-test) using the SPSS 10.0 (IBM Corporation) software, with a significance cutoff of P > 0.05.
Results
KDM4C is located in mitotic chromosomes and its NCDM-32b-specific inhibitor modulates the trimethylated form of lysine 9 on histone 3
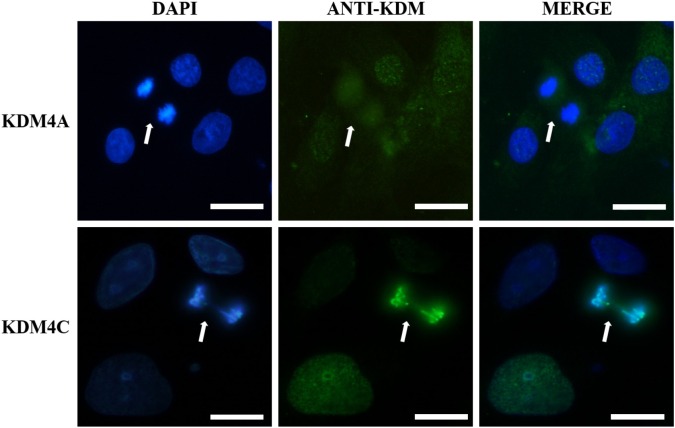
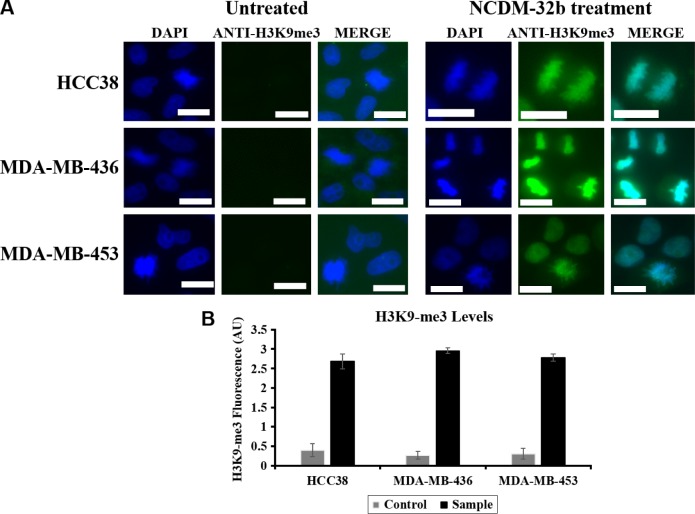
IFAs with anti-KDM4C and anti-KDM4A proteins were performed in the triple-negative breast cancer cell lines. KDM4A was not recruited to mitotic chromosomes and presented a diffuse cellular distribution, whereas KDM4C was able to interact with mitotic chromosomes, thus highlighting its potential relevance for chromosome segregation in contrast with the other members of KDM4 subfamily (Fig. 1). To evaluate the effect of KDM4C inhibition on breast neoplasms, a KDM4C-specific inhibitor was tested in three triple-negative breast cancer cell lines. IFA analysis confirmed a significant increase in the levels of H3K9-3me when the cells were exposed to NCDM-32b (Fig. 2), demonstrating that NCDM-32b was capable of modulating KDM4C activity under our experimental conditions.
Figure 1.
KDM4A and KDM4C cellular localization during mitosis. Representative immunofluorescent images of HCC38 cells in anaphase are presented (white arrows), illustrating a different distribution pattern for both proteins. Note that KDM4C colocalizes with DNA signal detected with DAPI, while KDM4A is excluded from mitotic chromosomes. The presented localization is conserved along all cell lines included in the study. White scale bar: 10 μm.
Figure 2.
KDM4C inhibition with NCDM-32b affects histone demethylation levels. (A) Immunofluorescence of HCC38, MDA-MB-436, and MDA-MB-453 cell lines treated (right) or untreated (left) with inhibitor. Note the significant increment in fluorescence intensity for treated samples with respect to the untreated cells. All images were recorded at 80 milliseconds under the same conditions of exposition. White scale bar: 10 μm. (B) Quantification of histone 3 lysine 9 trimethyl levels by ImageJ software (National Institutes of Health), showing a significant increment on H3K9-3me levels up to six times with respect to the control for all treatments.
Inhibition of demethylase activity of KDM4C increases the number of chromosome segregation errors
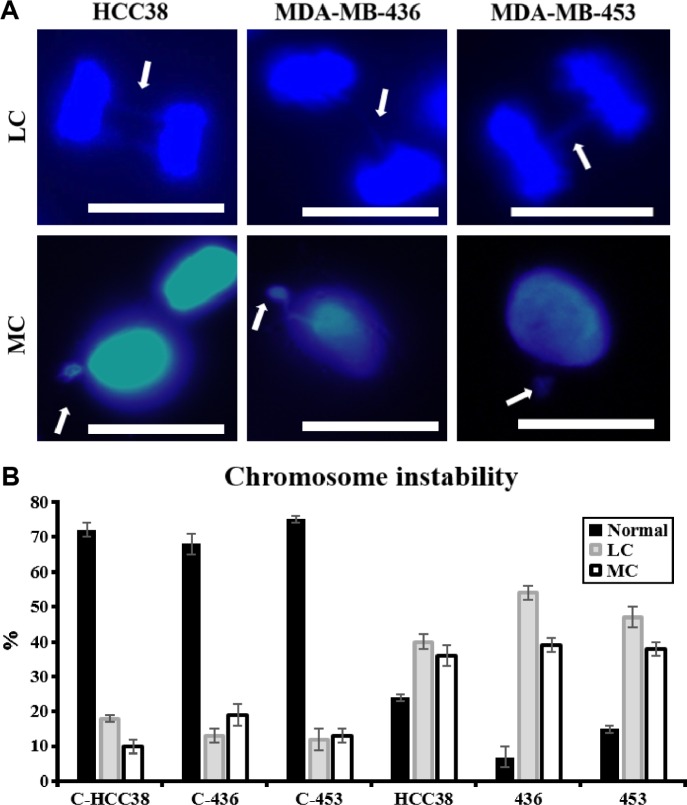
The relevance of modulation of KDM4C’s activity for the segregation of genetic information was analyzed by treating synchronized HCC38, MDA-MB-436, and MDA-MB-453 cell lines with NCDM-32b inhibitor (Fig. 3). Mitotic defects such as lagging chromosomes (LCs; associated with delayed movement during anaphase) increased by a mean of 38%, and the presence of micronuclei (resulting from mitotic segregation defects) increased by 20%–26% in the three breast cancer cell lines under KDM4C inhibition compared to untreated cells (Fig. 3B). Together, these observations demonstrated that KDM4C activity is highly relevant in the maintenance of proper gene distribution through mitosis.
Figure 3.
Effect of the activity of KDM4C on chromosomal stability. (A) Representative immunofluorescence at 100 times for triplicated samples analyzed as methods description for HCC38, MDA-MB-436, and MDA-MB-453 cell lines. A total of 30–40 random cells (1 × 105 cells/well) were analyzed per sample to evaluate different mitotic segregation errors. Upper line represents LC and lower line represents micronucleus. DNA was detected by using DAPI. White scale bar: 10 μm. (B) Quantification of chromosome segregation errors, determined for this experiment. Each sample was analyzed 48 hours post treatment and was compared to its corresponding control. Differences are presented as percentages.
Proliferation of triple-negative breast cancer cells is affected by KDM4C inhibition
The proliferation of NCDM-32b-treated and NCDM-32b-untreated triple-negative breast cancer cells was analyzed by an MTT assay. We observed a significant reduction in the proliferation of the three cell lines after 24 and 48 hours of KDM4C inhibition (means of 15% and 48%, respectively) compared to untreated cells (Fig. 4), indicating that KDM4C was associated with triple-negative breast cancer viability, which was consistent with previous observations with other KDM4 macromolecules.36,52
Figure 4.
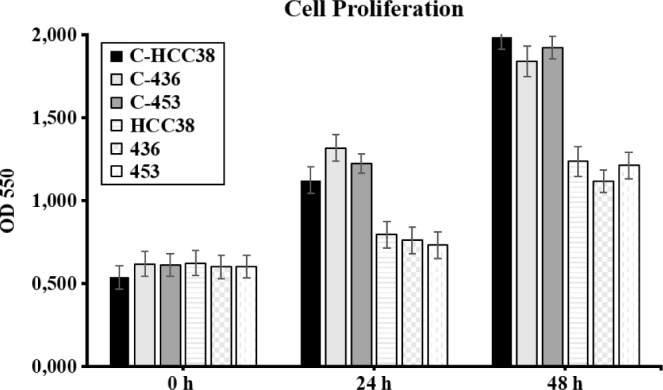
Cell proliferation assays. Optical density at 550 nm was evaluated at 24 and 48 hours by an MTT assay for unexposed HCC38, MDA-MB-436, and MDA-MB-453 cell lines (controls, labeled with C-) or samples exposed to NCDM-32b treatment. Note the significant reduction in the optical density of treated cells in comparison with their corresponding controls.
Discussion
This study presents the significance of inhibition of KDM4C demethylase activity in three triple-negative breast cancer cell lines. First, we show that the reduction of demethylase activity affects tumor cell growth along with an increase in the number of chromosome segregation errors in inhibitor-treated cells compared with untreated controls. Histone demethylases are epigenetic regulators that can remove methyl groups of specific lysine residues. Among these, the JMJ/KDM demethylases are of special interest because they can modify trimethylated lysine residues1 and are associated with the development of several forms of cancer.28,33–36 Accordingly, KDM4 histone demethylases are considered as promising therapeutic targets to treat some types of neoplasms.27,53 It has also been reported that commonly observed amplicons, such as 9p24.3, in breast cancer contain the kdm4c gene, which encodes a histone demethylase that is widely associated with the development of several cancers, including prostate and esophageal tumors.32,50,54
Previous studies have shown that KDM4C has a unique cellular distribution in the U2OS osteosarcoma cell line that is functionally related to the maintenance of chromosome stability, as inhibition of KDM4C’s expression promotes the occurrence of mitotic errors.46 Our experiments in triple-negative breast cancer cells have revealed that KDM4C is recruited to mitotic chromosomes as opposed to the structurally similar KDM4A demethylase, which is completely excluded (Fig. 1). Such localization is associated with the preservation of H3K9 trimethylation levels as seen by a significant increment in the corresponding signal of trimethylated lysine residues when compared to untreated cells (Fig. 2), indicating that KDM4C activity may be necessary for the correct distribution of genetic information in breast cancer cells.
This is consistent with the fact that an increase in mitotic errors is observed in triple-negative breast cancer cell lines that are treated with a KDM4-specific inhibitor such as NCDM-32b, similar to the use of RNA interference in other neoplastic cell lines in previous studies.46 A higher number of LCs and micronuclei are observed in treated cells under our experimental conditions (Fig. 3), which suggest that demethylation of H3K9-3me by KDM4C is a key process for maintaining chromosomal stability in triple-negative breast cancer. Interestingly, a recent article shows that micronuclei in some cell lines can be recovered by the daughter cells.55 However, the evidence suggesting the association between chromosome segregation errors and chromosome instability56–58 in conjunction with the exposition of external chromosomes in micronuclei to DNA damage that can be inherited by daughter cells59 underscores the need for additional studies to identify the specific role of KDM4C for the proper segregation of DNA content as part of the complex linkage between mitosis defects, aneuploidy, and chromosome instability. In this regard, previous reports have shown that a sequential process involving deacetylation and methylation of H3K9 is relevant for the condensation of chromosomes,60 as a potential epigenetic pathway associated with these relevant events.
On the other hand, we have performed similar analysis on the depletion of KDM4C by RNAi in triple-negative breast cancer cells where an increment in chromosome segregation errors is observed together with a significant reduction in cell proliferation and a differential modulation of the cell’s migration and invasion capacities (Garcia, Lizcano. Role of the Jumonji-domain containing protein KDM4C in triple-negative breast cancer. Submitted to J Breast Cancer, unpublished results). Together, these results highlight the importance of KDM4C and its demethylase activity for mitotic segregation.
In addition, the influence of KDM4C activity on the proliferation of triple-negative breast cancer cells was analyzed in which inhibition of the demethylase activity led to a significant reduction in cell multiplication (Fig. 4). This behavior has been observed for KDM4C in tumor cells with a different expression profile, including HER2/neu (+) cells.43,61,62 This important demethylase has been associated with regulation of cell growth in normal and tumoral cells,63 suggesting that KDM4C or its target genes are associated with the proliferation of triple-negative breast cancer in a HER2/neu-independent pathway that remains to be determined.
In summary, these observations highlight the relevance of histone demethylase, KDM4C, as a potential therapeutic target for the most aggressive form of breast cancer and support the importance of histone demethylases belonging to the LSD family64 and other members of the JMJ/KDM subgroup65–67 as part of a new generation of potential therapeutic targets against breast cancer.
Conclusion
This work presents the importance of KDM4C activity for triple-negative breast cancer, which is directly associated with the growth of the most aggressive form of breast cancer, represented by three triple-negative carcinoma cell lines. Although extended analysis is required to uncover the complete mechanism associated with this critical demethylase, our study determines that KDM4C’s activity is highly required for the correct segregation of chromosomes during mitosis in these cancer cell lines, which strongly supports the potential of KDM4C as a promising therapeutic target for new inhibitors and strategies against the main cause of cancer death in women worldwide.
Acknowledgments
We would like to thank Dr. Hernando Curtidor of the Fundación Instituto de Inmunología de Colombia—FIDIC and Dr. Maria Ximena Quintanilla of the agro-industrial processes (La Sabana University) for their help performing the IFA analysis.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1347 words, excluding any confidential comments to the academic editor.
FUNDING: We would like to thank Colciencias and La Sabana University (research department) for their support by the grant nos. 528 and 909, respectively. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript, conceived and designed the experiments, and analyzed the data: JG. Made critical revisions, jointly developed the structure and arguments for the article, and approved the final version: FL. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Lizcano F, Garcia J. Epigenetic control and cancer: the potential of histone demethylases as therapeutic targets. Pharmaceuticals (Basel) 2012;5(9):963–990. doi: 10.3390/ph5090963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaidi SK, Van Wijnen AJ, Lian JB, Stein JL, Stein GS. Targeting deregulated epigenetic control in cancer. J Cell Physiol. 2013;228(11):2103–2108. doi: 10.1002/jcp.24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badve S, Dabbs DJ, Schnitt SJ, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24(2):157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- 6.WHO . World Cancer Report. World Health Organization; Lyon, France: 2008. [Google Scholar]
- 7.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Reinberg D. Chromatin higher-order structures and gene regulation. Curr Opin Genet Dev. 2011;21(2):175–186. doi: 10.1016/j.gde.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Fang Y. Histone variants: the artists of eukaryotic chromatin. Sci China Life Sci. 2015;58(3):232–239. doi: 10.1007/s11427-015-4817-4. [DOI] [PubMed] [Google Scholar]
- 11.Westhorpe FG, Straight AF. The centromere: epigenetic control of chromosome segregation during mitosis. Cold Spring Harb Perspect Biol. 2015;7(1):a015818. doi: 10.1101/cshperspect.a015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicente JJ, Wordeman L. Mitosis, microtubule dynamics and the evolution of kinesins. Exp Cell Res. 2015;334(1):61–69. doi: 10.1016/j.yexcr.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez-Brauer C, Thu KL, Mason JM, Blaser H, Bray MR, Mak TW. Targeting mitosis in cancer: emerging strategies. Mol Cell. 2015;60(4):524–536. doi: 10.1016/j.molcel.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Hirota T. Chromosome segregation machinery and cancer. Cancer Sci. 2009;100(7):1158–1165. doi: 10.1111/j.1349-7006.2009.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H. How chromosome mis-segregation leads to cancer: lessons from BubR1 mouse models. Mol Cells. 2014;37(10):713–718. doi: 10.14348/molcells.2014.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Audia JE, Campbell RM. Histone modifications and cancer. Cold Spring Harb Perspect Biol. 2016;8(4):a019521. doi: 10.1101/cshperspect.a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubicek S, Schotta G, Lachner M, et al. The role of histone modifications in epigenetic transitions during normal and perturbed development. Ernst Schering Res Found Workshop. 2006;57:1–27. doi: 10.1007/3-540-37633-x_1. [DOI] [PubMed] [Google Scholar]
- 20.Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol Cell. 2012;48(4):491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schotta G, Lachner M, Sarma K, et al. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18(11):1251–1262. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen MT, Helin K. Histone demethylases in development and disease. Trends Cell Biol. 2010;20(11):662–671. doi: 10.1016/j.tcb.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Thrasher BJ, Hong LK, Whitmire JK, Su MA. Epigenetic dysfunction in Turner syndrome immune cells. Curr Allergy Asthma Rep. 2016;16(5):36. doi: 10.1007/s11882-016-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang QJ, Liu ZP. Histone methylations in heart development, congenital and adult heart diseases. Epigenomics. 2015;7(2):321–330. doi: 10.2217/epi.14.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hojfeldt JW, Agger K, Helin K. Histone lysine demethylases as targets for anticancer therapy. Nat Rev Drug Discov. 2013;12(12):917–930. doi: 10.1038/nrd4154. [DOI] [PubMed] [Google Scholar]
- 27.Thinnes CC, England KS, Kawamura A, Chowdhury R, Schofield CJ, Hopkinson RJ. Targeting histone lysine demethylases—progress, challenges, and the future. Biochim Biophys Acta. 2014;1839(12):1416–1432. doi: 10.1016/j.bbagrm.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou H, Yu H. Structural insights into histone lysine demethylation. Curr Opin Struct Biol. 2010;20(6):739–748. doi: 10.1016/j.sbi.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SY, Park JW, Chun YS. Jumonji histone demethylases as emerging therapeutic targets. Pharmacol Res. 2016;105:146–151. doi: 10.1016/j.phrs.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Wade MA, Jones D, Wilson L, et al. The histone demethylase enzyme KDM3A is a key estrogen receptor regulator in breast cancer. Nucleic Acids Res. 2015;43(1):196–207. doi: 10.1093/nar/gku1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li LL, Xue AM, Li BX, et al. JMJD2A contributes to breast cancer progression through transcriptional repression of the tumor suppressor ARHI. Breast Cancer Res. 2014;16(3):R56. doi: 10.1186/bcr3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, Liu S, Liu G, et al. Identification and functional analysis of 9p24 amplified genes in human breast cancer. Oncogene. 2012;31(3):333–341. doi: 10.1038/onc.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry WL, Janknecht R. KDM4/JMJD2 histone demethylases: epigenetic regulators in cancer cells. Cancer Res. 2013;73(10):2936–2942. doi: 10.1158/0008-5472.CAN-12-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry WL, Shin S, Lightfoot SA, Janknecht R. Oncogenic features of the JMJD2A histone demethylase in breast cancer. Int J Oncol. 2012;41(5):1701–1706. doi: 10.3892/ijo.2012.1618. [DOI] [PubMed] [Google Scholar]
- 35.Li BX, Luo CL, Li H, et al. Effects of siRNA-mediated knockdown of jumonji domain containing 2A on proliferation, migration and invasion of the human breast cancer cell line MCF-7. Exp Ther Med. 2012;4(4):755–761. doi: 10.3892/etm.2012.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li BX, Zhang MC, Luo CL, et al. Effects of RNA interference-mediated gene silencing of JMJD2A on human breast cancer cell line MDA-MB-231 in vitro. J Exp Clin Cancer Res. 2011;30:90. doi: 10.1186/1756-9966-30-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rui L, Emre NC, Kruhlak MJ, et al. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell. 2010;18(6):590–605. doi: 10.1016/j.ccr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TD, Fuchs JR, Schwartz E, et al. Pro-growth role of the JMJD2C histone demethylase in HCT-116 colon cancer cells and identification of curcuminoids as JMJD2 inhibitors. Am J Transl Res. 2014;6(3):236–247. [PMC free article] [PubMed] [Google Scholar]
- 39.Helias C, Struski S, Gervais C, et al. Polycythemia vera transforming to acute myeloid leukemia and complex abnormalities including 9p homogeneously staining region with amplification of MLLT3, JMJD2C, JAK2, and SMARCA2. Cancer Genet Cytogenet. 2008;180(1):51–55. doi: 10.1016/j.cancergencyto.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Italiano A, Attias R, Aurias A, et al. Molecular cytogenetic characterization of a metastatic lung sarcomatoid carcinoma: 9p23 neocentromere and 9p23–p24 amplification including JAK2 and JMJD2C. Cancer Genet Cytogenet. 2006;167(2):122–130. doi: 10.1016/j.cancergencyto.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Wissmann M, Yin N, Muller JM, et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9(3):347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 42.Margareto J, Leis O, Larrarte E, Pomposo IC, Garibi JM, Lafuente JV. DNA copy number variation and gene expression analyses reveal the implication of specific oncogenes and genes in GBM. Cancer Invest. 2009;27(5):541–548. doi: 10.1080/07357900802563044. [DOI] [PubMed] [Google Scholar]
- 43.Liu G, Bollig-Fischer A, Kreike B, et al. Genomic amplification and oncogenic properties of the GASC1 histone demethylase gene in breast cancer. Oncogene. 2009;28(50):4491–4500. doi: 10.1038/onc.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong Q, Yu S, Yang Y, Liu G, Shao Z. A polymorphism in JMJD2C alters the cleavage by caspase-3 and the prognosis of human breast cancer. Oncotarget. 2014;5(13):4779–4787. doi: 10.18632/oncotarget.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berdel B, Nieminen K, Soini Y, et al. Histone demethylase GASC1—a potential prognostic and predictive marker in invasive breast cancer. BMC Cancer. 2012;12:516. doi: 10.1186/1471-2407-12-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kupershmit I, Khoury-Haddad H, Awwad SW, Guttmann-Raviv N, Ayoub N. KDM4C (GASC1) lysine demethylase is associated with mitotic chromatin and regulates chromosome segregation during mitosis. Nucleic Acids Res. 2014;42(10):6168–6182. doi: 10.1093/nar/gku253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Y, Kim C, Mao Y. New insights into the mechanism for chromosome alignment in metaphase. Int Rev Cell Mol Biol. 2013;303:237–262. doi: 10.1016/B978-0-12-407697-6.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamada S, Suzuki T, Mino K, et al. Design, synthesis, enzyme-inhibitory activity, and effect on human cancer cells of a novel series of jumonji domain-containing protein 2 histone demethylase inhibitors. J Med Chem. 2010;53(15):5629–5638. doi: 10.1021/jm1003655. [DOI] [PubMed] [Google Scholar]
- 49.Khammanit R, Chantakru S, Kitiyanant Y, Saikhun J. Effect of serum starvation and chemical inhibitors on cell cycle synchronization of canine dermal fibroblasts. Theriogenology. 2008;70(1):27–34. doi: 10.1016/j.theriogenology.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Ye Q, Holowatyj A, Wu J, et al. Genetic alterations of KDM4 subfamily and therapeutic effect of novel demethylase inhibitor in breast cancer. Am J Cancer Res. 2015;5(4):1519–1530. [PMC free article] [PubMed] [Google Scholar]
- 51.Gallego-Paez LM, Tanaka H, Bando M, et al. Smc5/6-mediated regulation of replication progression contributes to chromosome assembly during mitosis in human cells. Mol Biol Cell. 2014;25(2):302–317. doi: 10.1091/mbc.E13-01-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawazu M, Saso K, Tong KI, et al. Histone demethylase JMJD2B functions as a co-factor of estrogen receptor in breast cancer proliferation and mammary gland development. PLoS One. 2011;6(3):e17830. doi: 10.1371/journal.pone.0017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labbe RM, Holowatyj A, Yang ZQ. Histone lysine demethylase (KDM) subfamily 4: structures, functions and therapeutic potential. Am J Transl Res. 2013;6(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- 54.Cloos PA, Christensen J, Agger K, et al. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442(7100):307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- 55.Huang Y, Jiang L, Yi Q, et al. Lagging chromosomes entrapped in micronuclei are not ‘lost’ by cells. Cell Res. 2012;22(5):932–935. doi: 10.1038/cr.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawyer JR, Swanson CM, Wheeler G, Cunniff C. Chromosome instability in ICF syndrome: formation of micronuclei from multibranched chromosomes 1 demonstrated by fluorescence in situ hybridization. Am J Med Genet. 1995;56(2):203–209. doi: 10.1002/ajmg.1320560218. [DOI] [PubMed] [Google Scholar]
- 57.Hatch EM, Hetzer MW. Linking micronuclei to chromosome fragmentation. Cell. 2015;161(7):1502–1504. doi: 10.1016/j.cell.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Matrka MC, Hennigan RF, Kappes F, et al. DEK over-expression promotes mitotic defects and micronucleus formation. Cell Cycle. 2015;14(24):3939–3953. doi: 10.1080/15384101.2015.1044177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giam M, Rancati G. Aneuploidy and chromosomal instability in cancer: a jackpot to chaos. Cell Div. 2015;10:3. doi: 10.1186/s13008-015-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park JA, Kim AJ, Kang Y, Jung YJ, Kim HK, Kim KC. Deacetylation and methylation at histone H3 lysine 9 (H3K9) coordinate chromosome condensation during cell cycle progression. Mol Cells. 2011;31(4):343–349. doi: 10.1007/s10059-011-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehrbrecht A, Muller U, Wolter M, et al. Comprehensive genomic analysis of desmoplastic medulloblastomas: identification of novel amplified genes and separate evaluation of the different histological components. J Pathol. 2006;208(4):554–563. doi: 10.1002/path.1925. [DOI] [PubMed] [Google Scholar]
- 62.Yang ZQ, Imoto I, Fukuda Y, et al. Identification of a novel gene, GASC1, within an amplicon at 9p23–24 frequently detected in esophageal cancer cell lines. Cancer Res. 2000;60(17):4735–4739. [PubMed] [Google Scholar]
- 63.Gregory BL, Cheung VG. Natural variation in the histone demethylase, KDM4C, influences expression levels of specific genes including those that affect cell growth. Genome Res. 2014;24(1):52–63. doi: 10.1101/gr.156141.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrari-Amorotti G, Chiodoni C, Shen F, et al. Suppression of invasion and metastasis of triple-negative breast cancer lines by pharmacological or genetic inhibition of slug activity. Neoplasia. 2014;16(12):1047–1058. doi: 10.1016/j.neo.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Z, Sun C, Li F, Han J, Li X, Song Z. Overexpression of histone demethylase JMJD5 promotes metastasis and indicates a poor prognosis in breast cancer. Int J Clin Exp Pathol. 2015;8(9):10325–10334. [PMC free article] [PubMed] [Google Scholar]
- 66.Bamodu OA, Huang WC, Lee WH, et al. Aberrant KDM5B expression promotes aggressive breast cancer through MALAT1 overexpression and downregulation of hsa-miR-448. BMC Cancer. 2016;16:160. doi: 10.1186/s12885-016-2108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kottakis F, Foltopoulou P, Sanidas I, et al. NDY1/KDM2B functions as a master regulator of polycomb complexes and controls self-renewal of breast cancer stem cells. Cancer Res. 2014;74(14):3935–3946. doi: 10.1158/0008-5472.CAN-13-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]