Abstract
Aim
The Danish Quality Database for Mammography Screening (DKMS) was established in 2007, with the aim to monitor, sustain, and improve the quality of the Danish national breast cancer screening program.
Study population
All Danish women aged 50–69 years who were invited every 2 years for breast cancer screening in the nationwide program since July 10, 2007.
Main variables
The DKMS consists of data retrieved from the five regional invitation systems, the National Pathology Registry, and the National Registry of Patients. The DKMS covers the entire screening process and includes variables required to determine the following eleven indicators: 1) radiation exposure, 2) participation among invited women and participation within the target population, 3) time between screening and result, 4) screening interval, 5) recall for further diagnostics, 6) interval cancers consisting of women diagnosed with breast cancer between screening rounds, 7) invasive breast tumors, 8) node-negative cancers, 9) invasive tumors ≤10 mm, 10) ratio of surgery for benign vs malignant lesions, and 11) breast-conserving therapy.
Descriptive data
As of August 10, 2015, the database included data from 888,151 unique women who have been invited to one or more screenings. In the first three screening rounds, 641,835 (round I), 580,452 (round II), and 641,938 (round III) women were invited, and participation increased from 79% to 84%. In the third round, 79% of the screened women received their result within ten working days, 2.7% of the screened women were recalled for further diagnostics, 82% of the women operated for invasive carcinomas were node negative, and 40% of the women had the tumor size of ≤10 mm.
Conclusion
The DKMS has successfully evaluated the quality of the nationwide Danish breast cancer screening program against international quality standards. The quality of the Danish program complies well with international standards particularly as regards to the clinical aspects.
Keywords: breast cancer, screening, epidemiology
Background
The overall goal of a breast cancer screening program is to reduce breast cancer-specific mortality and morbidity while minimizing the adverse effects. In Denmark, population based mammography screening began in Copenhagen in 1991, followed by four other counties/municipalities in the period 1993–2004.1,2 Nationwide mammography screening was implemented during the period 2007–2010 depending on the administrative region and screening capacity. Screening is offered free of charge every 2 years to all Danish women aged 50–69 years.3 The current screening program is administered by the five Danish regions following national guidelines for breast cancer screening4 and European guidelines for quality assurance.5 Reducing the breast cancer mortality with limited adverse effects via a national screening program requires high levels of clinical expertise and a well organized program. In 2007, a multidisciplinary steering committee was appointed to establish and run the Danish Quality Database for Mammography Screening (DKMS). To monitor the program, the committee identified three organizational and eight clinical quality indicators, each reflecting important aspects of the screening program (Tables 1 and 2).
Table 1.
Organizational quality indicator results from the first three national screening rounds in Denmark
| Indicator | Standard | Round III, missing (%) | Round I, proportion (95% CI) | Round II, proportion (95% CI) | Round III, proportion (95% CI) |
|---|---|---|---|---|---|
| Participation: invited women who participated in the screening program/all invited women | >75% | – | 79.0 (78.9–79.1) | 81.7 (81.6–81.8) | 83.9 (83.8–84.0) |
| Participation: invited women who participated/target populationa | Not determined | – | 74.3 (74.2–74.4) | 81.7 (81.4–81.6) | 75.3 (75.5–75.7) |
| Time from screening to result: proportion of women who received their result ≤10 days after screening/all women screened | >95% | 2.0 | 69.0 (68.8–69.1) | 84.3 (84.2–84.4) | 78.5 (78.3–78.6) |
| Screening interva: women who are reinvited to screening within 2 years ±3 months/all women reinvited for screening | Minimum 98% | – | Not relevant | 51.5 (51.3–51.6) | 74.4 (74.3–74.6) |
Notes: Standard: the acceptable level of quality for each indicator defined by the steering committee for DKMS.
The number of women aged 50–69 years residing in Denmark in January 1, 2008, 2010, and 2012, obtained from Statistics Denmark.
Abbreviations: DKMS, Danish Quality Database for Mammography Screening; CI, confidence interval.
Table 2.
Clinical quality indicator results from the first three national screening rounds in Denmark
| Indicator | Standard | Round III, missing (%) | Round I, proportion or ratio (95% CI) | Round II, proportion or ratio (95% CI) | Round III, proportion or ratio (95% CI) |
|---|---|---|---|---|---|
| Recall: women recalled for clinical mammography/all women screened | <3% | – | 3.0 (2.9–3.0) | 2.7 (2.6–2.7) | 2.7 (2.7–2.7) |
| Interval cancer: women with cancer detected within 12 months after screening/underlying incidence of cancera | <30% | – | Not relevant | 26.4 (23.9–29.0) | 25.7 (23.3–28.4) |
| Interval cancer: women with cancer detected within 12–24 months after screening/underlying incidence of cancera | <50% | – | Not relevant | 37.3 (34.4–40.4) | 50.0 (46.5–53.6) |
| Invasive breast tumors: women with invasive breast tumors/women with any breast cancer including DCIS | ≥80% and ≤90% | – | 87.4 (86.5–88.4) | 86.3 (85.0–87.5) | 86.4 (85.2–87.5) |
| Node-negative cancer: women operated for node-negative invasive carcinomas/all women operated for invasive carcinomas | >75% | 1.9 | 69.8 (68.4–71.2) | 79.1 (77.5–80.7) | 82.0 (80.6–83.3) |
| Small cancers: women operated for invasive carcinomas ≤10 mm/all women operated for invasive carcinomas | >30% | 5.8 | 36.1 (34.4–37.8) | 39.9 (38.0–41.9) | 40.1 (38.3–41.8) |
| Ratio of surgery for benign vs malignant lesions: women with surgery for benign breast tumor/women with surgery for malignant breast tumor | Maximum 1:4 | – | 1:5.8 (1:5.5–1:6.2) | 1:6.9 (1:6.3–1:7.6) | 1:8.1 (1:7.5–1:8.9) |
| Breast-conserving therapy: women with invasive carcinomas treated with breast-conserving therapy/women operated for invasive carcinomas | >60% | – | 80.0 (78.7–81.2) | 81.3 (79.7–82.8) | 83.0 (81.5–84.4) |
Notes: Standard: the acceptable level of quality for each indicator defined by the steering committee for DKMS.
The nominator is the incidence of interval cancer per 100,000 women within 12 months and 12–24 months of screening. The denominator is the background incidence of breast cancer in Denmark in 2006 per 100,000 women.
Abbreviations: DCIS, ductal carcinoma in situ; DKMS, Danish Quality Database for Mammography Screening; CI, confidence interval.
Aim
The primary aim of the database is to monitor, sustain, and improve the quality of the Danish national breast cancer screening program.6
Study population
Data have been collected since July 10, 2007, and the database includes all Danish women aged 50–69 years who were invited to breast cancer screening in the nationwide program. As of August 10, 2015, the database consisted of 888,151 women who have been invited to one or more screenings. In the first three completed screening rounds, 641,835 (round I), 580,452 (round II), and 641,938 (round III) women were invited for screening.
Main variables
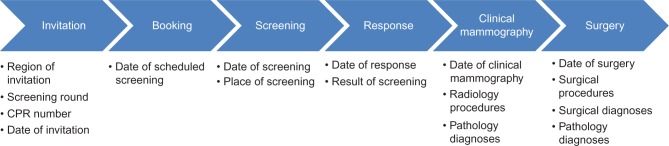
Based on information from the Civil Registration System,7 which is updated on a daily basis with vital status, immigration, and addresses on all Danish residents, the five regions are responsible for inviting the relevant women with a time interval of 2 years. Women can decline participation or rejoin the program at any time. Invitation letters with a fixed appointment and an information brochure are sent to the women. A woman can reschedule her appointment by mail, Internet, or telephone. At each screening session, two standardized X-ray images of each breast are performed. The images are read independently by two radiologists. In the case of a suspicious finding on a screening mammogram, the woman is recalled for a clinical mammography, including ultrasound and clinical examination, possibly additional imaging and needle biopsy. If the clinical mammography reveals a suspected malignancy, the woman is referred to surgery. The DKMS includes data covering the entire process from the day of invitation to the final surgery (Figure 1).
Figure 1.
The course of breast cancer screening including data available in the DKMS at each stage.
Abbreviations: CPR, civil registration; DKMS, Danish Quality Database for Mammography Screening.
No primary data collection on an individual level takes place in relation to the DKMS; thus, the database solely depends on data collected by other systems and registries. The most updated data sources are used, in order to report from the DKMS as close to real time as possible.
Regional invitation systems
First, the DKMS relies on data retrieved from five regional invitation systems, which contain the civil registration (CPR) number, invitation date, booking date, and number of screening round for all women invited for screening. The CPR number is a unique ten-digit personal identification number assigned at birth or upon immigration to all Danish residents permitting unambiguous linkage among all Danish registries.8 The CPR number encodes sex and date of birth. Number of screening round indicates the organizational screening round in which a woman is screened, in contrast to a number indicating how many screens a woman may have undergone.
National Pathology Registry
The DKMS obtains data from the National Pathology Registry (NPR), which holds detailed data on all cytological and histological specimens analyzed in Denmark since 1997. The NPR is updated daily, and data completeness is close to 100%.9 Data are compiled using the Systematized Nomenclature of Medicine codes. Thus, the DKMS includes Systematized Nomenclature of Medicine codes for breast cancer diagnoses including tumor size and node status based on examination of material (cells and tissue) retrieved from needle biopsy and surgery.
The Danish National Patient Registry
The Danish National Patient Registry (DNPR) contains extensive data on the date of hospital admission and discharge and up to 20 discharge diagnoses and procedures for all patients admitted to nonpsychiatric hospitals in Denmark since 1977 (including all outpatient and emergency contacts since 1995).10 The DKMS obtains data from DNPR on dates and procedures for radiology and surgery, including results of mammography screening (normal and abnormal) and International Classification of Diseases codes for breast cancer diagnoses.
Radiation exposure
Since radiation exposure may be a negative side effect of mammography screening, the goal is to obtain the best possible image using the lowest possible radiation dose. At present, it is not possible to obtain data on radiation dose at an individual level. Instead, in accordance with the European guidelines, the DKMS obtains data on radiation dose measured once a week on the X-ray machines using test phantoms.5
Indicators
Annually, the DKMS reports on eleven quality indicators and compares the results with predefined standards based on the European guidelines for quality assurance.5 For the indicator “radiation dose”, the results are reported as average glandular dose using a test phantom equal to a 53 mm EU-standard breast. All other indicator results are calculated as proportions with 95% confidence intervals (exact binomial method).
Over the past three completed screening rounds, 641,835 (round I), 580,452 (round II), and 641,938 (round III) women have been invited for screening, and participation among the invited women has increased from 79% to 84% (Table 1). Thus, the standard of 75% is achieved. Women who actively refuse to participate in the screening program are no longer invited; hence, participation in the target population (all women aged 50–69 years and living in Denmark since January 1, 2012) is lower than in the invited population (75% vs 84%) for round III. The European guidelines do not provide a standard for participation in the target population. The number of women comprising the target population is obtained from Statistics Denmark (Table 2).
To avoid unnecessary anxiety, women should receive their result as soon as possible. To enhance the detection of tumors at an early stage, screening should be provided at regular intervals (2 years ±3 months). In the third round, 79% of the screened women received their result within ten working days, and 74% were reinvited for screening within the specified interval (Table 1). These two indicators do not reach the standards, which may be explained by insufficient capacity in the screening departments and by the fact that the DKMS defines the screening interval as 2 years ±3 months, whereas the European guidelines use a specified interval in years ±6 months. In screening round III, all X-ray machines used in the Danish breast cancer screening program adhere to the standard of average glandular dose <2.0 mGy (data not shown).11
The proportion of all women recalled for further diagnostics was 2.7% in the last two completed screening rounds, including both true and false positives (Table 2). The effectiveness of a screening program depends on its ability to detect cancer at an early stage, ie, the tumor should be small (≤10 mm) and the lymph nodes should be unaffected at the time of surgery. In the most recent completed screening round (round III), 3,646 women with invasive tumors, including ductal carcinoma in situ, were identified. In total, 82% of the women operated for invasive carcinomas were node negative, and 40% of the women had the tumor size of ≤10 mm. The term interval cancer refers to the number of women who are diagnosed with breast cancer between screening rounds, ie, before a subsequent screening or 2 years whichever comes first, and consists of overlooked, fast growing, and radiologically undetectable invasive malignant tumors. Between screening rounds II and III, 1,180 women were diagnosed with interval cancer, which is equal to 32% of the total number of invasive cancers registered (screen detected plus interval cancers). The conventional way of expressing interval cancer is to divide the total number of interval cancers with the expected incidence without screening.5 Here, the number of interval cancers detected within 12 months and 12–24 months after screening is related to the breast cancer incidence in Denmark in 2006, when only local screening programs were running, and the estimates related to the period between rounds II and III are 26% and 50%, respectively. In total, the results of all clinical indicators point toward a quality close to or above the standards.
Validity and completeness
Completeness and validity of data in the DKMS depend highly on the systems and registries that provide data for the DKMS. The completeness of DNPR for surgically treated diseases is considered high. However, it has not been evaluated specifically for breast cancer diagnoses.10 Overall, data completeness is high in the NPR.9 Thus, whenever data on the same parameter are available both from the NPR and the DNPR, the NPR is used as the data source. For tumor size and node status, there are 1.9% and 5.8% missing values, respectively (Table 2).
Completeness in the DKMS in relation to the number of women invited and screened is continuously compared with local data obtained in the regions, and if possible, missing data are subsequently reported to the DKMS. A number of internal validation projects have shown that the regional invitation systems, particularly in rounds I and II, have had difficulties providing valid data for the following variables: screening round, invitation date, and booking date. However, this has been corrected to some extent, and any remaining errors are not expected to have any major impact on the clinical indicators (annual reports 2010, 2012, and 2014).11
Follow-up
Via the DNPR and the Civil Registration System, it is possible to establish practically complete follow-up on breast cancer status and vital status for all participants and nonparticipants in the DKMS cohort.
Examples of research
Thus far, four articles primarily describing the DKMS and the indicator results from the first and second screening rounds have been published.4,6,12,13
Conclusion
The DKMS has successfully collected the relevant data and evaluated the quality of the nationwide Danish mammography screening program against international quality standards. The quality of the Danish program complies well with international standards, particularly in regards to the clinical aspects. DKMS data can be a valuable tool for future research.
Administrative issues and funding
The steering committee for the DKMS is responsible for publishing a yearly report on the quality of the breast cancer screening program and for providing comments and suggestions for quality improvement. Due to the biennial screening procedure, the database has published three reports, each covering a complete screening round (2010, 2012, and 2014)11 in addition to four intermediate reports. The database is publicly funded by The Danish Clinical Registries. The database is supported by the Registry Support Centre of Epidemiology & Biostatistics (North) and Registry Support Centre of Clinical Quality & Health Informatics (West). Researchers can retrieve data from the DKMS upon application to the Registry Support Centre of Clinical Quality & Health Informatics (West) and acceptance from the Danish Data Protection Agency.
Acknowledgments
The authors would like to acknowledge: the present members of the steering committee Ute Hoyer, Anders Lernevall, Walter Schwartz, Nikolaj Borg Mogensen, and Martin Bak, the former head of the steering committee Jens Peter Garne, the former biostatistician for the database Heidi Larsson, and the former contact person for the database Lea Grey Haller. This article was funded by the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by the Danish Regions.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lynge E. Mammography screening for breast cancer in Copenhagen April 1991-March 1997. Mammography Screening Evaluation Group. APMIS Suppl. 1998;83:1–44. [PubMed] [Google Scholar]
- 2.Njor SH, Olsen AH, Bellstrom T, et al. Mammography screening in the county of Fyn. November 1993-December 1999. APMIS Suppl. 2003;111(110):1–33. [PubMed] [Google Scholar]
- 3.Retsinformation [webpage on the Internet] Sundhedsloven Kapitel 18 § 85: Særlige Sygehusydel m.v. 2010. 2015. [Accessed December 17, 2015]. Available from: https://www.retsinformation.dk/Forms/r0710.aspx?id=130455#K18.
- 4.Vejborg I, Mikkelsen E, Garne JP, et al. Mammography screening in Denmark. Dan Med Bull. 2011;58(6):C4287. [PubMed] [Google Scholar]
- 5.European Breast Cancer Network [webpage on the Internet] European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. 4. European Breast Cancer Network; 2006. [Accessed December 17, 2015]. Available from: http://www.euref.org/european-guidelines. [Google Scholar]
- 6.Langagergaard V, Garne JP, Vejborg I, et al. Existing data sources for clinical epidemiology: the Danish Quality Database of Mammography Screening. Clin Epidemiol. 2013;5:81–88. doi: 10.2147/CLEP.S40484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 8.Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287(5462):2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 9.Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39(7 Suppl):72–74. doi: 10.1177/1403494810393563. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundhed [webpage on the Internet] DKMS Årsrapport 2015. 2015. [Accessed December 17, 2015]. Available from: https://www.sundhed.dk/content/cms/78/4678_dkms-rapport-version-4_final_29012015.pdf.
- 12.Larsen MB, Langagergaard V, Larsson H, Mikkelsen EM, Andersen B. Screening mammography may be a rapid and effective investigation of mamma cancer in asymptomatic women. Ugeskr Laeger. 2014;176(36):2–4. [PubMed] [Google Scholar]
- 13.Vejborg I, Mikkelsen EM, Schwartz W, et al. Dansk Kvalitetsdatabase for Mammografiscreening. Ugeskr Laeger. 2012;174(42):2533. [PubMed] [Google Scholar]