Abstract
γδ T cells play critical roles in host defense against infections and cancer. Although advances have been made in identifying γδ TCR ligands, it remains essential to understand molecular mechanisms responsible for in vivo expansion of γδ T cells in periphery. Recent findings identified the expression of the inducible NO synthase (NOS2) in lymphoid cells and highlighted novel immunoregulatory functions of NOS2 in αβ T cell differentiation and B cell survival. In this context, we wondered whether NOS2 exerts an impact on γδ T cell properties. Here, we show that γδ T cells express NOS2 not only in vitro after TCR triggering, but also directly ex vivo. Nos2 deficient mice have fewer γδ T cells in peripheral lymph nodes (pLNs) than their wild-type counterparts, and these cells exhibit a reduced ability to produce IL-2. Using chemical NOS inhibitors and Nos2 deficient γδ T cells, we further evidence that the inactivation of endogenous NOS2 significantly reduced γδ T cell proliferation and glycolysis metabolism that can be restored in presence of exogenous IL-2. Collectively, we demonstrate the crucial role of endogenous NOS2 in promoting optimal IL-2 production, proliferation and glycolysis of γδ T cells that may contribute to their regulation at steady state.
Introduction
Nitric oxide (NO) is a short half-life molecule that can diffuse through the membranes and act in an autocrine or paracrine manner [1]. The production of NO is catalyzed by three distinct isoforms of nitric oxide synthase (NOS) from the amino-acid arginine. Nitric oxide synthase 2 (NOS2) is the inducible form of these enzymes and its expression is found in various cell types such as myeloid, stromal and tumor cells [2–7]. Recent studies established that primary B and αβ T lymphocytes could also express NOS2 after IL-6 stimulation or T cell receptor (TCR) triggering respectively [8–11]. While NOS2 is required for the survival of plasma cells [10], two opposite effects were described on activated CD4+ αβ T cells. Autocrine NOS2 inhibits the differentiation of murine CD4+ T cells into IL-17-producing T helper cells (Th17) [8], whereas it is required for inducing and maintaining Th17 phenotype in cells derived from human CD4+ T lymphocytes [9].
Unlike αβ T cells, γδ T cells develop early in ontogeny [12,13] and most of them do not recognized peptides bound to conventional MHC molecules [14–17]. While advances have been made in characterizing γδ TCR ligands, little is known about their activation processes in periphery, thus limiting the use of their functional properties in therapy. Due to their pleiotropic functions, γδ T cells exhibit key roles in host defense against infection, sterile stress and cancer [18,19]. γδ T cells could directly lyse target cells through the production of granzymes, cooperate with αβ T, B and dendritic cells and also secrete a large range of chemokines and cytokines (i.e. IFN-γ or IL-17). Indeed, their IFN-γ secretion triggers protective responses to the West Nile virus infection [20] and tumor immunity [21–23], whereas their IL-17 production is important to control various bacterial infections [24,25].
While NOS2 expression affects αβ T cell differentiation and plasmocyte survival, its potential impact on γδ T cells at steady state remains unexplored. The aim of this study is to explore the role of NOS2 on γδ T cell functions. To raise this issue, we analyzed the pool of γδ T cells in wild-type (WT) and Nos2-deficient (Nos2KO) mice. In order to unravel how NOS2 regulates the peripheral γδ T cell pool in vivo, we used γδ T cells, either competent or deficient for NOS2, in presence or not of chemical NOS inhibitors. We compared their capacity to proliferate in vitro and investigated for the first time their metabolism.
Materials and Methods
Mice and Ethics statement
C57BL/6J (designated as WT) mice were purchased from Harlan and Jackson Laboratories. C57BL/6J Nos2-/- (designated as Nos2KO) described elsewhere [26] were kindly provided by Dr. H-J Garchon (Inserm U1173 and University of Versailles Saint-Quentin, France) and bred locally. WT and Nos2KO mice were euthanized at 6 to 12 weeks of age by cervical elongation. The animal experiment protocol approval number is CEEA34.AB.038.12 and was delivered by the Institutional Animal Ethics Committee of the Descartes University of Paris. All mice were maintained under specific pathogen-free conditions in our animal facility which also received an approval number (A75-14-02).
Single cell suspension procedures
LNs and thymus were mechanically dissociated, homogenized, and passed through a 100 μM cell strainer in 5% (vol/vol) FCS and 0.5% EDTA in phosphate-buffered saline (PBS). For skin suspensions, ears were collected and cut in small parts and digested with 0.4 mg ml-1 liberase, 0.05 mg ml-1 collagenase D, and 0.1 mg ml-1 DNase I (Roche) for 1h at 37°C.
Culture of γδ T cells
γδ T cells were sorted from pLNs. CD4+, CD8+ and CD19+ cells were depleted using Dynabeads (Invitrogen) before a negative sorting using Aria III cytometer (BD Biosciences). Highly purity of γδ T cells with untouched TCR was obtained. γδ T cells were cultured in RPMI 1640+Glutamax (Gibco) with 10% FCS, 100 U ml-1 penicillin and streptomycin, 10 mM HEPES, 1 mM sodium pyruvate, non-essential amino acids, 50 μM 2-mercaptoethanol in 96 well plates at 37°C, 5% CO2. When indicated 15–30 U ml-1 of rIL-2 and 15 μg ml-1 rIL-7 (R&D Systems) were used. Cells were cultured on plate-bound with 0.1 μg ml-1 anti-CD3ε (145-2C11) and 10 μg ml-1 anti-CD28 (37.51) (both from eBioscience).
Antibodies
Following anti-mouse Abs were used for cytometry analysis and cell sorting: FITC–conjugated anti-B220 (RA3-632), PE–conjugated anti-δ TCR (GL3) and anti-NK1.1 (PK136), APC- conjugated anti-CD45.2 (104), anti-IL-2 (JES6-5H4), PerCP-Cy5.5 –conjugated anti-CD3 (145-2C11), anti-β TCR (H57-597), and anti-CD45.2 (104), Pacific Blue- conjugated anti-CD4 (RM4-5), APC-H7-conjugated anti-CD8 (53–6.7). Abs were purchased from BD Biosciences except anti-B220 and anti-β TCR from eBioscience. NOS2 staining was performed using a primary goat anti-mouse Ab (M19 Santa Cruz) following by anti-goat PerCP conjugated Ab (Jackson immuno research).
Following purified anti-mouse Abs were purchased from eBioscience and used to deplete cells before γδ T cells cell sorting: anti-CD19 (eBio1D3), anti-CD8 (53–6.7), anti-CD4 (GK 1.5).
Microscopy was performed using primary Abs; purified hamster anti-mouse γδ TCR (GL3, BD Biosciences) and rabbit anti-mouse NOS2 (Calbiochem). Alexa fluor 488-conjugated goat anti-rabbit (Jackson immuno research), and Alexa fluor 647-conjugated goat anti-hamster (Biolegend) were used as secondary Abs.
Cell staining and flow cytometry
Surface staining was performed by incubating cells on ice, for 20 min, with saturating concentrations of labeled Abs in PBS, 5% FCS and 0.5% EDTA. Mouse cell-staining reactions were preceded by a 15-min incubation with purified anti-CD16/32 Abs (FcγRII/III block; 2.4G2) obtained from hybridoma supernatants. Intracellular cytokine staining were performed after stimulation of single cell suspensions with Phorbol 12-myristate 13-acetate (PMA) (50 ng ml-1) (Sigma), ionomycin (0.5 μg ml-1) (Sigma) and 1 μL ml-1 Golgi Plug (BD Biosciences) for 4h at 37°C 5% CO2. Cells were incubated with Live/Dead Blue stain (Invitrogen), according to the manufacturer protocol prior to Ab surface staining. Then, intracellular staining was performed using Cytofix/Cytoperm kit (BD biosciences) following the manufacturer’s instructions. γδ T cell apoptosis were assessed ex vivo by staining pLNs for FITC-conjugated annexin V (BioLegend) according to the manufacturer’s instructions. Data files were acquired and analyzed on LSRII using Diva software (BD Biosciences).
Microscopy
First, single cell suspensions from pLNs were incubated for 15 min with purified anti-CD16/32 Abs. γδ T cell staining was performed by incubating cells with purified hamster anti-mouse γδ TCR for 20 min at 4°C. After washes, cells were incubated with the goat anti-hamster Ab for 20 min at 4°C. Then, intracellular NOS2 staining was performed using Cytofix/Cytoperm kit. Cells were incubated with anti-NOS2 Ab overnight at 4°C. After washes, cells were incubated with the goat anti-rabbit Ab for 20 min at 4°C. Then cells were centrifuged onto a microscope slide using Cellspin 1 (Tharmac). Slides were washed in PBS before being labeled with DAPI. Slides were mounted in Vectashield mounting medium (Vector Labs). Images were acquired using an automated high-resolution scanning system (Lamina, PerkinElmer) with 40X objective. Images were analyzed with Pannoramic Viewer (3DHISTECH).
Functional assays
For γδ T cell proliferation, cells were labeled with 2.5 μM CFSE (Molecular Probes) at 37°C for 7 min. Cells were seeded in a 96-well plate pre-coated with anti-CD3ε and anti-CD28. After incubation at 37°C for 48h, cells were stained with Live/Dead Blue Stain (Invitrogen) before being analyzed by flow cytometry for CFSE dilution. When indicated 15 U ml-1 rIL-2 (R&D Systems) or 10 mM L-NMMA (Calbiochem), 0.5 mM N6-(1-iminoethyl)-l-lysine dihydrochloride (L-NIL, Calbiochem) were added to the cultures.
For metabolic assays, γδ T cells were sorted from WT and Nos2KO mice and cultured for 4 days in 0.1 μg ml-1 anti-CD3ε, 10 μg ml-1 anti-CD28, 15 μg ml-1 rIL-7 and 15 U ml-1 rIL-2. Metabolism analyses were performed directly or after 18h of resting followed by 4h of stimulation in media containing 5 mM L-NMMA and or 15 U ml-1 rIL-2 when indicated. 5.105 cells per well were plated in Seahorse plates coated with CellTak (Corning). The OCR and ECAR were measured in XF medium (unbuffered RPMI containing 2 mM glutamine, pH 7.4) under basal conditions and in response to glucose (25 mM), oligomycin (1 μM), FCCP (1.5 M) plus pyruvate (1 mM) and antimycin A (1 μM) plus rotenone (0.1 μM) with an XF-24 Extracellular Flux Analyzer (Seahorse Bioscience). Rate of glycolysis was defined as the difference between ECAR following the injection of glucose and the basal ECAR reading.
Protein quantification
Quantitative determination of IL-2 were performed in culture supernatants during γδ T cell proliferation experiments using kit from R&D Systems according to supplier instructions.
Statistics
Data are expressed as mean ± SEM. The significance of differences between two series of results was assessed using the Mann-Whitney test. (*, p < 0.05; **, p < 0.01; ***, p < 0.001). All statistical analyses were performed using Prism 5 software (GraphPad).
Results
NOS2 regulates the pool of peripheral lymph node γδ T cells in vivo
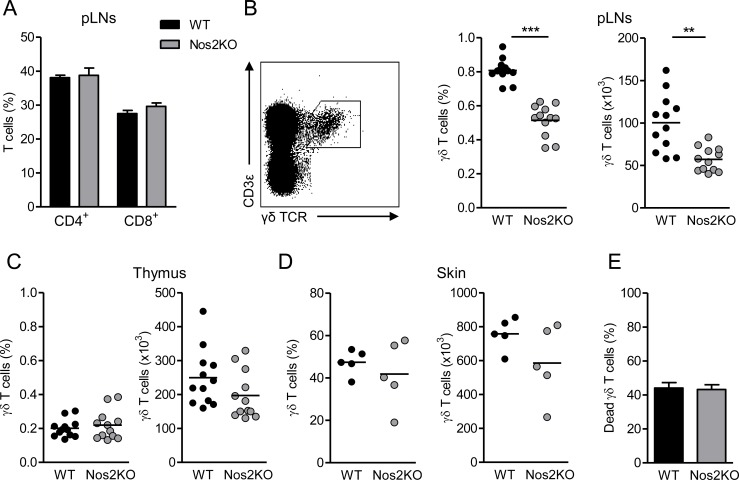
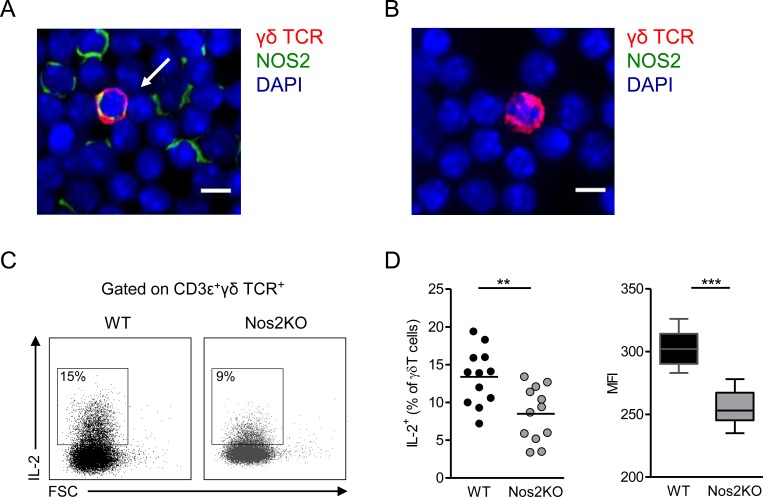
We have recently shown that NOS2 improves the recruitment of γδ T cells within the tumor microenvironment in a murine model of spontaneous melanoma [27]. Here we wondered whether NOS2 also impacts γδ T cell proportions at steady state. While the pools of CD4+ and CD8+ αβ T cells are similar in peripheral LNs (pLNs) of Nos2KO and WT mice (Fig 1A), the proportion and absolute number of γδ T cells were significantly reduced in pLNs of Nos2KO mice compared to WT animals (Fig 1B). Thymus and skin contained the same γδ T cell pool excluding both thymus retention and skin relocalization of γδ T cells in Nos2KO mice (Fig 1C and 1D). Moreover, similar percentages of pLN derived γδ T cells positive for annexin V were observed in both groups (Fig 1E), indicating that NOS2 deficiency did not increase γδ T cell death. To assess whether the difference of γδ T cell pool observed in vivo may be due to NOS2 expression by γδ T cell themselves at steady state, we performed double stainings from single cell suspensions directly ex vivo. As illustrated in Fig 2A, we detected few double stained cells in pLNs of WT mice, whereas no γδ T cell positive for NOS2 was found in pLNs derived from Nos2KO mice (Fig 2B). Next, we compared the ability of peripheral γδ T cells to produce IL-2 known to promote γδ T cell expansion [28]. Consistent with the reduced pool of γδ T cells in Nos2KO mice, γδ T cells from these mice produced significantly less IL-2 than their WT counterparts in response to PMA/ionomycin stimulation (Fig 2C and 2D). Both the percentage of IL2+ among γδ T cells and their capacity to produce IL-2 as revealed by mean fluorescence intensity (MFI) are reduced in Nos2KO mice (Fig 2D). These results suggest that NOS2 regulates the pool of γδ T cells in vivo through an IL-2 dependent mechanism.
Fig 1. Influence of NOS2 deficiency on γδ T cells in vivo.
(A) Percentages of CD4+ and CD8+ αβ T cells from pLNs of WT (n = 12) and Nos2KO (n = 12) mice. (B-D) Representative dot plot of peripheral γδ T cells defined as CD3ε+TCRγδ+ cells (B—left). Percentages and absolute numbers of γδ T cells from pLNs (B), thymus (C) and skin (D) of WT (n = 12, except for E n = 5) and Nos2KO (n = 12, except for E n = 5) mice. (E) Proportion of dead γδ T cells from pLNs of WT (n = 10) and Nos2KO (n = 9) mice analyzed ex vivo by annexin V staining. Data are pooled from one (D), two (B, C) or three (A, E) experiments. Bars are mean or mean ± SEM and each point represents one mouse. ** p<0.01, *** p<0.001 (Mann-Whitney’s test).
Fig 2. Effect of NOS2 deficiency on IL-2 production by pLNs γδ T cells.
(A-B) Representative microscopy images showing a γδ T cell positive (A) or negative (B) for NOS2 derived respectively from pLNs of WT and Nos2KO mice and stained with antibodies to TCR γδ (red), NOS2 (green) and counterstained with DAPI (blue). The arrow indicates a NOS2+ γδ T cell. Bars 10 μM. 40 X objective. The experiment was performed from WT (n = 3) and Nos2KO (n = 2) mice. (C-D) IL-2 production by γδ T cells after 4h PMA/ionomycin stimulation of pLNs from WT (n = 12) and Nos2KO (n = 12) mice. (C) Representative dot plots of IL-2 staining among γδ T cells in WT and Nos2KO mice. (D) Percentages of IL-2+ cells among γδ T cells are shown in left and geometric mean of fluorescence intensity (MFI) in right. Data are pooled from two experiments. Point represents individual mouse, bars are mean and box and whiskers are min to max values and median. ** p<0.01, *** p<0.001 (Mann-Whitney’s test).
Autocrine NOS2 improves proliferation of pLN γδ T cells
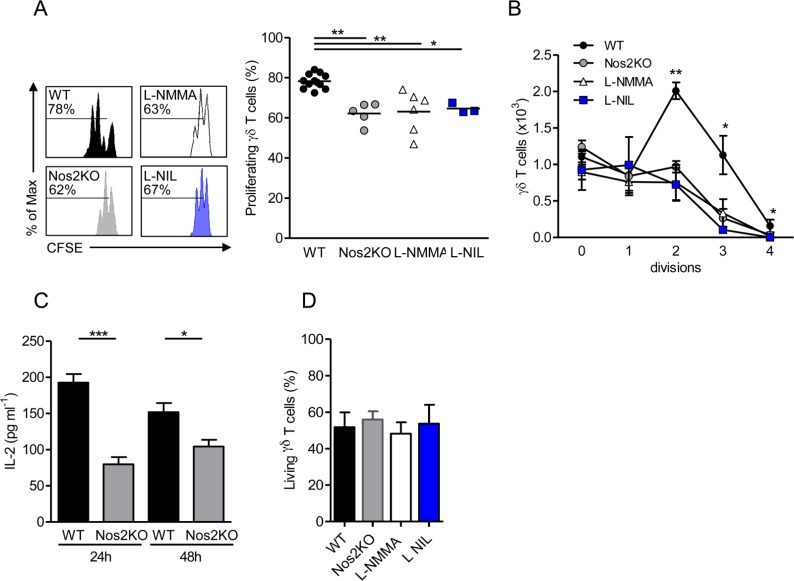
Next, we evaluated whether NOS2 is induced in response to T cell receptor (TCR) triggering for two days in vitro. We cultured cells in presence of IL-2 to expand them before the intracellular NOS2 staining and FACS analysis. γδ T cells sorted from pLNs of WT mice revealed that almost 15% of γδ T cells express NOS2 after stimulation with CD3- and CD28-specific antibodies, whereas only 2% of NOS2+ γδ T cells are detected in response to TCR sub-optimal stimulation with anti-CD28 (S1 Fig). Next, we analyzed the proliferation capacity of γδ T cells in vitro. After 48h of TCR triggering in the absence of IL-2, 78% of WT γδ T cells proliferated as determined by CFSE dilution, whereas only 62% of NOS2-deficient γδ T cells did (Fig 3A). WT γδ T cells treated with L-NMMA, a NOS inhibitor, or with L-NIL, a specific NOS2 inhibitor, proliferated to the same extent as their counterparts from Nos2KO mice (Fig 3A and 3B). These results showed that NOS2 deficiency impairs the proliferation via a direct or indirect effect. Taking into account the ability of γδ T cells to express NOS2 at steady state (Fig 2A) and that our proliferation assay is performed from purified γδ T cells, our results suggest a direct effect of endogenous NOS2 on γδ T cell proliferation (Fig 3A and 3B). In agreement with data shown in Fig 2, we quantified lower IL-2 levels in supernatants of NOS2-deficient γδ T cells after 24h and 48h of culture (Fig 3C). In addition, the decreased percentage of proliferation in NOS2-deficient cells or following L-NMMA and L-NIL treatments was not due to higher cell mortality (Fig 3D). Taken together our data suggest that NOS2 expression in γδ T cells promotes their proliferation in vitro that may explain the lesser γδ T cell number observed in vivo in Nos2 deficient mice.
Fig 3. Effect of autocrine NOS2-derived NO on γδ T cell proliferation.
γδ T cells sorted from pLNs of WT or Nos2KO mice and labeled with CFSE were cultured for 2 days in presence of CD3- and CD28-specific antibodies, 0,5 mM L-NIL and 10 mM L-NMMA when indicated. (A) Representative histograms of CFSE dilution. Numbers above line on CFSE plots indicate percent of proliferating cells (left). Percentages of γδ T cell proliferation undergoing division (right). (B) Number of γδ T cells by division. (C) IL-2 levels quantified in supernatants by ELISA after 24h and 48h of γδ T cell cultures. (D) Proportion of living γδ T cells after 48h culture. Data are from five independent experiments with 12 WT, 5 Nos2KO, 3 L-NIL and 6 L-NMMA replicates. Point represents individual replicate, bars mean ± SEM (except in B right mean) * p<0.05 ** p<0.01, *** p<0.001 (Mann-Whitney’s test).
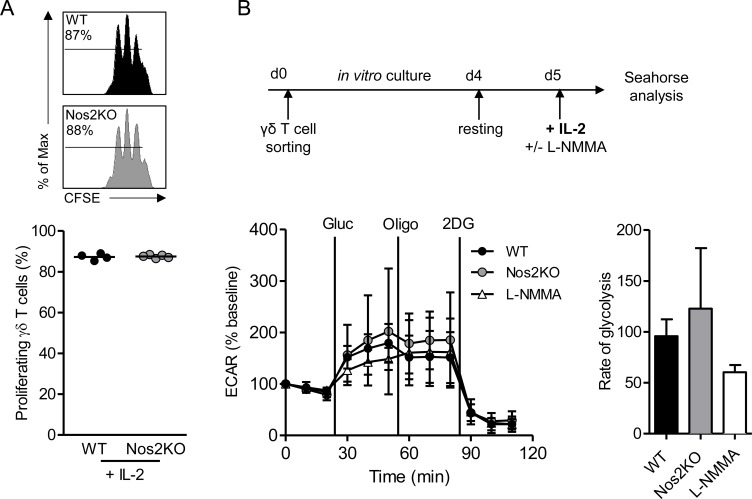
Autocrine NOS2 up-regulates glycolytic metabolism in pLN γδ T cells
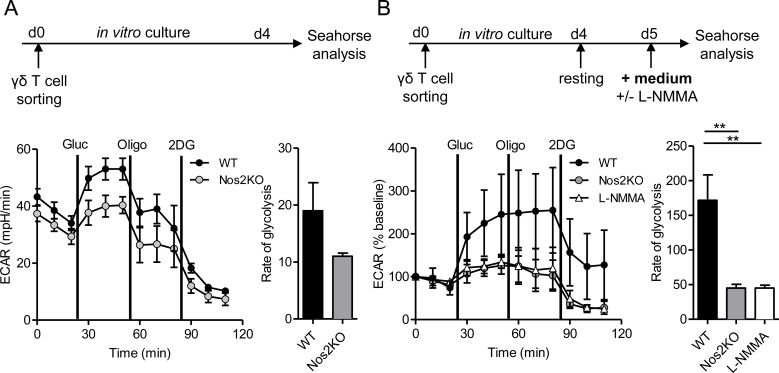
To investigate whether the reduced proliferation capacity of NOS2-deficient γδ T cells relies on a metabolic failure, we compared the metabolic phenotypes of γδ T cells from WT and Nos2KO mice. We evaluated the oxygen-consumption rate (OCR) and extracellular acidification rate (ECAR), proportional to mitochondrial respiration and aerobic glycolysis, respectively. WT and NOS2-deficient γδ T cells displayed the same OCR at basal level and after addition of metabolic inhibitors (S2 Fig), suggesting that mitochondrial respiration is NOS2 independent. Both γδ T cells displayed increased ECAR following glucose addition (Fig 4A), but the glycolysis rate was about two fold less for NOS2-deficient γδ T cells (Fig 4A). Oligomycin was added to promote maximal ECAR. However, γδ T cells, expressing NOS2 or not, did not show any ECAR increase, indicating that they had already reached their maximal glycolytic capacity after glucose addition. Because of high levels of ECAR at baseline, γδ T cells were then starved for 18h before metabolic analysis (Fig 4B). WT γδ T cells performed significantly more glycolysis than NOS2-deficient γδ T cells or L-NMMA treated WT γδ T cells (Fig 4B) revealing a role of endogenous NOS2 in the control of this metabolic pathway. Collectively, these data show that autocrine NOS2 is essential for optimal glycolysis of γδ T cells and suggest that a more efficient glycolysis in NOS2 expressing γδ T cells may contribute to their better capacity to proliferate.
Fig 4. Glycolytic metabolism assessed in competent or NOS2 deficient γδ T cells.
Sorted γδ T cells from pLNs of WT or Nos2KO mice were expanded in vitro for 4 days in presence of CD3- and CD28- specific antibodies, 15 μg/mL IL-7 and 15U/mL IL-2. Glycolytic metabolism was analyzed using a Seahorse XF-24 analyzer either directly (A), or after 18 h of resting followed by an additional 4h of stimulation with media containing 5 mM L-NMMA when indicated (B). ECAR was assessed after glucose (gluc) addition and in response to metabolic inhibitors oligomycin (oligo) and 2-Deoxy-D-glucose (2DG). Shown are time courses (A left), normalized time courses as % of baseline (B left) and calculations of rate of glycolysis (A, B right panels). Data are from one experiment with 3 (Nos2KO) and 4 (WT) replicates (A) and are pooled from three independent experiments with 7 (WT), 5 (Nos2KO) and 5 (L-NMMA) replicates (B). Mean ± SEM are shown. ** p < 0.01 (Mann-Whitney’s test).
IL-2 overrides the impairment of proliferation and glycolysis in NOS2-deficient γδ T cells
To assess if the deficiency of proliferation and glycolysis in NOS2-deficient γδ T cells could be rescued, γδ T cells were respectively cultured or stimulated after resting with IL-2 (Fig 5A and 5B). NOS2-deficient γδ T cells proliferate as efficiently as WT γδ T cells after addition of exogenous IL-2 at the beginning of the culture (Fig 5A). The IL-2 stimulation globally increased ECAR at basal levels and after glucose and oligomycin addition in all conditions tested (S3 Fig). Interestingly, WT γδ T cells, L-NMMA treated WT γδ T cells, and NOS2-deficient γδ T cells displayed similar ECAR and glycolysis rates (Fig 5B), indicating that exogenous IL-2 rescued the impairment of glycolytic metabolism in NOS2-deficient γδ T cells. These results demonstrate that IL-2 reverses glycolysis and proliferation defects in NOS2-deficient γδ T cells.
Fig 5. Proliferation and glycolysis of NOS2-deficient γδ T cells in presence of IL-2.
(A) γδ T cells sorted from pLNs of WT and Nos2KO mice and labeled with CFSE were cultured for 2 days in presence of CD3 and CD28-specific antibodies and 15U/mL IL-2. Numbers above line on CFSE plots indicate percent of proliferating cells. Percentages of γδ T cell proliferation are shown below. Data are representative of two experiments with 4 WT and 5 Nos2KO replicates. (B) Sorted γδ T cells from pLNs were expanded in vitro for 4 days in presence of CD3 and CD28-specific antibodies, 15 μg/mL IL-7 and 15U/mL IL-2. ECAR was analyzed after 18h of resting followed by an additional 4h of stimulation with 15U/mL IL-2. Media contain 5 mM L-NMMA when indicated. Shown are normalized time courses as % of baseline and calculations of rate of glycolysis Data are pooled from three independent experiments with 7 (WT), 5 (Nos2KO) and 5 (L-NMMA) replicates Mean ± SEM are shown. (Mann-Whitney’s test).
Discussion
In this study, we show that NOS2 favors IL-2 production by γδ T cells that may regulate their expansion in vivo. To support these results, we demonstrate the crucial role of NOS2 in promoting efficient glycolysis and proliferation of γδ T cells in vitro.
To our knowledge, we provide the first evidence that primary murine γδ T cells display the capacity to express NOS2 in situ and in vitro after TCR activation at steady state. Two previous studies investigated the function of autocrine NO production by human γδ T cells and produced conflicting results. The NOS3 protein, but neither NOS1 nor NOS2, was detected in γδ T cells and endogenous NOS3-derived NO was involved in γδ T cell protection from apoptosis [29]. On the other hand, NOS2 expression was found in γδ T cells upon stimulation with heat shock protein that led to their apoptosis through the mitochondrial death pathway [30]. Our study was performed on primary γδ T cells, whereas the quoted studies used γδ T cell lines. This difference may explain why we failed to observe any effect on γδ T cell apoptosis, but rather highlighted an effect on γδ T cell expansion.
Recently, we detected NOS2 expressing γδ T cells within the primary tumor of melanoma patients, but also in the primary tumor and tumor draining LNs in mice transgenic for the Ret oncogene developing a spontaneous metastatic melanoma (model Ret) [27]. γδ T cells in Ret mice lysed less efficiently tumor cells, and produced less IFN-γ than their counterparts in Ret mice deficient for NOS2. NOS2 also promoted γδ T cell polarization toward a pro-tumorigenic profile, in particular through regulating the balance between CD27-/CD27+ favoring their capacity to produce IL17. Collectively, our data indicate that NOS2 drives the expansion of γδ T cells in pLNs at steady state, but may also support, at least in part, their accumulation within the tumor microenvironment.
In vitro TCR activation results in the expression of NOS2 by murine CD4+ T cells [8]. Nevertheless and consistent with a previous study, we found a similar proportion of peripheral CD4+ T cells in WT and in Nos2KO mice [8]. In striking contrast, we show that NOS2 regulates the pool of peripheral γδ T cells in vivo by enhancing their IL-2 production. Recently, Niedbala et al found a link between NO and the up-regulation of IL-2 in Th9 cells, an αβ T cell subset, by investigating the effect of NO donor on Th9 polarization in vitro. Indeed, nitrosylation of cysteine residues induces a signaling cascade leading to higher IL-2 production by Th9 cells [31]. Although no evidence for Th9 cell-intrinsic expression and function of NOS2 was provided, it is tempting from these data and ours to envisage that such a NOS2 dependent post-translational modification drives IL-2 production in γδ T cells.
Proliferating T cells require adaptation of their metabolic programs to provide sufficient biosynthetic precursors and adequate energy. In particular, activated αβ T lymphocytes up-regulate aerobic glycolysis to rapidly grow and divide [32]. Here, we found that endogenous NOS2 enhanced glycolysis in γδ T cells consistent with their efficient ability to proliferate in vitro. The effects of NOS2 on immune cell metabolism remain poorly described. Nevertheless, NOS2-derived NO was identified as a metabolic regulator in inflammatory DC [33]. Autocrine NO production by DC leads to a switch towards glycolysis as a consequence of inhibition of mitochondrial respiration. Glycolysis is linked to availability of nutrients such as glucose and depends on glucose uptake. We hypothesized that NOS2 defective γδ T cells express fewer glucose transporters than WT γδ T cells, resulting in less capacity for glycolysis and subsequently reduced proliferation. To support this hypothesis, NO has been shown to enhance glucose uptake in the HEK293T cell line via up-regulation of the glucose transporter (GLUT) 3 [34]. Moreover, we show that the addition of exogenous IL-2 restored the rate of glycolysis and proliferation in NOS2 defective γδ T cells. Consistent with our results, IL-2 promotes GLUT1 expression and glucose uptake in activated αβ T cells [35–37]. Thus, NOS2, by up-regulating IL-2, could maximize expression of GLUT on γδ T cells resulting in their efficient proliferation in vivo. These hypotheses need to be investigated elsewhere.
This study show for the first time that NOS2 affects metabolism of γδ T cells. Metabolic regulations of immune cells are closely linked to their functions and changes in metabolism have been shown to enhance or suppress T cell functions and fate [36]. Understanding the metabolic regulations of αβ and γδ T cells appears to be a critical step toward the development of more efficient immunotherapy strategies based on these cells [38].
Supporting Information
Sorted γδ T cells from pLNs of WT mice were cultured for 2 days in presence of 30 U/mL IL-2 and CD3- and CD28-specific antibodies when indicated (n = 4 replicates each condition). Cells were stained for NOS2 and a viability marker. Flow cytometry representative of NOS2 staining (left) and percentages of NOS2+ γδ T cells among living cells (right) are shown. Numbers above line indicate percent of NOS2+ γδ T cells. * p<0.05 (Mann-Whitney’s test).
(TIF)
Sorted γδ T cells from pLNs of WT and Nos2KO mice were expanded in vitro for 4 days in presence of CD3 and CD28- specific antibodies, 15 μg/mL IL-7 and 15U/mL IL-2. Metabolism was analyzed using a Seahorse XF-24 analyzer. OCR was assessed in response to mitochondrial inhibitors: oligomycin (oligo), Carbonyl cyanide 4—(trifluoromethoxy) phenylhydrazone (FCCP), and rotenone and antimycin A (Rot/AntiA). Shown are time courses. Data are from one experiment with 3 (Nos2KO) and 4 (WT) replicates.
(TIF)
γδ T cells sorted from pLNs of WT and Nos2KO mice were expanded in vitro for 4 days in presence of CD3 and CD28-specific antibodies, 15 μg/mL IL-7 and 15U/mL IL-2. Glycolytic metabolism analysis was performed after 18 h of resting following by an additional 4 h of stimulation with media containing 5mM L-NMMA and/or 15U/mL IL-2 when indicated. ECAR was assessed after adding glucose and in response to metabolic inhibitors oligo and 2DG. Time courses are pooled from three independent experiments.
(TIF)
Acknowledgments
This work was supported by the SILAB Jean Paufique foundation. L. Douguet was supported by the “Ligue contre le Cancer” and Paris VII University. J. Cherfils-Vicini was supported by the “Fondation de France”. A. Prévost-Blondel’s team is supported by ARC and Comité “Ile de France” from Ligue contre le Cancer. CytoMed were supported by le Conseil général, FEDER, le Ministère de l’Enseignement Supérieur, la Région PACA and INSERM.
We are grateful to V. Molinier-Frenkel, Y. Richard, and F. Castellano for critical review of the manuscript. We thank B. Lucas for helpful discussions, HJ. Garchon for providing Nos2KO mice, and E. Morgan for technical support. We acknowledge the Cytometry and Immunobiology (CYBIO) and the Animal core facilities of the Cochin Institute (Paris) and the IRCAN flow cytometry facility (CytoMed, Nice).
Data Availability
All relevant data are within the paper and its Supporting Information files
Funding Statement
This work was supported by the SILAB Jean Paufique foundation. LD was supported by the “Ligue contre le Cancer” and Paris VII University. JCV was supported by the “Fondation de France”. APB’s team is supported by the french fondation "ARC" for the Cancer Research and Comité “Ile de France” from Ligue contre le Cancer. CytoMed were supported by le Conseil général, FEDER, le Ministère de l’Enseignement Supérieur, la Région PACA and INSERM.
References
- 1.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–78. Epub 2015/02/18. 10.1016/j.it.2015.01.003 . [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. Epub 2012/03/23. nri3175 [pii] 10.1038/nri3175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayaraman P, Parikh F, Lopez-Rivera E, Hailemichael Y, Clark A, Ma G, et al. Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release. J Immunol. 2012;188(11):5365–76. Epub 2012/04/25. 10.4049/jimmunol.1103553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nature immunology. 2011;12(11):1096–104. Epub 2011/09/20. 10.1038/ni.2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell stem cell. 2008;2(2):141–50. Epub 2008/03/29. 10.1016/j.stem.2007.11.014 . [DOI] [PubMed] [Google Scholar]
- 6.Siegert S, Huang HY, Yang CY, Scarpellino L, Carrie L, Essex S, et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PloS one. 2011;6(11):e27618 10.1371/journal.pone.0027618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(22):7738–42. Epub 1985/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jianjun Y, Zhang R, Lu G, Shen Y, Peng L, Zhu C, et al. T cell-derived inducible nitric oxide synthase switches off Th17 cell differentiation. The Journal of experimental medicine. 2013;210:1447–62. 10.1084/jem.20122494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obermajer N, Wong JL, Edwards RP, Chen K, Scott M, Khader S, et al. Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. The Journal of experimental medicine. 2013;210:1433–445. Epub 2013/06/26. 10.1084/jem.20121277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini AS, Shenoy GN, Rath S, Bal V, George A. Inducible nitric oxide synthase is a major intermediate in signaling pathways for the survival of plasma cells. Nature immunology. 2014;15:275–82. 10.1038/ni.2806 . [DOI] [PubMed] [Google Scholar]
- 11.Tumurkhuu G, Koide N, Dagvadorj J, Noman AS, Khuda II, Naiki Y, et al. B1 cells produce nitric oxide in response to a series of toll-like receptor ligands. Cellular immunology. 2010;261(2):122–7. 10.1016/j.cellimm.2009.11.009 . [DOI] [PubMed] [Google Scholar]
- 12.Carding SR, Kyes S, Jenkinson EJ, Kingston R, Bottomly K, Owen JJ, et al. Developmentally regulated fetal thymic and extrathymic T-cell receptor gamma delta gene expression. Genes & development. 1990;4(8):1304–15. Epub 1990/08/01. . [DOI] [PubMed] [Google Scholar]
- 13.McVay LD, Carding SR. Extrathymic origin of human gamma delta T cells during fetal development. J Immunol. 1996;157(7):2873–82. Epub 1996/10/01. . [PubMed] [Google Scholar]
- 14.Crowley MP, Reich Z, Mavaddat N, Altman JD, Chien Y. The recognition of the nonclassical major histocompatibility complex (MHC) class I molecule, T10, by the gammadelta T cell, G8. The Journal of experimental medicine. 1997;185(7):1223–30. Epub 1997/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matis LA, Fry AM, Cron RQ, Cotterman MM, Dick RF, Bluestone JA. Structure and specificity of a class II MHC alloreactive gamma delta T cell receptor heterodimer. Science. 1989;245(4919):746–9. Epub 1989/08/18. . [DOI] [PubMed] [Google Scholar]
- 16.Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22(1):71–80. Epub 2005/01/25. 10.1016/j.immuni.2004.11.012 . [DOI] [PubMed] [Google Scholar]
- 17.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, et al. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308(5719):252–5. Epub 2005/04/12. 10.1126/science.1106480 . [DOI] [PubMed] [Google Scholar]
- 18.Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat Rev Immunol. 2015;15(11):683–91. Epub 2015/10/10. 10.1038/nri3904 . [DOI] [PubMed] [Google Scholar]
- 19.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13(2):88–100. Epub 2013/01/26. 10.1038/nri3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Gao Y, Scully E, Davis CT, Anderson JF, Welte T, et al. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J Immunol. 2006;177(3):1825–32. Epub 2006/07/20. . [DOI] [PubMed] [Google Scholar]
- 21.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67(1):425; author reply 6. 10.1158/0008-5472.CAN-06-3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. The Journal of experimental medicine. 2003;198(3):433–42. Epub 2003/08/06. 10.1084/jem.20030584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanca T, Costa MF, Goncalves-Sousa N, Rei M, Grosso AR, Penido C, et al. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic gammadelta T lymphocytes to tumor beds. J Immunol. 2013;190(12):6673–80. Epub 2013/05/21. 10.4049/jimmunol.1300434 . [DOI] [PubMed] [Google Scholar]
- 24.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. The Journal of clinical investigation. 2010;120(5):1762–73. Epub 2010/04/07. 10.1172/JCI40891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F 3rd, Schubert WD, et al. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39(1):184–95. Epub 2013/07/31. 10.1016/j.immuni.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81(4):641–50. Epub 1995/05/19. . [DOI] [PubMed] [Google Scholar]
- 27.Douguet L, Bod L, Lengagne R, Labarthe L, Kato M, Avril MF, et al. Nitric oxide synthase 2 is involved in the pro-tumorigenic potential of gammadelta17 T cells in melanoma. Oncoimmunology. 2016;5(8):e1208878 10.1080/2162402X.2016.1208878 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribot JC, Debarros A, Mancio-Silva L, Pamplona A, Silva-Santos B. B7-CD28 costimulatory signals control the survival and proliferation of murine and human gammadelta T cells via IL-2 production. J Immunol. 2012;189(3):1202–8. Epub 2012/06/27. 10.4049/jimmunol.1200268 . [DOI] [PubMed] [Google Scholar]
- 29.Sciorati C, Rovere P, Ferrarini M, Heltai S, Manfredi AA, Clementi E. Autocrine nitric oxide modulates CD95-induced apoptosis in gammadelta T lymphocytes. The Journal of biological chemistry. 1997;272(37):23211–5. Epub 1997/09/12. . [DOI] [PubMed] [Google Scholar]
- 30.Atre N, Thomas L, Mistry R, Pathak K, Chiplunkar S. Role of nitric oxide in heat shock protein induced apoptosis of gammadeltaT cells. International journal of cancer Journal international du cancer. 2006;119(6):1368–76. Epub 2006/04/19. 10.1002/ijc.21966 . [DOI] [PubMed] [Google Scholar]
- 31.Niedbala W, Besnard AG, Nascimento DC, Donate PB, Sonego F, Yip E, et al. Nitric oxide enhances Th9 cell differentiation and airway inflammation. Nature communications. 2014;5:4575 Epub 2014/08/08. 10.1038/ncomms5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. Journal of leukocyte biology. 2008;84(4):949–57. Epub 2008/06/26. 10.1189/jlb.0108024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–31. 10.1182/blood-2012-03-419747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cidad P, Almeida A, Bolanos JP. Inhibition of mitochondrial respiration by nitric oxide rapidly stimulates cytoprotective GLUT3-mediated glucose uptake through 5'-AMP-activated protein kinase. Biochem J. 2004;384:629–36. 10.1042/BJ20040886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coloff JL, Mason EF, Altman BJ, Gerriets VA, Liu T, Nichols AN, et al. Akt requires glucose metabolism to suppress puma expression and prevent apoptosis of leukemic T cells. The Journal of biological chemistry. 2011;286(7):5921–33. Epub 2010/12/17. 10.1074/jbc.M110.179101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83. Epub 2013/01/10. 10.1146/annurev-immunol-032712-095956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–303. Epub 2011/02/15. 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CH, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nature immunology. 2016;17(4):364–8. Epub 2016/03/24. 10.1038/ni.3415 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sorted γδ T cells from pLNs of WT mice were cultured for 2 days in presence of 30 U/mL IL-2 and CD3- and CD28-specific antibodies when indicated (n = 4 replicates each condition). Cells were stained for NOS2 and a viability marker. Flow cytometry representative of NOS2 staining (left) and percentages of NOS2+ γδ T cells among living cells (right) are shown. Numbers above line indicate percent of NOS2+ γδ T cells. * p<0.05 (Mann-Whitney’s test).
(TIF)
Sorted γδ T cells from pLNs of WT and Nos2KO mice were expanded in vitro for 4 days in presence of CD3 and CD28- specific antibodies, 15 μg/mL IL-7 and 15U/mL IL-2. Metabolism was analyzed using a Seahorse XF-24 analyzer. OCR was assessed in response to mitochondrial inhibitors: oligomycin (oligo), Carbonyl cyanide 4—(trifluoromethoxy) phenylhydrazone (FCCP), and rotenone and antimycin A (Rot/AntiA). Shown are time courses. Data are from one experiment with 3 (Nos2KO) and 4 (WT) replicates.
(TIF)
γδ T cells sorted from pLNs of WT and Nos2KO mice were expanded in vitro for 4 days in presence of CD3 and CD28-specific antibodies, 15 μg/mL IL-7 and 15U/mL IL-2. Glycolytic metabolism analysis was performed after 18 h of resting following by an additional 4 h of stimulation with media containing 5mM L-NMMA and/or 15U/mL IL-2 when indicated. ECAR was assessed after adding glucose and in response to metabolic inhibitors oligo and 2DG. Time courses are pooled from three independent experiments.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files