Abstract
Light has long been used to image the heart, but now it can be used to modulate its electrophysiological function. Imaging modalities and techniques have long constituted an indispensable part of arrhythmia research and treatment. Recently, advances in the fields of optogenetics and photodynamic therapy have provided scientists with more effective approaches for probing, studying and potentially devising new treatments for cardiac arrhythmias. This article is a review of research toward the application of these techniques. It contains (a) an overview of advancements in technology and research that have contributed to light-based cardiac applications and (b) a summary of current and potential future applications of light-based control of cardiac cells, including modulation of heart rhythm, manipulation of cardiac action potential morphology, quantitative analysis of arrhythmias, defibrillation and cardiac ablation.
Keywords: optogenetics, cardiac arrhythmia, optical therapy, pacing, photodynamic therapy
Introduction
Normally, illumination does not have an effect on the heart’s electrical activity. One way to render cardiac cells sensitive to illumination is through delivery of genes that encode light-sensitive proteins. Such proteins, called opsins, are normally expressed in animals’ eyes, as well as in certain photosensitive bacteria and algae. Some opsins form membrane-bound light-sensitive ion channels and pumps.1 In the last 15 years, researchers have been able to express opsin genes in mammalian neurons in order to control neural activity through the use of external light sources, thus introducing the field of optogenetics.2,3 Since its inception, optogenetics has become an indispensable method for neuroscience and further research has contributed to the discovery and development of a wide range of opsins. More recently, optogenetics has been applied in cardiac research,4 allowing elicitation and inhibition of cardiomyocyte electrical excitation with light.
A second way to render cardiac cells sensitive to light is through the direct insertion of fluorescent dye molecules, which damage and/or kill cells when illuminated.5 This technique is called photodynamic therapy and has been a popular method of cancer treatment, but it has not been clinically applied in the heart yet.5 Recent research suggests that photodynamic therapy can be used for cardiomyocyte-specific ablation in the heart6 in order to stop abnormal electrical conduction and treat arrhythmias.7
Research into these two methods, optogenetics and photodynamic therapy, is the subject of this review. We review (a) the scientific tools that allow researchers to interrogate and probe the heart with light and (b) the research on the use of these tools to study and treat cardiac arrhythmias.
Tools for Optical Control of the Heart
What are the tools that allow us to use light to modulate, rather than just measure, cardiac electrical activity? In this section, we discuss the scientific advancements that have enabled the use of light to induce changes in cardiac electrophysiology.
Light-sensitive proteins and molecules
Cardiac tissue can be made sensitive to light by the following two main methods: with opsin genes, used in optogenetics, and with small photosensitizer molecules, used in photodynamic therapy.
Several opsins have been introduced to cardiac cells in optogenetics experiments. One of the best-studied opsins is channelrhodopsin-2 (ChR2). ChR2 is derived from algae and is sensitive to blue light.1 When activated by illumination, it quickly becomes conductive to the flow of cations (including Na+, K+ and H+) in and out of the cell. The directionality of cation flow, and consequently of the current flow, depends on the cell membrane potential. When the membrane potential is below the reversal potential, which is 0 mV for ChR2, depolarizing current flows into the cell, while the current is outward and weaker at membrane potentials above 0 mV.8 At the cardiomyocyte resting potential (around −80 mV, depending on the species), ChR2 current is inward and leads to cardiomyocyte depolarization and excitation. Thus, ChR2 is considered an excitatory opsin. ChR2 has been used for numerous cardiac applications4 and its voltage-dependent kinetics has been well studied.8 Importantly, it has been shown that the addition of ChR2 to cells does not affect their electrical functionality in the absence of illumination.9 However, ChR2 can be toxic to cells in the long term at high expression rates.4,10
Discoveries and genetic engineering efforts have resulted in new opsins, with characteristics that make them well suited for various cardiac applications. Modifications in ChR2 have led to variants with increased photocurrent.11 A variant that has been used for cardiac research is CatCh.12 CatCh exhibits high permeability to Ca2+ flow. As a result, higher current is elicited when CatCh is stimulated by blue light compared to wild-type ChR2 and, more importantly, the illumination threshold for activation is significantly lower.13 This theoretically allows for in vivo stimulation deeper in the heart, overcoming the limitation of poor light penetration through the cardiac muscle. Yu et al.14 reported that the illumination threshold for ChR2 stimulation can further be decreased by addition of all-trans-retinal (the chromophore that imparts light sensitivity to ChR2) in ChR2-expressing cells.
Another opsin property that is significant for optical stimulation below the cardiac surface is spectral sensitivity. Red light can reach deeper in the heart than blue light.15 Therefore, use of opsins with red-shifted absorption spectrum, such as ReaChR16 and the ChR2 variant ChRimson,17 enables the use of light that penetrates deeper and can enhance optical applications.18
While ChR2 and its variants are excitatory opsins, other opsins are inhibitory. This means that, when activated at membrane voltages near the cardiomyocyte resting potential, they elicit outward current that can counteract excitatory inward current. Thus, they increase the current threshold required to activate fast sodium channels and excite the cell. Halorhodopsin, derived from archaea, is a light-sensitive chloride pump.19 Variants of the yellow light-sensitive Natronomonas pharaonis halorhodopsin (NpHR) have been used as inhibitory opsins.20 When activated, they pump Cl− anions into the cell, resulting in an outward current. Archaerhodopsin-T (ArchT), also derived from archaea, is a proton pump that is sensitive to green light; when activated, it elicits an outward current, like NpHR.21,22 The irradiance required to activate these inhibitory opsins is higher than that required for ChR2.23 Recently, anion channelrhodopsins have been discovered. These are inhibitory opsins that exhibit higher light sensitivity.24
Optogenetics can also be used to regulate signaling pathways. Melanopsin is an opsin found in the mammalian retina that normally regulates the day-and-night circadian rhythm.25 When it is expressed in pacemaker cardiomyocytes, it can allow control of the Gq pathway via illumination, with downstream cascade effects on automaticity and heart rate.26 Other opsins regulate shuttling of proteins in and out of the nucleus,27,28 allowing partial control of the gene expression. These opsins can potentially be used for the directed differentiation of stem cells or for the manipulation of ion channel expression in the heart.
A different family of opsins can be used for the control of reactive oxygen species (ROS) in the heart. ROS release leads to cell damage and cell death. KillerRed29 is a genetically encoded red light-sensitive protein,30 which produces ROS when activated. As such, it can be used to effect cardiac damage and to ablate specific regions of the heart for the study and treatment of arrhythmias. A comprehensive review of ROS-generating opsins has been written by Wojtovich and Foster.30
In addition to opsin proteins, the heart can be made sensitive to light through the insertion of small fluorescent light-sensitive molecules that are not encoded to the genome. For instance, clorin e6 is a small fluorescent dye molecule that can be inserted in cells and is sensitive to near-infrared light. When illuminated, it enhances the production of ROS.31 As such, clorin e6 has been used as a photosensitizer to enable cell ablation during photodynamic therapy.6 Other photosensitizers, including talaporfin sodium32 and methylene blue,33 have also been used for cardiac ablation applications.
Delivery of light-sensitive genes and molecules
Genes and molecules that impart light sensitivity need to be delivered in a targeted manner to cardiac cells and tissues prior to their actuation via illumination.
There are various methods to achieve gene delivery to cells in vitro, including electroporation9 and viral delivery.34 After an opsin gene is inserted in a cell’s nucleus, it becomes a part of the cell’s genome. Its transcription and translation through cellular mechanisms enable the expression of the light-sensitive protein, in addition to the normal protein expression of the cell (Fig. 1A and B).
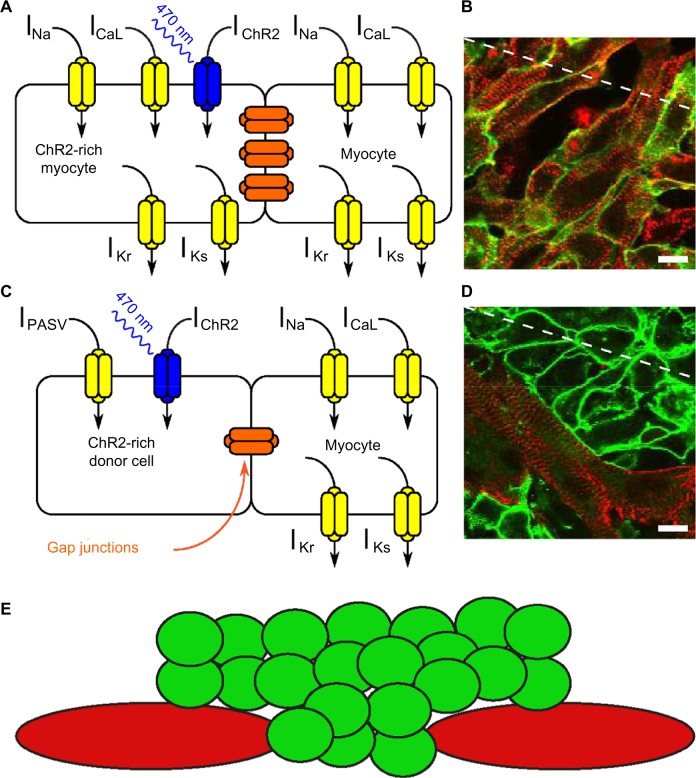
Figure 1.
(A) Simplified schematic representation of ionic currents in a myocyte expressing the opsin ChR2 following gene delivery. (B) Superimposed immunofluorescence images in a cardiomyocyte monolayer following gene delivery of ChR2. Red: α-actinin, a myocyte marker; green: ChR2, marked with the reporter protein EYFP. (C) Schematic representation describing the cell delivery approach. A light-excitable donor cell expressing ChR2 (left) is connected with a myocyte (right) by gap junctions. (D) Superimposed immunofluorescence images in a cardiomyocyte monolayer following cell delivery of ChR2-expressing donor HEK cells. Red: α-actinin, a myocyte marker; green: ChR2. (E) Two-dimensional representation of donor cell (green) distribution over a cardiomyocyte (red) monolayer. Reproduced with permission from Ambrosi et al.34
Achieving expression in the intact heart is more challenging. For experimental purposes, transgenic animals have been typically used.9,35 To generate transgenic animals, embryonic stem cells are first transfected with the gene encoding the light-sensitive protein by electroporation and then aggregated with early-stage animal embryos. Selective breeding of the resulting animals can lead to animals that have the light-sensitive protein in their genome and express it in all their cells.9
However, for potential therapeutic applications of optogenetics, the opsin genes need to be inserted into millions or billions of cells in a grown heart,36 rather than a few stem cells. A method to achieve targeted gene delivery is through the use of viral vectors. The opsin genes, along with promoters that enhance their expression in the targeted tissue, can be inserted in nonpathogenic viral vectors, such as the adeno-associated virus (AAV).37 large number of these vectors can then be inserted in the heart, either by direct injection or through an injection to the bloodstream.37,38 The viral vectors can be optimized to specifically target the heart,39 minimizing the expression in other organs.40 Gene delivery to the beating human heart has been attempted in clinical trials, with AAV delivery by coronary artery infusion.38 Another method, which is specific to the atria, involves painting the epicardium with a mixture of modified adenovirus carrying the target gene, along with a polymer gel that allows it to stick to cardiac walls and the protease trypsin, which enhances penetration of the virus through the cardiac walls.41 This procedure resulted in expression throughout the atrial walls in pig hearts but had short-term deleterious effects on atrial mechanical function.
An additional method to inscribe light sensitivity involves the delivery of donor or spark cells (Fig. 1C–E).34,42,43 These are small somatic cells (eg, fibroblasts44 or HEK cells42), which are normally not excitable but are made sensitive to light through transfection with opsin genes. They can be added to a cardiomyocyte monolayer tissue culture, where, after short incubation, they form a functional syncytium with existing cells, allowing the indirect optical control of myocytes (Fig. 1E).
Small molecules used for photodynamic therapy (ie, photosensitizers) can be inserted into the body by infusion in the blood. However, systemic delivery of photosensitizers has drawbacks, due to their non-specificity. Delivery to the skin results in skin photosensitivity.45 In addition, photosensitizers do not accumulate in cardiac cells and get cleared from the body relatively quickly. Therefore, continuous infusion near the cardiac capillaries is required to increase their concentration in the vicinity of cardiomyocytes and make them more effective for cardiac ablation.32
Nanoparticle-mediated delivery can be used to increase photosensitizer delivery to the heart. Organic nanoparticles can bind multiple photosensitizer molecules. In addition, they can bind small targeting proteins, such as the cardiac targeting peptide,46 which selectively targets cardiomyocytes, allowing delivery of the photosensitizers to specific cells. The nanoparticles can be injected intravenously and will localize in the heart, as long as they are small enough to move through the cardiac capillaries.6
Illumination
The light-sensitive proteins and molecules and the delivery methods described earlier enable the photosensitization of cardiac tissue for light-based cardiac therapies. To control the photosensitized cardiac tissue, light sources of various kinds (eg, LED, CFL and laser) can be used, as long as the wavelength of the emitted light matches the absorption spectrum of the light-sensitive proteins and molecules. The preferred method of light delivery depends on the specific application.
In vitro experiments, which involve illumination of cardiomyocyte monolayers, utilize epifluorescent illumination in setups similar to those used for cellular imaging.42 The light source can be set to illuminate the whole cell layer or only a subset thereof. The timing, duration and frequency of these light pulses can be controlled via a computer. Complex spatial and temporal illumination patterns allow investigation of cardiac cell behavior under specific circumstances for various light-based applications.13,47 Similar illumination techniques can be applied for small vertebrates, such as embryonic zebrafish,35 whose hearts are small enough to fit inside a microscope’s field of view.
Light delivery to mammalian hearts can be performed with the use of an optical fiber, which allows delivery of light from a laser or an LED source to a specific part of the cardiac tissue. For experiments in vivo, the tip of an optical fiber can be placed on the animal heart epicardial wall following thoracotomy. In addition, the heart can be isolated and perfused in a Langendorff preparation (Fig. 2A),48 which enables the stabilization of the heart, removes the complications of neural and hormonal feedback from a living animal and allows easier access of an optical fiber to all areas of the epicardial wall. These in vivo and ex vivo techniques have been applied in mice and rats in optogenetics experiments9,40,49–51 and also in larger mammals (pigs,52 dogs,32 and sheep6) for cardiac photodynamic therapy studies.
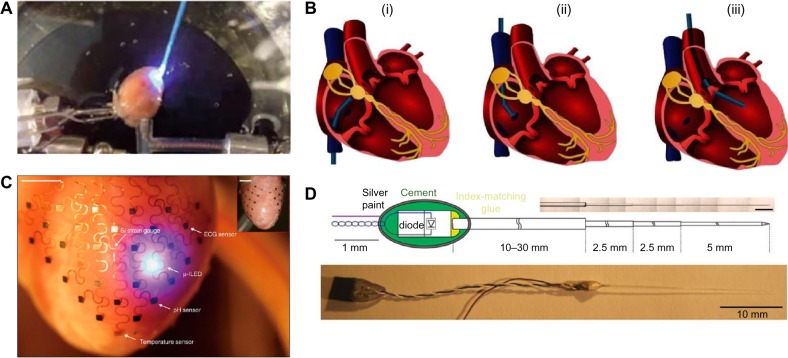
Figure 2.
(A) Illumination of Langendorff-perfused mouse heart ex vivo. Reproduced from Richter et al.51 © 2016, Springer Science+Business Media New York, with permission of Springer. (B) Schematic representation illustrating endoscopic access routes for illumination of the human heart through the inferior/superior vena cava (i/ii) or the aorta (iii). Reproduced with permission from Klimas and Entcheva.53 (C) Integumentary 3D-printed membrane on the epicardium of a rabbit heart with sensors and a micro-LED. Reproduced by permission from Macmillan Publishers Ltd, from Xu et al.54 © 2014. (D) Design of a light-emitting diode-optical fiber assembly. Reproduced with permission from Stark et al.55
Illumination of the endocardial walls is more challenging. It requires endoscopic methods, typically involving a catheter, to place an optical fiber tip inside the heart.53 The catheter can be inserted through an artery or a vein and reaches the left ventricle via the aorta or the right atrium via the vena cava (Fig. 2B). Illumination through endoscopy is a less invasive method, as it does not require opening the chest and, therefore, would constitute a better choice for therapeutic applications. However, it has not yet been applied in optogenetics experiments in animals.
A long-term therapeutic application of optogenetics, eg, an optical pacemaker, would require a different kind of optical apparatus, as an external light source would be not be feasible in that case. Implantable micro-LEDs offer a potential approach for long-term illumination at a rate and intensity controlled by a microprocessor. Such light sources could be held in place by 3D-scaffolded integumentary membranes (Fig. 2C), along with sensors and other electronics, or implanted deep in the tissue (Fig. 2D).54,55
Current progress in biocompatible optoelectronics has led to the development of bioabsorbable implantable microelectronics56 and optical waveguides.57 Such technologies enable short-term implantation of the optoelectronic apparatus needed for optogenetic interrogation or photodynamic ablation of the heart and can eliminate the need for surgical removal of illumination devices after an optical procedure.
Modeling approaches
Computational modeling is an important part of cardiac research and has numerous applications, from the research of ionic currents to the optimization of common clinical procedures.58–66 Computational simulations of cardiac electrophysiology span multiple scales: from the single protein to the cellular, tissue and finally organ level.
For cardiac optogenetics, protein-scale modeling is concerned with simulating the photocycles of light-sensitive proteins and the currents elicited by opsin activation. ChR2 is the light-sensitive protein whose light activation8,67,68 and electrical kinetics8 have been most closely studied. Simple models of other opsins, such as NpHR, have also been developed.18,69
At the cellular scale, the effect of currents elicited by illumination of opsins on the cardiac action potential (AP) is delineated. Differences between inscription of light sensitivity by gene delivery and cell delivery must also be modeled at this scale.34 To study the effects of gene delivery, the kinetics of the opsin current can be integrated into a system of ordinary differential equations, which describes the kinetics of the cardiomyocytes’ native ion channels.8,70,71 Cell delivery methods can be modeled by the addition of the opsin current model to a passive model of the donor cell, without other active ionic channels. Further differential equations govern the communication of these cells with the cardiomyocytes through gap junctions.
Electrophysiological simulations at larger scales reproduce the effects of cell-to-cell conduction and the propagation of electrical activity through the cardiac muscle. Modeling cardiac optogenetics at the tissue scale takes into account the nonuniform delivery of light-sensitive genes in cardiac tissue. The expression of the light-sensitive proteins in the heart depends on the delivery method and can be modeled with a stochastic approach.72
The heterogeneity of illumination through cardiac tissue is modeled at the organ level. Illumination depends on the anatomy of the heart, for which 3D models based on magnetic resonance imaging (MRI) are available,64,73–75 and on the placement of light sources or optical fibers relative to the heart. The irradiance incident upon each cell in the heart can be estimated by calculating the light absorption and scattering in cardiac tissue.18,76,77
Realistic approximation of cardiac optogenetics requires a simulation approach that integrates modular approaches at all of the above-mentioned scales. Boyle et al.72 developed a detailed framework for the multiscale modeling of optogenetics in the heart. This framework has been validated by experiments34 and can be used to make predictions on the effects of optogenetics-based therapies in human hearts.
To our knowledge, similar models for the simulation of photodynamic therapy in the heart are not currently available. However, photodynamic therapy targeting tumors in noncardiac tissues has been modeled previously. Modeling of photodynamic therapy involves the simulation of photosensitizer delivery in the tissue78 and the calculation of illumination and light attenuation at the organ scale and aims to optimize illumination protocols.79 The optogenetics simulation framework described earlier can be adapted to model and study cardiac-specific effects of ablation based on photodynamic therapy.
Light-Based Applications in Arrhythmia Research and Treatment
Cardiac pacing
Optogenetics is a method that can be used as an alternative to current injection for in vivo pacing of the heart and cardiac resynchronization therapy. Optogenetics-based pacing allows contactless and uniform excitation with long duration stimuli, unlike electrical pacing, and is free of Faradaic reactions and their adverse effects on cardiac tissue.9 Moreover, optogenetics is expected to facilitate the selective modulation of electrophysiological properties in specific subpopulations of cells or tissue regions targeted (eg, via cardiomyocyte-specific promoter) to express light-sensitive proteins. Work conducted in simulations72 has prompted speculation that this could lead to the development of novel approaches for cardiac arrhythmia treatment.80
Several researchers have reported pacing of the intact heart with light. Bruegmann et al.9, in the first reported application of cardiac optogenetics, successfully paced the hearts of ChR2+ transgenic adult mice by applying short blue light pulses. Arrenberg et al.35 used light to pace embryonic zebrafish hearts expressing ChR2. When the sinoatrial region of zebrafish hearts was subjected to brief optical stimuli of varying frequencies, the beating rate adapted to the externally imposed frequencies. Later, Vogt et al.40 were able to optically pace normal mouse hearts after systemic gene delivery of ChR2 with AAV. They quantified excitability by pacing area and found that epicardial optical pacing of the left atrium failed to capture pacing, while optical stimulation of other areas of the heart led to successful pacing. Nussinovitch and Gepstein50 performed in vivo transfection of ChR2 by local injection of AAV in a rat heart. They then paced it in vivo, as well as ex vivo after extraction of the heart, with an optical fiber delivering blue light. Finally, Alex et al.81 demonstrated in vivo optical pacing with blue light in transgenic fruit flies (of the species Drosophila melanogaster) expressing ChR2.
Simulations have contributed to the optimization of cardiac pacing techniques. Using a computational model of the ChR2-expressing heart, Boyle et al.72 predicted that selective stimulation of the Purkinje system would lead to a significantly lower excitation threshold. Zaglia et al.49 verified these predictions in vivo. In a different model, Wong et al.71 showed that biventricular pacing, which can be used in cardiac resynchronization therapy for synchronizing the beats of the ventricles, can be achieved by illumination in two locations, one in each ventricle. Resynchronization therapy in rat hearts was later applied in experiments.50
In addition to these applications, it was shown in a simulation study82 that constant illumination can affect the inherent frequency of oscillating cells, such as those found in the sinoatrial node and the Purkinje system. The beating frequency of oscillating cells transfected with ChR2 can be potentiated externally by the intensity of the illumination. Zhu et al.83 used constant illumination to increase the heart rate of transgenic fruit fly larval hearts expressing ChR2-XXL,84 a variant of ChR2 with enhanced expression and photocurrent. They found that the heart rate increased even at low temperature and during calcium depletion, conditions that resemble those that human hearts undergo during transplant. The native beating rate of oscillating cells can also be affected by opsins that control signaling cascades. Beiert et al.26 achieved melanopsin expression in cardiomyocytes by creating transgenic mice and then applied illumination to control the Gq signaling pathway. They found that even very weak illumination (0.04 mW/mm2 irradiance) activated Gq signaling and resulted in an increase in the native heart rate.
Manipulation of the cardiac AP
Unlike typical electrical stimulation, optogenetics allows for precise manipulation of the cardiac rhythm beyond simple pacing. Modification of the length, duration and location of optical pulses, along with careful selection of light-sensitive proteins, enables the direct manipulation of the cardiac AP. This section will consider techniques used to prolong, shorten, or completely suppress the cardiac AP.
When cells transfected with excitatory opsins are at resting potential, they can be excited by illumination, as mentioned in the previous section. If illumination is applied while a cell’s membrane is depolarized, the morphology and the shape of the AP will change. Due to the voltage-dependent properties of opsin ion channels,8,85 the nature of this change is determined by the cell’s native AP morphology, as well as the timing, duration and intensity of illumination. In general, a long light stimulus will lead to the prolongation of the AP, as has been shown in simulations82,86 and experiments in vitro.87 Since excitatory opsins such as ChR2 have reversal potentials near 0 mV,8 they only create a depolarizing current when the cell’s membrane voltage is below 0 mV. Therefore, only limited AP prolongation can be achieved in adult human ventricular cardiomyocytes, in which the AP exhibits a plateau around +10 mV. The technique can be more effectively applied to cells without a prominent plateau, such as human atrial cells and neonatal rat ventricular cardiomyocytes.
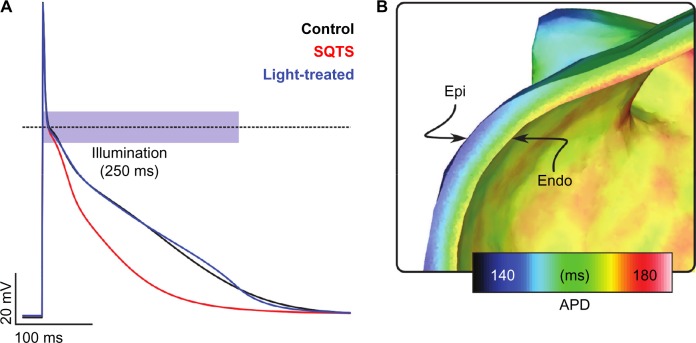
Simulations performed by our group86 suggest that a constant light stimulus of varying intensity can successfully prolong the AP in a model of ChR2-transfected atrial cardiomyocyte with abbreviated AP duration, so that it closely matches a healthy cardiomyocyte’s AP (Fig. 3A). However, the applicability of such a technique for therapeutic purposes is constrained by the limited penetration depth of blue light. We conducted electrophysiological simulations in a ChR2-expressing 3D model of the human left atrium and simulated uniform illumination of the whole endocardial wall with blue light. We observed that the AP was prolonged in a thin layer of tissue close to the light sources, while it was not significantly prolonged farther from the endocardial light sources (Fig. 3B).
Figure 3.
Simulations of cardiac AP modulation with light. (A) Human atrial cells with short QT syndrome (SQTS) are characterized by shortened AP. A ChR2-expressing cell can be treated with a controlled light pulse, so that its AP (blue) closely matches the AP of wild-type atrial cells (black). (B) Tissue-scale simulations showed AP prolongation only near the endocardium when a light pulse with similar temporal and irradiance characteristics was applied uniformly in the endocardium of the ChR2-expressing left atrium. Reproduced from Karathanos et al.86 with permission from the European Society of Cardiology.
While prolongation of the AP can be achieved with excitatory opsins, its shortening and suppression can be achieved through the use of hyperpolarizing opsins. Arrenberg et al.35 used transgenic zebrafish carrying the NpHR gene to locate the pacemaker region in embryonic zebrafish hearts. Using a precise beam focusing apparatus including a mirror system, they illuminated every region of the embryonic zebrafish heart. While illumination of other areas of the same hearts had negligible results on the heart rate, illumination of the sinoatrial region ceased all electrical activity in the zebrafish embryonic hearts, thus identifying the main pacemaking region. Nussinovitch et al.43 reported that illumination of a tissue culture transfected with ArchT fully arrested electrical activity. However, the effect of light pulses of moderate intensity (below 10 mW/mm2) on cells transfected with inhibitory opsins is not as pronounced.23,87 In particular, Park et al.87 reported that illumination of cells transfected with NpHR or ArchT prior to electrical activation led to a shorter and dampened AP. In simulations conducted by our group,18 based on parameters calculated by Nikolic et al.69, illumination of cardiac cells transfected with NpHR with 1.4 mW/mm2 orange light counteracted weak electrical pacing to keep the cell membranes at resting potential but was not effective when the excitatory electrical currents were stronger or longer. Cardiac electrophysiological experiments with some of the more recently developed hyperpolarizing opsins have not yet been attempted but may result in more effective silencing of cardiomyocytes.
Drug testing
Drugs in development need to be screened for safety prior to clinical trials. Cardiotoxicity due to arrhythmogenic alterations of the cardiac AP is a major risk for a new drug. Klimas et al.88 introduced an optogenetics-based automated technique for the investigation of drug effects on the cardiac AP. Coupled with fluorescent dyes that report the levels of intracellular Ca2+ and membrane potential, optogenetics allows for a fully optical, contactless system that can replace cumbersome patch clamp experiments.
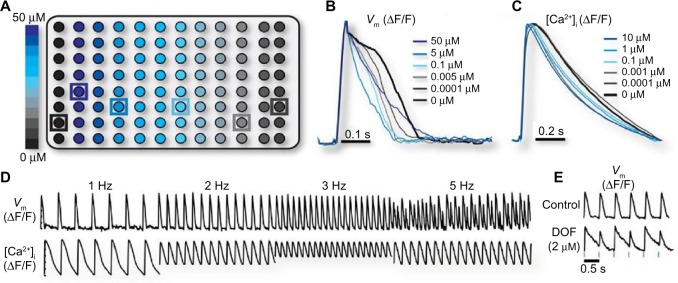
In an experimental validation of the method, isolated cardiomyocytes were placed in 96-well plates and rendered light-sensitive, either by adenovirus-mediated ChR2 gene delivery or by cell delivery, with the addition of somatic cells transfected with ChR2. Then, the plates were treated with different doses of drug compounds (Fig. 4A). Blue light illumination of the cell cultures inside each well initiated cardiac APs. The AP and the calcium transient were recorded after the addition of fluorescent dyes. The voltage-sensitive dye and the calcium-sensitive dye that were used have absorption and emission spectra that do not overlap with each other or with the absorption spectrum of ChR2, which allowed simultaneous actuation and imaging. For each of the wells, the automatic screening system produced the average voltage (Fig. 4B) and calcium (Fig. 4C) traces by averaging the levels of the voltage-sensitive and calcium-sensitive dye illumination in each well. This system allowed optical pacing at different frequencies and the detection of arrhythmogenic beat-to-beat variance in the AP, which can be the result of rapid pacing (Fig. 4D) or the addition of an AP-altering drug (Fig. 4E).
Figure 4.
(A) Schematic representation of a 96-well plate, with a ChR2-expressing culture of neonatal rat ventricular cardiomyocytes in each plate. Nifedipine, an L-type calcium blocker, was added in some of the cultures in varying concentrations (0.0001–50 µM). Action potential (B) and calcium transient (C) recordings (averaged by nifedipine concentration) during 1 Hz optical pacing of the cell cultures with blue light. (D) Membrane potential and calcium transient recordings following optical pacing at different frequencies; instabilities appear at 5 Hz optical pacing. (E) Action potentials recorded after treatment of ChR2-expressing cardiomyocytes with 2 µM dofetilide, a drug that causes AP prolongation, and 2 Hz optical pacing. Reproduced with permission from Klimas et al.88
Determining cardiac tissue excitability
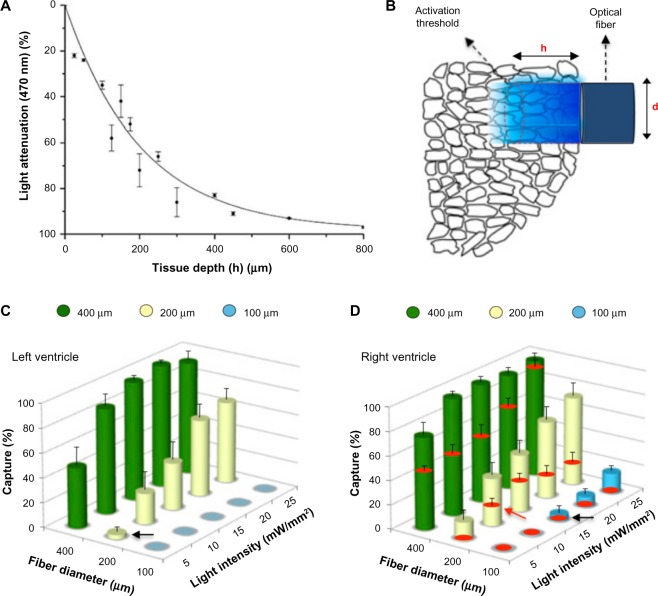
Ectopic beats originating in the ventricles can cause sustained cardiac arrhythmias. Early and delayed afterdepolarizations are AP abnormalities that increase the membrane potential of cardiomyocytes. Simultaneous depolarization of multiple cardiomyocytes can lead to a wave of propagating excitation and result in an ectopic beat. Despite its importance for studying and understanding arrhythmias, the minimum number of cardiomyocytes that need to be depolarized in order to elicit an ectopic beat cannot be identified using conventional electrical methods and has only been approximated computationally.89 Zaglia et al.49 used an optogenetics-based experimental approach to calculate this number. First, they measured the light penetration through mouse heart slices of varying thicknesses. From these measurements, they calculated the attenuation profile for blue light through cardiac tissue (Fig. 5A). Then, they used optical fibers (Fig. 5B) to illuminate the following two different groups of transgenic mouse hearts in vivo: one group with cardiomyocyte-specific expression of ChR2 and one group with Purkinje fiber-specific expression of ChR2. After illuminating hearts of mice in the former group, they determined the ectopic beat capture rate for varying values of fiber diameter and light irradiance (Fig. 5C and D). They calculated the minimum irradiance threshold to trigger a propagating ectopic beat (black arrows in Fig. 5C and D) and, by combining this information with the light attenuation profile and the previously known threshold for ChR2 excitation, they were able to estimate the volume of tissue that was light excited. They calculated that approximately 1300–1800 cells in the left ventricle or 511–570 cells in the right ventricle need to be excited in order to initiate an ectopic beat. When they repeated the same experiment in mice with Purkinje fiber-specific expression of ChR2, they estimated that only 90–160 Purkinje fiber cells need to be excited in order to induce ectopic activity, suggesting that abnormal electrical activity originating in the conduction system is more likely to cause ectopic beats.
Figure 5.
Interrogation of the minimal myocardial volume to trigger ectopic beats. (A) Attenuation of blue light at various tissue depths. (B) Schematic representation of tissue illumination by an optical fiber of diameter, d; the penetration depth, h, is a function of light intensity. (C and D) The cylinders show the capture rate of ectopic beat induction with light as a function of the fiber diameter d and the light intensity. The red disks in (D) indicate the capture after application of Lugol’s solution, which ablates the Purkinje system along with parts of the endocardium. Reproduced with permission from Zaglia et al.49
Evaluating efficiency of biological methods for restoring cardiac excitability
Biological approaches to restore cardiac tissue excitability via gene or cell therapy have been proposed,90 and some have even advanced to the stage of clinical trials,91–93 but there is no systematic way to quantitatively evaluate either the mode of delivery (viral gene delivery, GD or cell delivery, CD) or the geometric constraints (ie, clustered or diffuse spatial patterns of transgene-expressing cells) in terms of their functional impact on therapeutic outcome (ie, efficiency). Ambrosi et al.34 used experiments in cardiac cell monolayers complemented by biophysically detailed simulations to explore the feasibility of overcoming this shortcoming by directly, selectively and exclusively engaging cells expressing an optogenetic construct in order to derive a surrogate measure of the therapeutic efficiency associated with different delivery modes and spatial patterns. They measured the optical threshold required to induce propagating activation in cardiomyocyte monolayers transfected with ChR2 with different spatial parameters and used this measurement as a surrogate for the efficacy of a genetic therapy for restoration of excitability. After conducting experiments featuring gene delivery and cell delivery both in vitro and in silico, they determined that gene delivery was more effective than cell delivery, especially when the transfected cells were distributed in a uniform pattern. To confirm the applicability of this approach in the context of biological methods for restoring cardiac excitability, they used further simulations: in a computational model of a regionally heterogeneous reduction in Na+ channel expression, they directly calculated the therapeutic efficiency of GD- and CD-based restoration of Na+ (ie, similarity of activation times in treated models to control models) with a wide variety of spatial delivery patterns. Their analysis revealed that, regardless of delivery mode, there was an inverse correlation between true therapeutic efficiency and threshold optogenetic stimulation energy levels associated with different spatial patterns. This provided the first compelling validation of the concept that light-based methods could indeed be used as a surrogate tool for assessing therapeutic efficiency of such approaches in vitro and, potentially, in vivo.
Induction of arrhythmias
Optogenetics can be used to transiently induce arrhythmias in an experimental setting. Thus, it can be utilized to study the nature of various arrhythmias and test treatment modalities. The high precision of optogenetic control allows interrogation of the role of specific cells in the generation of arrhythmia, leading to useful insights for arrhythmia prevention and treatment.
Arrenberg et al.35 successfully suppressed propagation of cardiac activation in NpHR-expressing zebrafish embryos by illuminating the atrioventricular canal that connects the atria and ventricles. As a result, electrical conduction between the atria and the ventricles was blocked, leading to irregular and slower beating of the ventricles (atrioventricular block).
Zaglia et al.49 attempted to induce sustained arrhythmia in mice with cardiomyocyte-specific expression of ChR2. Initially, they were not able to induce sustained arrhythmia through rapid (10–20 Hz) optical pacing. However, after they rendered parts of the mouse ventricles ischemic (by stopping blood supply through the coronary arteries), they were able to induce sustained arrhythmias with application of the same optical stimulation protocol to the ischemic parts of the heart. Longer and more complex arrhythmias were induced after pacing the right ventricular outflow tract, a result that is consistent with observations on right ventricular outflow tract arrhythmogeneicity in humans.94
Burton et al.47 used gene delivery to express ChR2 in a cardiomyocyte monolayer. With an illumination system controlled by a computer, they were able to excite parts of the monolayer ahead of a propagating electrical wave, thus increasing the apparent conduction velocity. They used patterned blue light generated by a micromirror device to control the refractoriness of cardiac tissue and transiently stop the conduction of electrical waves in one direction (unidirectional block) or both directions (bidirectional block). In addition, they were able to induce sustained spiral waves (which, when observed in the heart, are an indicator of arrhythmia) by applying spiral wave-shaped illumination patterns. Using a different pattern of illumination on the spiral waves, they were able to stop the spiral waves altogether or reverse their chirality.
Defibrillation
Patients at high risk of life-threatening arrhythmias are currently treated with an implantable cardioverter-defibrillator, which can deliver a strong electrical shock upon detection of arrhythmia. Despite its life-saving potential, electrical defibrillation has drawbacks, the most prominent of which is the pain caused by electrical activation of tissue surrounding the heart.95–97 A defibrillation method based on optogenetics has the potential to alleviate some of those drawbacks through the selective excitation of myocytes.
Bingen et al.13 studied the potential of light to terminate arrhythmia in vitro. They expressed CatCh in neonatal rat atrial myocyte monolayers via lentiviral delivery. They then induced reentrant arrhythmia in the monolayers with rapid electrical pacing and attempted to defibrillate with illumination. A weak (0.038 mW/mm2) but long (500 ms) light pulse was sufficient to terminate reentrant electrical activity in the monolayers transfected with CatCh but not in control, non-CatCh-expressing monolayers (Fig. 6). The arrhythmias were terminated after the reentrant spiral waves drifted to the edge of the CatCh-expressing cardiomyocyte monolayer.
Figure 6.
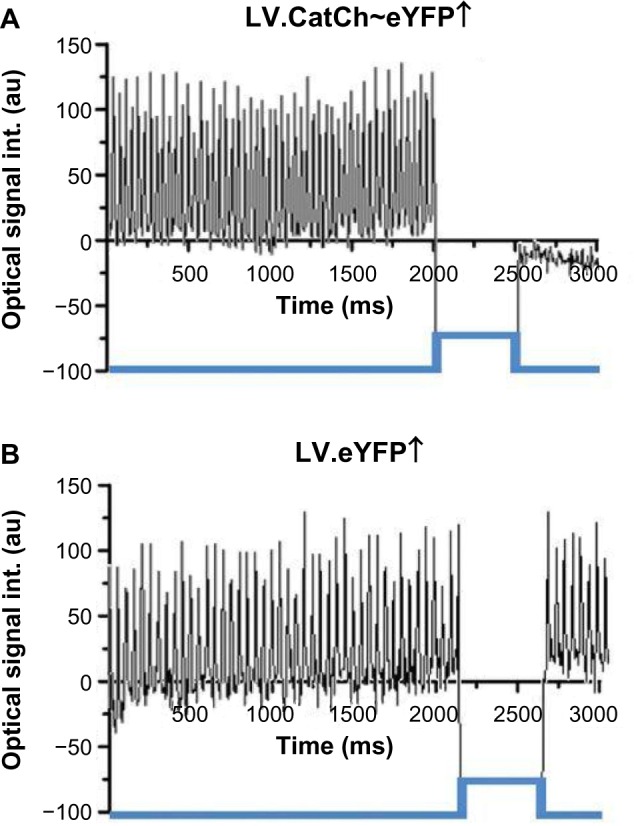
Measured electrical activity before and after blue light illumination in a cardiomyocyte monolayer with reentrant arrhythmia. (A) After CatCh transfection. (B) In the absence of CatCh. Reproduced from Bingen et al.13 with permission from the European Society of Cardiology.
Bruegmann et al.98 were able to terminate arrhythmias for the first time with epicardial illumination of ChR2-expressing mouse hearts ex vivo. A single one-second blue light pulse terminated induced reentrant arrhythmia in transgenic ChR2 mouse hearts (n = 36) 85% of the time. Similar experiments in wild-type mice transfected with ChR2 using AAV led to successful defibrillation a year after the viral injection.98
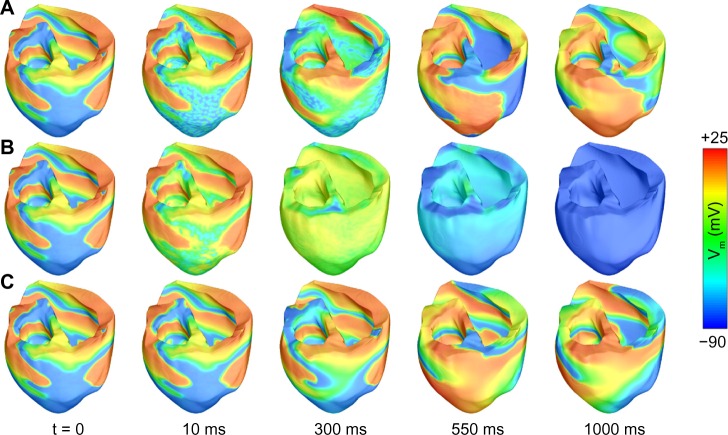
Even though arrhythmia termination with light is attainable in experiments, the translation of this method to large animals and humans is not straightforward. In a computational study, we simulated optogenetic delivery in an MRI-derived model of the failing human ventricles and demonstrated the theoretical feasibility of optogenetics-based defibrillation in clinical settings.99 Parameters regarding the efficacy of gene delivery and the expressions of opsins were extracted from animal models.40 The heart model was rendered photosensitive via systemic gene delivery. We simulated illumination through arrays of equally spaced LEDs placed on the endocardial and epicardial walls. Ventricular fibrillation (Fig. 7A), initiated by rapid pacing, was characterized by chaotic electrical activity throughout the ventricles. We attempted defibrillation with different illumination timings and durations, number of LEDs and opsin models in order to evaluate which parameters were more important to achieve successful defibrillation. The opsin characteristics were the most important determinant of defibrillation efficacy. Defibrillation attempts with the original blue light-sensitive ChR2 model failed (Fig. 7B), because light did not penetrate deep enough in the mid-myocardium. We subsequently modified the ChR2 model to make it sensitive to red light and lower its excitation threshold; these theoretical modifications were based on existing opsins, for which detailed mathematical models are not currently available. After these modifications, simulation of illumination with red light led to successful arrhythmia termination in some cases (Fig. 7C). Therefore, we highlighted the importance of discovering or designing new opsins sensitive to red light for cardiac applications. Defibrillation with red light was more effective with increased duration of illumination and higher number of LEDs on the cardiac surface. More recently, we showed that optogenetics-based defibrillation can also stop ventricular tachycardia in a different human heart model, derived from a patient with myocardial infarction.98 In other simulations, we identified that the atrial walls, which are not as thick as the ventricular walls, can be more effectively penetrated by light and, therefore, constitute a better target for arrhythmia treatment.100
Figure 7.
Simulations of defibrillation using optogenetics. (A) Ventricular fibrillation in the absence of illumination. (B) Failed defibrillation attempt with a blue light stimulus (t = 0–500 ms) after ChR2 delivery. (C) Successful defibrillation with a red light stimulus (t = 0–500 ms) after simulated delivery of a ChR2 variant with high photocurrent and red light sensitivity. Reproduced with permission from Karathanos et al.99
Ablation
Ablation is the therapy of choice for many arrhythmias, particularly in the atria.101 Catheter ablation typically involves killing heart cells by extreme heating or cooling. These methods of ablation have well-studied complications, such as cardiac rupture,102 creation of bubbles due to overheating,103 and injury of the phrenic nerve,104 which is located adjacent to the heart. Optical ablation, which can be more specific to the myocardium and less harmful to other tissues, has the potential to mitigate such risks.
Nontargeted photodynamic therapy has been used for cardiac ablation in large mammals.32,52 Kimura et al.32 delivered the red light-sensitive photosensitizer talaporfin sodium to the cardiac capillaries of adult dogs. They then used an intravenous catheter with an optical fiber to deliver illumination from a red light (663 nm) laser to excite photosensitizer molecules in each dog’s cavotricuspid isthmus, located in the right atrium. They applied point-by point illumination with a duration of 30 seconds at each point and irradiance of 100 mW/mm2 and achieved acute conduction block, which indicated successful ablation. They did not observe any adverse effects such as edema, but nonspecific application of the photosensitizer could potentially cause adverse effects such as skin photosensitivity.
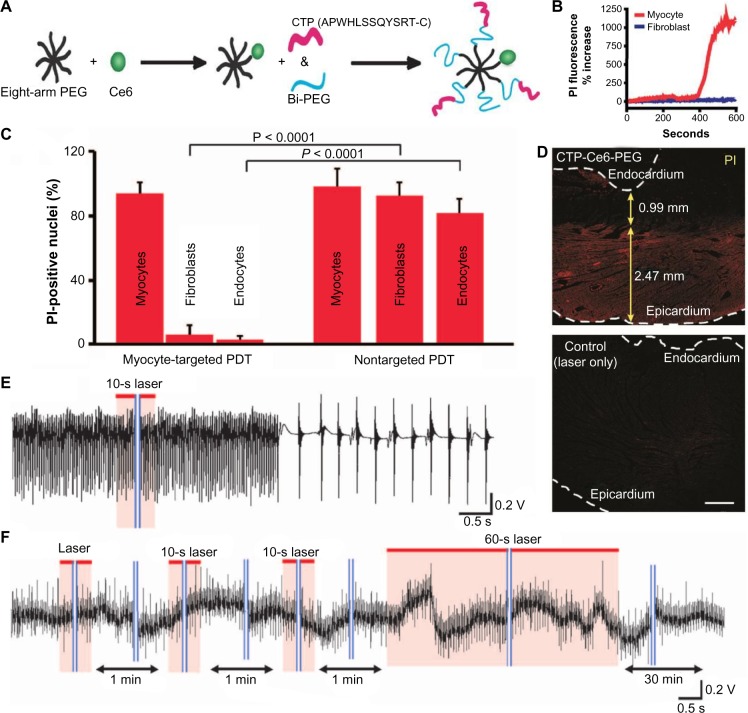
To avoid damage in remote tissues and to ensure that ablation only targets cardiomyocytes, Avula et al.6 performed nanoparticle-based delivery of photosensitizers. They loaded nanoparticles with the photosensitizer clorin e6 and rendered them specific to cardiomyocytes with the cardiac targeting peptide (Fig. 8A). After adding nanoparticles to a coculture of cardiomyocytes and fibroblasts, they observed that these nanoparticles preferentially localized in cardiomyocytes. After red light illumination, cardiomyocytes showed signs of cell death, while fibroblasts were not affected (Fig. 8B). In experiments in vivo, they performed injection of either nanoparticles or clorin e6 in the rats’ tail veins. In rats treated with nanoparticle injection, illumination of a specific area of the heart led to cell death of the cardiomyocytes only, as measured by the intensity of propidium iodide (PI), a cell death indicator. Illumination following the injection of clorin e6 only, however, led to the indiscriminate ablation of all cell types in the heart (Fig. 8C), which led to decreased viability. Epicardial illumination in the nanoparticle-treated rats led to cardiomyocyte-specific ablation of a layer of cardiac tissue (~2.5 mm thick, as seen in Fig. 8D). To test whether this ablation method can treat arrhythmias, they performed ex vivo experiments in Langendorff-perfused rat hearts. After chemically inducing atrial arrhythmia, they attempted ablation of the left atrial appendage in the presence and, as a control, in the absence of the nanoparticles. Arrhythmia was terminated within less than a minute of illumination with a red laser in the nanoparticle-treated hearts (Fig. 8E), while arrhythmia persisted in the control hearts (Fig. 8F). Finally, the applicability of this ablation technique in larger animals was shown in the same study,6 with cardiomyocyte-targeted ablation in Langendorff-perfused sheep hearts that led to conduction block in ablated zones.
Figure 8.
Myocyte-specific ablation. (A) Nanoparticle for cardiac photodynamic ablation; PEG, polyethylene glycol nanoparticle; Ce6, clorin e6; CTP, cardiac targeting peptide; Bi-PEG, heterobifunctional PEG used as a cross-linker. (B) Levels of PI, a cell death indicator, in rat myocytes and fibroblasts in vitro following treatment with nanoparticles and illumination. (C) Levels of PI in fibroblasts and endothelial cells vs cardiomyocytes following photodynamic ablation after systemic nanoparticle injection in living rat hearts. (D) PI levels (red color) following laser epicardial illumination of rat heart with (top) and without (bottom) nanoparticle infusion; scale bar: 1 mm. (E and F) Electrophysiological recordings from Langendorff-perfused atria after initiation of atrial arrhythmia; recordings shown before and after illumination of the left atrial appendage in the presence (E) or absence (F) of nanoparticles, showing successful arrhythmia termination only in the former. Reproduced from Avula et al.6 with permission from AAAS.
Cell-specific optical ablation can also be achieved with optogenetics. In particular, He et al.105 performed experiments in transgenic zebrafish embryos. Following illumination of zebrafish hearts expressing the ROS-producing opsin KillerRed, they reported significant alteration in the heart characteristics, which was attributable to photodamage induced by KillerRed. In particular, tranduced embryos exhibited marked edema and significantly reduced heart rate and contractility compared to control zebrafish embryos that were illuminated in the absence of KillerRed. To our knowledge, ablation with KillerRed or other optogenetic proteins has not been attempted as an antiarrhythmic therapy, but it offers a vehicle for studying arrhythmia and heart failure via induced heart damage.
Conclusions and Future Directions
This review summarized the main approaches for the control of cardiac electrical activity with light. Recent research in cardiac optogenetics and cardiac photodynamic therapy has enabled the development of optical systems for the study and, potentially, the treatment of cardiac arrhythmias.
Although remarkable progress in these experimental techniques has been achieved recently, further advances are required before these approaches can be applied to the beating human heart in vivo. As shown in simulation studies, research toward proteins and molecules with improved light sensitivity and red-shifted absorption spectrum could dramatically enhance the potential clinical applicability of these techniques. In addition, the efficacy and potential side effects of opsin gene delivery or photosensitizer infusion in the human heart remain to be quantified and investigated in clinical trials. Existing methods of gene delivery need to be improved upon and new modalities need to be devised in order to overcome shortcomings, such as the reported immunity to AAV delivery in part of the population.38 Finally, to fully unravel the potential of light-based arrhythmia treatment, novel illumination methods are required to robustly deliver light deep in the human heart.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 330 words, excluding any confidential comments to the academic editor.
FUNDING: This review was supported by the following grants from the US National Institutes of Health (DP1-HL123271 and R01-HL126802). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the article: TVK. Contributed to the writing of the article: TVK, PMB, NAT. Agreed with the article results and conclusions: TVK, PMB, NAT. Jointly developed the structure and arguments for the article: TVK, PMB, NAT. Made critical revisions and approved the final version: TVK, PMB, NAT. All the authors reviewed and approved the final article.
REFERENCES
- 1.Nagel G, Szellas T, Huhn W, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100(24):13940–5. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 3.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3(10):785–92. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 4.Boyle PM, Karathanos TV, Trayanova NA. “Beauty is a light in the heart”: the transformative potential of optogenetics for clinical applications in cardiovascular medicine. Trends Cardiovasc Med. 2015;25(2):73–81. doi: 10.1016/j.tcm.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis. 2010;15(9):1050–71. doi: 10.1007/s10495-010-0479-7. [DOI] [PubMed] [Google Scholar]
- 6.Avula UM, Yoon HK, Lee CH, et al. Cell-selective arrhythmia ablation for photo modulation of heart rhythm. Sci Transl Med. 2015;7(311):311ra172. doi: 10.1126/scitranslmed.aab3665. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14(4):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 8.Williams JC, Xu J, Lu Z, et al. Computational optogenetics: empirically-derived voltage- and light-sensitive channelrhodopsin-2 model. PLoS Comput Biol. 2013;9(9):e1003220. doi: 10.1371/journal.pcbi.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruegmann T, Malan D, Hesse M, et al. Optogenetic control of heart muscle in vitro and in vivo. Nat Methods. 2010;7(11):897–900. doi: 10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- 10.Miyashita T, Shao YR, Chung J, Pourzia O, Feldman DE. Long-term channelrhodopsin-2 (ChR2) expression can induce abnormal axonal morphology and targeting in cerebral cortex. Front Neural Circuits. 2013;7:8. doi: 10.3389/fncir.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15(24):2279–84. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Kleinlogel S, Feldbauer K, Dempski RE, et al. Ultra light-sensitive and fast neuronal activation with the Ca(2)+-permeable channelrhodopsin CatCh. Nat Neurosci. 2011;14(4):513–8. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- 13.Bingen BO, Engels MC, Schalij MJ, et al. Light-induced termination of spiral wave arrhythmias by optogenetic engineering of atrial cardiomyocytes. Cardiovasc Res. 2014;104(1):194–205. doi: 10.1093/cvr/cvu179. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Chen K, Lucero RV, Ambrosi CM, Entcheva E. Cardiac optogenetics: enhancement by all-trans-retinal. Sci Rep. 2015;5:16542. doi: 10.1038/srep16542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesradi M, Genoux A, Cuplov V, et al. Experimental and analytical comparative study of optical coefficient of fresh and frozen rat tissues. J Biomed Opt. 2013;18(11):117010. doi: 10.1117/1.JBO.18.11.117010. [DOI] [PubMed] [Google Scholar]
- 16.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16(10):1499–508. [Google Scholar]
- 17.Klapoetke NC, Murata Y, Kim SS, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11(3):338–46. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle PM, Karathanos TV, Entcheva E, Trayanova NA. Computational modeling of cardiac optogenetics: methodology overview & review of findings from simulations. Comput Biol Med. 2015;65:200–8. doi: 10.1016/j.compbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schobert B, Lanyi JK. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982;257(17):306–13. [PubMed] [Google Scholar]
- 20.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8(8):577–81. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 21.Han X, Chow BY, Zhou H, et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5(18):18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow BY, Han X, Dobry AS, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Entcheva E, Bub G. All-optical control of cardiac excitation: combined high-resolution optogenetic actuation and optical mapping. J Physiol. 2016;594(9):2503–10. doi: 10.1113/JP271559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. Natural light-gated anion channels: a family of microbial rhodopsins for advanced optogenetics. Science. 2015;349(6248):647–50. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panda S, Sato TK, Castrucci AM, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213–6. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 26.Beiert T, Bruegmann T, Sasse P. Optogenetic activation of Gq signalling modulates pacemaker activity of cardiomyocytes. Cardiovasc Res. 2014;102(3):507–16. doi: 10.1093/cvr/cvu046. [DOI] [PubMed] [Google Scholar]
- 27.Niopek D, Benzinger D, Roensch J, et al. Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nat Commun. 2014;5:4404. doi: 10.1038/ncomms5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niopek D, Wehler P, Roensch J, Eils R, Di Ventura B. Optogenetic control of nuclear protein export. Nat Commun. 2016;7:10624. doi: 10.1038/ncomms10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulina ME, Chudakov DM, Britanova OV, et al. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24(1):95–9. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 30.Wojtovich AP, Foster TH. Optogenetic control of ROS production. Redox Biol. 2014;2:368–76. doi: 10.1016/j.redox.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomer CJ, Ferrario A. Tissue distribution and photosensitizing properties of mono-L-aspartyl chlorin e6 in a mouse tumor model. Cancer Res. 1990;50(13):3985–90. [PubMed] [Google Scholar]
- 32.Kimura T, Takatsuki S, Miyoshi S, et al. Nonthermal cardiac catheter ablation using photodynamic therapy. Circ Arrhythm Electrophysiol. 2013;6(5):1025–31. doi: 10.1161/CIRCEP.113.000810. [DOI] [PubMed] [Google Scholar]
- 33.Avula UM, Kim G, Lee YE, Morady F, Kopelman R, Kalifa J. Cell-specific nanoplatform-enabled photodynamic therapy for cardiac cells. Heart Rhythm. 2012;9(9):1504–9. doi: 10.1016/j.hrthm.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrosi CM, Boyle PM, Chen K, Trayanova NA, Entcheva E. Optogenetics-enabled assessment of viral gene and cell therapy for restoration of cardiac excitability. Sci Rep. 2015;5:17350. doi: 10.1038/srep17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science. 2010;330(6006):971–4. doi: 10.1126/science.1195929. [DOI] [PubMed] [Google Scholar]
- 36.Adler C, Costabel U. Cell number in human heart in atrophy, hypertrophy, and under the influence of cytostatics. Recent Adv Stud Cardiac Struct Metab. 1974;6:343–55. [PubMed] [Google Scholar]
- 37.Zacchigna S, Zentilin L, Giacca M. Adeno-associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ Res. 2014;114(11):1827–46. doi: 10.1161/CIRCRESAHA.114.302331. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg B, Butler J, Felker GM, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387(10024):1178–86. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 39.Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99(4):e3–9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 40.Vogt CC, Bruegmann T, Malan D, et al. Systemic gene transfer enables optogenetic pacing of mouse hearts. Cardiovasc Res. 2015;106(2):338–43. doi: 10.1093/cvr/cvv004. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi K, McDonald AD, Sasano T, Donahue JK. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005;111(3):264–70. doi: 10.1161/01.CIR.0000153338.47507.83. [DOI] [PubMed] [Google Scholar]
- 42.Jia Z, Valiunas V, Lu Z, et al. Stimulating cardiac muscle by light: cardiac optogenetics by cell delivery. Circ Arrhythm Electrophysiol. 2011;4(5):753–60. doi: 10.1161/CIRCEP.111.964247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nussinovitch U, Shinnawi R, Gepstein L. Modulation of cardiac tissue electrophysiological properties with light-sensitive proteins. Cardiovasc Res. 2014;102(1):176–87. doi: 10.1093/cvr/cvu037. [DOI] [PubMed] [Google Scholar]
- 44.Yu J, Entcheva E. Inscribing optical excitability to non-excitable cardiac cells: viral delivery of optogenetic tools in primary cardiac fibroblasts. Methods Mol Biol. 2016;1408:303–17. doi: 10.1007/978-1-4939-3512-3_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55(1):145–57. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 46.Zahid M, Phillips BE, Albers SM, Giannoukakis N, Watkins SC, Robbins PD. Identification of a cardiac specific protein transduction domain by in vivo biopanning using a M13 phage peptide display library in mice. PLoS One. 2010;5(8):e12252. doi: 10.1371/journal.pone.0012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burton RA, Klimas A, Ambrosi CM, et al. Optical control of excitation waves in cardiac tissue. Nat Photonics. 2015;9(12):813–6. doi: 10.1038/nphoton.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50(6):940–50. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Zaglia T, Pianca N, Borile G, et al. Optogenetic determination of the myocardial requirements for extrasystoles by cell type-specific targeting of ChannelRhodopsin−2. Proc Natl Acad Sci U S A. 2015;112(32):E4495–504. doi: 10.1073/pnas.1509380112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nussinovitch U, Gepstein L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat Biotechnol. 2015;33(7):750–4. doi: 10.1038/nbt.3268. [DOI] [PubMed] [Google Scholar]
- 51.Richter C, Christoph J, Lehnart SE, Luther S. Optogenetic light crafting tools for the control of cardiac arrhythmias. Methods Mol Biol. 2016;1408:293–302. doi: 10.1007/978-1-4939-3512-3_20. [DOI] [PubMed] [Google Scholar]
- 52.Ito A, Miyoshi S, Kimura T, et al. Myocardial electrical conduction block induced by photosensitization reaction in exposed porcine hearts in vivo. Lasers Surg Med. 2011;43(10):984–90. doi: 10.1002/lsm.21136. [DOI] [PubMed] [Google Scholar]
- 53.Klimas A, Entcheva E. Toward microendoscopy-inspired cardiac optogenetics in vivo: technical overview and perspective. J Biomed Opt. 2014;19(8):080701. doi: 10.1117/1.JBO.19.8.080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu L, Gutbrod SR, Bonifas AP, et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat Commun. 2014;5:3329. doi: 10.1038/ncomms4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stark E, Koos T, Buzsaki G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J Neurophysiol. 2012;108(1):349–63. doi: 10.1152/jn.00153.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu KJ, Kuzum D, Hwang SW, et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat Mater. 2016;15(7):782–91. doi: 10.1038/nmat4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nizamoglu S, Gather MC, Humar M, et al. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat Commun. 2016;7:10374. doi: 10.1038/ncomms10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez B, Trayanova N. Upper limit of vulnerability in a defibrillation model of the rabbit ventricles. J Electrocardiol. 2003;36(suppl):51–6. doi: 10.1016/j.jelectrocard.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 59.Trayanova N, Li W, Eason J, Kohl P. Effect of stretch-activated channels on defibrillation efficacy. Heart Rhythm. 2004;1(1):67–77. doi: 10.1016/j.hrthm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Roux B, Allen T, Berneche S, Im W. Theoretical and computational models of biological ion channels. Q Rev Biophys. 2004;37(1):15–103. doi: 10.1017/s0033583504003968. [DOI] [PubMed] [Google Scholar]
- 61.Trayanova NA, Tice BM. Integrative computational models of cardiac arrhythmias – simulating the structurally realistic heart. Drug Discov Today Dis Models. 2009;6(3):85–91. doi: 10.1016/j.ddmod.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trayanova NA, O’Hara T, Bayer JD, et al. Computational cardiology: how computer simulations could be used to develop new therapies and advance existing ones. Europace. 2012;14(suppl 5):v82–9. doi: 10.1093/europace/eus277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trayanova NA, Boyle PM. Advances in modeling ventricular arrhythmias: from mechanisms to the clinic. Wiley Interdiscip Rev Syst Biol Med. 2014;6(2):209–24. doi: 10.1002/wsbm.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arevalo HJ, Vadakkumpadan F, Guallar E, et al. Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat Commun. 2016;7:11437. doi: 10.1038/ncomms11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vadakkumpadan F, Rantner LJ, Tice B, et al. Image-based models of cardiac structure with applications in arrhythmia and defibrillation studies. J Electrocardiol. 2009;42(2):157.e1–10. doi: 10.1016/j.jelectrocard.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trayanova NA, Boyle PM, Arevalo HJ, Zahid S. Exploring susceptibility to atrial and ventricular arrhythmias resulting from remodeling of the passive electrical properties in the heart: a simulation approach. Front Physiol. 2014;5:435. doi: 10.3389/fphys.2014.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hegemann P, Ehlenbeck S, Gradmann D. Multiple photocycles of channelrhodopsin. Biophys J. 2005;89(6):3911–8. doi: 10.1529/biophysj.105.069716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikolic K, Grossman N, Grubb MS, Burrone J, Toumazou C, Degenaar P. Photocycles of channelrhodopsin−2. Photochem Photobiol. 2009;85(1):400–11. doi: 10.1111/j.1751-1097.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- 69.Nikolic K, Jarvis S, Grossman N, Schultz S. Computational models of optogenetic tools for controlling neural circuits with light. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5934–7. doi: 10.1109/EMBC.2013.6610903. [DOI] [PubMed] [Google Scholar]
- 70.Abilez OJ, Wong J, Prakash R, Deisseroth K, Zarins CK, Kuhl E. Multiscale computational models for optogenetic control of cardiac function. Biophys J. 2011;101(6):1326–34. doi: 10.1016/j.bpj.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong J, Abilez OJ, Kuhl E. Computational optogenetics: a novel continuum framework for the photoelectrochemistry of living systems. J Mech Phys Solids. 2012;60(6):1158–78. doi: 10.1016/j.jmps.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boyle PM, Williams JC, Ambrosi CM, Entcheva E, Trayanova NA. A comprehensive multiscale framework for simulating optogenetics in the heart. Nat Commun. 2013;4:2370. doi: 10.1038/ncomms3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ukwatta E, Arevalo H, Rajchl M, et al. Image-based reconstruction of three-dimensional myocardial infarct geometry for patient-specific modeling of cardiac electrophysiology. Med Phys. 2015;42(8):4579–90. doi: 10.1118/1.4926428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prakosa A, Malamas P, Zhang S, et al. Methodology for image-based reconstruction of ventricular geometry for patient-specific modeling of cardiac electrophysiology. Prog Biophys Mol Biol. 2014;115(2–3):226–34. doi: 10.1016/j.pbiomolbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashikaga H, Arevalo H, Vadakkumpadan F, et al. Feasibility of image-based simulation to estimate ablation target in human ventricular arrhythmia. Heart Rhythm. 2013;10(8):1109–16. doi: 10.1016/j.hrthm.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bishop MJ, Rodriguez B, Eason J, Whiteley JP, Trayanova N, Gavaghan DJ. Synthesis of voltage-sensitive optical signals: application to panoramic optical mapping. Biophys J. 2006;90(8):2938–45. doi: 10.1529/biophysj.105.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bishop MJ, Plank G. Simulating photon scattering effects in structurally detailed ventricular models using a Monte Carlo approach. Front Physiol. 2014;5:338. doi: 10.3389/fphys.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang KK-H, Mitra S, Foster TH. Erratum: “A comprehensive mathematical model of microscopic dose deposition in photodynamic therapy” [Med Phys 34, 282–93 (2007)] Med Phys. 2008;35(9):4278. doi: 10.1118/1.2401041. [DOI] [PubMed] [Google Scholar]
- 79.Cassidy J, Betz V, Lilge L. Treatment plan evaluation for interstitial photodynamic therapy in a mouse model by Monte Carlo simulation with FullMonte. Front Phys. 2015;6(3) doi: 10.3389/fphy.2015.00006. [DOI] [Google Scholar]
- 80.Boyle PM, Entcheva E, Trayanova NA. See the light: can optogenetics restore healthy heartbeats? And, if it can, is it really worth the effort? Expert Rev Cardiovasc Ther. 2014;12(1):17–20. doi: 10.1586/14779072.2014.864951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alex A, Li A, Tanzi RE, Zhou C. Optogenetic pacing in Drosophila melanogaster. Sci Adv. 2015;1(9):e1500639. doi: 10.1126/sciadv.1500639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams JC, Entcheva E. Optogenetic versus electrical stimulation of human cardiomyocytes: modeling insights. Biophys J. 2015;108(8):1934–45. doi: 10.1016/j.bpj.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu YC, Uradu H, Majeed ZR, Cooper RL. Optogenetic stimulation of Drosophila heart rate at different temperatures and Ca2+ concentrations. Physiol Rep. 2016;4(3):e12695. doi: 10.14814/phy2.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dawydow A, Gueta R, Ljaschenko D, et al. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc Natl Acad Sci U S A. 2014;111(38):13972–7. doi: 10.1073/pnas.1408269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Entcheva E, Williams JC. Channelrhodopsin2 current during the action potential: “Optical AP Clamp” and approximation. Sci Rep. 2014;4:5838. doi: 10.1038/srep05838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karathanos TV, Boyle PM, Trayanova NA. Optogenetics-enabled dynamic modulation of action potential duration in atrial tissue: feasibility of a novel therapeutic approach. Europace. 2014;16(suppl 4):iv69–76. doi: 10.1093/europace/euu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park SA, Lee SR, Tung L, Yue DT. Optical mapping of optogenetically shaped cardiac action potentials. Sci Rep. 2014;4:6125. doi: 10.1038/srep06125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klimas A, Ambrosi CM, Yu J, Williams JC, Bien H, Entcheva E. OptoDyCE as an automated system for high-throughput all-optical dynamic cardiac electrophysiology. Nat Commun. 2016;7:11542. doi: 10.1038/ncomms11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99(5):1408–15. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circ Res. 2012;110(5):777–93. doi: 10.1161/CIRCRESAHA.111.252981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jessup M, Greenberg B, Mancini D, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124(3):304–13. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs. autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malliaras K, Makkar RR, Smith RR, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63(2):110–22. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dobrzynski H, Anderson RH, Atkinson A, et al. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol Ther. 2013;139(2):260–88. doi: 10.1016/j.pharmthera.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 95.Pelletier D, Gallagher R, Mitten-Lewis S, McKinley S, Squire J. Australian implantable cardiac defibrillator recipients: quality-of-life issues. Int J Nurs Pract. 2002;8(2):68–74. doi: 10.1046/j.1440-172x.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 96.Jayam V, Zviman M, Jayanti V, Roguin A, Halperin H, Berger RD. Internal defibrillation with minimal skeletal muscle activation: a new paradigm toward painless defibrillation. Heart Rhythm. 2005;2(10):1108–13. doi: 10.1016/j.hrthm.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 97.Marcus GM, Chan DW, Redberg RF. Recollection of pain due to inappropriate versus appropriate implantable cardioverter-defibrillator shocks. Pacing Clin Electrophysiol. 2011;34(3):348–53. doi: 10.1111/j.1540-8159.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bruegmann T, Boyle P, Vogt C, et al. Optogenetic defibrillation of ventricular arrhythmia in mouse hearts and human simulations. J Clin Invest. 2016;126(10):3894–904. doi: 10.1172/JCI88950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karathanos TV, Bayer JD, Wang D, Boyle PM, Trayanova NA. Opsin spectral sensitivity determines the effectiveness of optogenetic termination of ventricular fibrillation in the human heart: a simulation study. J Physiol. 2016 doi: 10.1113/JP271739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boyle PM, Murphy M, Karathanos TV, et al. Pulse duration determines efficacy of arrhythmia termination via targeted optogenetic stimulation. Biophys J. 2016;110(3):585a. [Google Scholar]
- 101.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33(21):2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 102.Kono T, Kitahara H, Sakaguchi M, Amano J. Cardiac rupture after catheter ablation procedure. Ann Thorac Surg. 2005;80(1):326–7. doi: 10.1016/j.athoracsur.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 103.Eick OJ, Gerritse B, Schumacher B. Popping phenomena in temperature-controlled radiofrequency ablation: when and why do they occur? Pacing Clin Electrophysiol. 2000;23(2):253–8. doi: 10.1111/j.1540-8159.2000.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 104.Sacher F, Monahan KH, Thomas SP, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol. 2006;47(12):2498–503. doi: 10.1016/j.jacc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 105.He J, Wang Y, Missinato MA, et al. A genetically targetable near-infrared photosensitizer. Nat Methods. 2016;13(3):263–8. doi: 10.1038/nmeth.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]