ABSTRACT
Non-coding RNAs (ncRNAs) including microRNAs (miRNAs) and small interfering RNAs (siRNAs) are important players in the control of gene regulation and represent novel promising therapeutic targets or agents for the treatment of various diseases. While synthetic ncRNAs are predominately utilized, the effects of excessive artificial modifications on higher-order structures, activities and toxicities of ncRNAs remain uncertain. Inspired by recombinant protein technology allowing large-scale bioengineering of proteins for research and therapy, efforts have been made to develop practical and effective means to bioengineer ncRNA agents. The fermentation-based approaches shall offer biological ncRNA agents with natural modifications and proper folding critical for ncRNA structure, function and safety. In this article, we will summarize current recombinant RNA platforms to the production of ncRNA agents including siRNAs and miRNAs. The applications of bioengineered ncRNA agents for basic research and potential therapeutics are also discussed.
KEYWORDS: bioengineering, cancer, miRNA, non-coding RNAs, siRNA, therapy
Introduction
Non-coding RNA (ncRNA) is a term for RNA molecule that is derived from genome while not translated into a protein.1 Indeed over 95% of human genome is composed of non-coding DNA sequences which may be transcribed into various categories of ncRNAs including small interfering RNAs (siRNAs), microRNAs (miRNAs), and long non-coding RNAs (lncRNAs).1-3 With the rapid development of molecular and cellular technologies, ncRNAs are revealed to have far more important functions than previously recognized. Much attention has been drawn onto these small RNAs (sRNAs) due to their important roles in the regulation of gene expression and potentials for the development of novel therapies. There is accumulating evidence supporting that siRNAs and miRNAs are promising targets for the treatment of different diseases including cancers and infections, and many siRNA/miRNA therapeutics are under clinical investigation.4-6 As an example, MRX34, a liposome formulated miR-34a mimic, exhibits antiproliferative activities against various types of human carcinoma cells via repressing multiple oncogenes, and MRX34 has entered into Phase 1 clinical trials to treat unresectable primary liver tumor.7 Patisiran, a lipid nanoparticle (LNP)-formulated 25-bp siRNA agent targeting Transthyretin (TTR) mRNA, is in Phase 3 clinical trial for the treatment of TTR-mediated amyloidosis.8 The siRNAs and miRNAs can be designed and/or employed to control virtually the expression of any gene of interest, and thus they have the advantage of acting on targets inaccessible by conventional small-molecule therapeutics.
Large quantities of pure homogeneous RNA agents are essential for delineation of RNA functions in vivo and development of RNA-based therapies. Currently, RNA agents are commonly produced through chemical synthesis or in vitro transcription with recombinant T7 RNA polymerase.9 The major limitation of those RNA agents is the addition of excessive artificial modifications and/or the lack of necessary posttranscriptional modifications occurring in natural RNAs, which may lead to different folding properties, biological activities, and safety profiles. Another approach to introduce target ncRNAs into mammalian cells is the use of DNA materials such as viral or non-viral vector-based ncRNA expression plasmids. However, such DNA agents need extra processes to produce functional ncRNAs in cells which makes the process rather more complicated. In addition, ncRNA expression plasmids often offer a rather low and even unpredictable level of target ncRNAs in human cells. It is also noteworthy that protein therapeutics are mainly produced via bioengineering approaches10-12 or isolated directly from plants or animals since the bioengineering of human insulin almost 40 y ago.12,13 Therefore, the use of natural ncRNA molecules and the development of ncRNA bioengineering approaches are highly demanded for RNA research and development.6,14 In vivo fermentation approaches are expected to provide large quantities of biological ncRNA agent with proper folding and natural modifications that are critical for RNA higher-order structure, stability, activity and safety. In this article, we provide an overview of newly-developed in vivo approaches to the production of ncRNAs agents. The applications of bioengineered ncRNA agents (BERAs) to basic and translational research are also discussed.
Mechanistic actions and therapeutic potentials of siRNAs and miRNAs
Both siRNA and miRNA are short ncRNA duplexes. The actions of miRNAs and siRNAs may be unified as RNA interference (RNAi) process that silences target gene expression in a sequence dependent manner in cells,15-17 yet their specific mechanisms may differ (Fig. 1). After processed by Dicer from transcribed- or artificially introduced-dsRNA, siRNA is loaded into the RISC (siRNA- or miRNA-induced silencing complex). While the passenger strand of siRNA is cleaved by AGO2 (Argonaute 2), a component of RISC, the guide strand within the active RISC binds to the target mRNA and leads to the cleavage of mRNA.2 The miRNA gene is transcribed by RNA polymerase II in the nucleus to pri-miRNA, which is then cleaved by Drosha to form pre-miRNA. The pre-miRNA is transported by Exportin-5 to the cytoplasm and then processed by Dicer into mature miRNA, which is loaded into the RISC. The RISC removes the passenger strand and then the remaining strand guides the RISC to target the mRNA through partially complementary binding, leading to translational repression, or target mRNA degradation or cleavage.3,18
Figure 1.
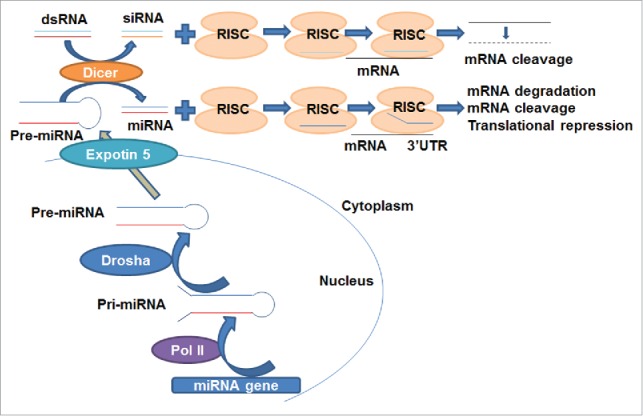
The mechanistic actions of siRNA and miRNA derived from genome. siRNA: Dicer processes dsRNA (either transcribed or artificially introduced) into siRNA, which is then loaded into the RISC. AGO2, a component of RISC, cleaves the passenger strand of siRNA. The guide strand guides the active RISC to the target mRNA and binds to the target mRNA completely, leading to the cleavage of mRNA. miRNA: miRNA gene is transcribed by RNA polymerase II in the nucleus to pri-miRNA, which is then cleaved by Drosha to form pre-miRNA. The pre-miRNA is transported by Exportin-5 to the cytoplasm and then processed by Dicer to miRNA, which is loaded into the RISC. The passenger strand is removed and the remaining strand guides the RISC to the target mRNA through partially complementary binding to the miRNA response elements within 3'UTR, leading to translational repression, degradation or cleavage of the target mRNA.
Because siRNAs and miRNAs can downregulate the expression of virtually any genes in human genome and overcome the limitation of classic small-molecule drugs that target only certain classes of proteins, the sRNAs have huge potentials as therapeutic agents. Several siRNA or miRNA-based therapeutic approaches have been developed.6 For siRNA, a synthetic siRNA that targets a specific mRNA (mRNA) can be introduced into cells to elicit RNAi, which is expected to inhibit the expression of target mRNA, produce a gene silencing effect, and thus manage disease progression.19 By contrast, miRNA-based therapies consist of miRNA inhibition and miRNA replacement strategies.20,21 The former approach delivers single stranded, synthetic RNA into cells, which targets particular miRNA to produce miRNA antagonism. Similar to the mechanism of siRNAs, miRNA inhibitor is anticipated to suppress the function of target miRNA and thus control disease progression. Conversely, the miRNA replacement approach reintroduces miRNAs into the cells which may reactivate miRNA pathways and thus lead to target mRNA degradation or translation inhibition, and produce a gene silencing effect to combat disease.
Bioengineering of RNAi agents in vivo
Currently RNAi molecules are mainly produced by chemical synthesis or enzymatic methods in vitro.6 Although the broad applications of RNAi have led to the improvement of RNA chemistry and synthetic RNAs are relatively more accessible than before, it is still costly to obtain larger quantity (e.g., milligrams) of siRNA or miRNA materials and the length or size of synthetic RNA is also limited. Therefore, there are growing interests in developing more cost-effective approaches to producing ready-to-use ncRNA agents on a large scale.
Fermentation-based approaches for in vivo production of ncRNAs (Table 1) have attracted attentions as the biological ncRNAs made in cells do not carry artificial but necessary posttranscriptional modifications that are important for ncRNA higher-order structure, stability, and biological function. In principle, the target ncRNA coding sequence is introduced into a vector, and the resulting plasmid is transformed into host cells grown in appropriate conditions. The ncRNA of interest is thus generated by intrinsic transcription and ncRNA processing machineries in host cells. However, heterogeneous RNAs are very susceptible to cellular RNases, and ncRNAs of interest may not be accumulated to a desirable level in host cells. Therefore, BERAs should be assembled into stable RNA entities and/or protected within steady complexes or storages. Under such conditions, target BERAs are nondegradable by cellular RNases and thus accumulated to significant levels in cytosol. Cells are then harvested and target BERAs may be purified by appropriate methods (e.g., fast protein liquid chromatography or FPLC) and subjected to structure, function, efficacy and safety tests (Fig. 2).
Table 1.
Summary of current fermentation-based approaches to bioengineering of ncRNAs.
| Approaches for the production of ncRNAs | Product size (nt) | Applications | References |
|---|---|---|---|
| The siRNA-binding protein p19 | ∼21 | Production of fully processed siRNAs at small or median scale for functional study | 22 |
| The rRNA as scaffold | < 100 | Production of small RNAs including siRNAs, shRNAs and aptamers at medium or large scale for various utilities | 30,31 |
| The tRNA as scaffold | < 400 | Production of a variety of ncRNAs at large scale for various studies | 32,33,35-38,43 |
| Optimal ncRNA (tRNA/pre-miRNA) as scaffold | 100–400 | Production of various ncRNAs at high yield and large scale for in vitro and in vivo studies | 40,44,45 |
Figure 2.
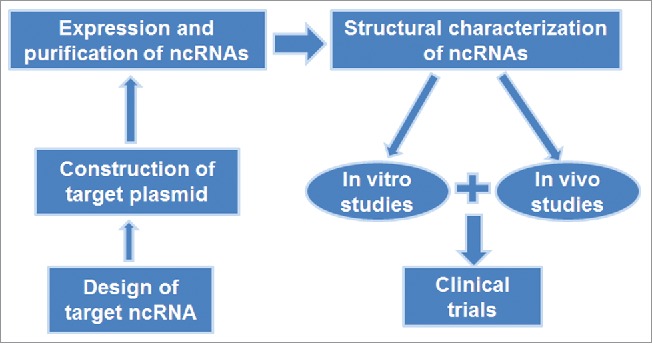
Bioengineering of ncRNA agents for research and therapy. The sequence of target ncRNA is cloned into a target vector. Overexpression of target ncRNA is verified and then purified from total RNAs. Bioengineered ncRNA is subjected to structural characterization, and then used for in vitro and in vivo studies before clinical investigations.
One reported approach is to use siRNA-binding protein to stabilize and enrich target siRNA molecules in cells22 (Table 1). A 19 kD siRNA-binding protein, p19,23-25 was employed to bind to recombinant siRNA to form a siRNA-p19 complex and then purified by nickel affinity chromatography and followed by anion exchange high performance liquid chromatography (HPLC).22 While this method allows the production of fully-processed, ready-to-use siRNAs or miRNAs, the overall yield is very low and it unlikely provides a much desired large quantity of target siRNA agents.
Utilization of stable RNA scaffold or carrier represents a novel strategy for the production of BERAs. tRNA (tRNA) and ribosome RNA (rRNA), the most abundant and stable sRNA entities in cells, have been used as scaffolds to achieve heterogeneous expression of some ncRNAs of interest (Table 1). The 5S rRNA scaffold can accommodate target ncRNA sequences under 80 nt within its stem II, resulting in replacement of its stem III and loops B and C by the ncRNA insert, which has been utilized for bioengineering of several ncRNAs.26-30 The 5S rRNA approach has been shown to offer as much as 2.5-7.5 mg of purified RNA per gram of cells under optimal conditions29-31 while its utility awaits further evaluations. The tRNA is proven as a simple scaffold for successful production of large quantities of RNA molecules in vivo, i.e., milligrams of RNA from 1 litter bacterial culture.32,33 The tRNA-carried ncRNAs include a number of viral RNAs, RNA aptamers, hammerhead riboswitch RNAs, and human pre-miRNAs.32-39 Nevertheless, levels of recombinant ncRNAs accumulated in cells are largely variable and inevitably dependent upon the structures and metabolic stabilities of chimeric RNAs.34,35,40
An optimal ncRNA scaffold (OnRS) approach has been developed toward a more general, versatile, and robust high-yield and large-scale production of RNAi agents40 (Table 1). Because the yields of biological pre-miRNAs produced by genetic engineering were very low (unpublished data),6,41,42 we added a tRNA scaffold to the pre-miRNAs to increase the yields. However, most of target tRNA/pre-miRNA chimeras were still not or minimally expressed. Nevertheless, we did identify that several tRNA/pre-miRNAs including tRNA/mir-34a and tRNA/mir-1291 were able to accumulate in bacteria to significantly high levels,34,40 likely due to the intrinsic stabilities of chimeric ncRNAs. Furthermore, chimeric tRNA/pre-miRNAs showed good cellular stability, and were selectively processed to mature miRNAs in various types of human cancer cells to regulate target gene expression and cell functions. Therefore, such high-yield expressing tRNA/pre-miRNA chimeras were developed as OnRS for the production of target ncRNAs.40 In particular, the mature miRNA duplex sequence in the plasmid is replaced with target siRNA/miRNA sequences. Following the construction of target plasmid and transformation, target ncRNA is expressed in bacteria and purified to a high degree of homogeneity by anion exchange FPLC method.40,43
We have further demonstrated that many chimeric ncRNAs (e.g., OnRS/miR-27b, OnRS/miR-124 and OnRS/GFP-siRNA, etc.) can be produced in E.coli on large scale, and be processed to target sRNA agents (e.g., miR-27b, miR-124 and GFP-siRNA, etc.) in human cells and animals by both unbiased RNA sequencing study and targeted quantitative real-time PCR analysis.40 Consequently, BERAs are able to selectively reduce target genes expression in vitro and in vivo while the tRNA segment within OnRS is processed to the same tRNA fragments at similar levels as control tRNA scaffold. Therefore, this OnRS offers a robust platform which not only offers high-yield, large-scale and cost-effective production of BERAs carrying various types of sRNAs but also delivers such functional sRNAs into mammalian cells.6,40
Applications of bioengineered RNAi agents
BERAs are derived in cells and they are more relevant to natural and highly-structured RNAs for biological studies, which are distinguished from current synthetic RNA and recombinant DNA materials.6 Therefore, these RNAs represent a new family of agents for functional, diagnostic and therapeutic investigations. For instance, chimeric RNA aptamers produced through tRNA scaffold method (Table 1) have been successfully used as sensors for the detection/imaging of target molecules in the cells.37,38 In addition, recombinant ncRNAs from, such as the siRNAs isolated from p19 complex and miRNAs/siRNAs from OnRS platform (Table 1), are biologically active in the regulation of target gene expression in mammalian cells, which have been demonstrated in many studies.22,34,35,40,43,44 Specifically, bioengineered tRNA/mir-27b was found to be processed to mature miR-27b in human carcinoma cells, which consequently reduced CYP3A4 protein expression and led to a lower midazolam 1'-hydroxylase activity.35 The tRNA/mir-1291 was readily processed to mature miR-1291 in breast cancer MCF-7 cells and pancreatic cancer PANC-1 cells.34 Consequently, recombinant tRNA/mir-1291 reduced the protein levels of miR-1291 target genes and increased the sensitivity of carcinoma cells to chemotherapeutics. In addition, BERA miRNAs/siRNAs were more effective than synthetic miRNA/siRNA agents at same concentrations in the regulation of target gene expression and cell functions,40,43 indicating that natural BERA agents could be valuable for functional and therapeutic studies.
Bioengineered tRNA/mir-34a agent indeed acted as a prodrug in suppressing tumor growth in both subcutaneous A549 xenograft43 and orthotopic 143B xenograft45 mouse models, while BERAs were well tolerated in mice. Combined with DNA or protein targeting agents, RNA targeting BERAs could have synergistic effects to combat lethal cancer, especially those lacking effective target therapeutics. As an example, strong synergistic effects in the suppression of osteosarcoma cell proliferation were demonstrated for bioengineered tRNA/mir-34a and doxorubicin, a DNA intercalator.44 Much greater degrees of late apoptosis, necrosis, and G2 cell cycle arrest as well as suppression of miR-34a target gene expression were also elucidated for combination therapy. In addition, systemic co-administration of bioengineered miR-34a prodrug and doxorubicin was revealed to be more effective than single drug treatment to control tumor growth in an orthotopic osteosarcoma xenograft mouse model.44 These findings support the use of biological ncRNAs as novel prodrugs for monotherapy or combination therapy.
Conclusions and future prospects
Improved understanding of ncRNA functions has opened new doors to development of RNA-based therapy. There are a number of RNA-based therapeutics currently in clinical use or under clinical development. Given the limitations of synthetic RNA agents consisting of excessive artificial modifications, it is urgent to develop new approaches toward high-yield, large-scale and cost-effective production of biological RNAs. Bioengineering ncRNAs carry no or natural posttranscriptional modifications, and these BERAs are biological active in mammalian cells and animal models. Therefore, BERAs represent novel RNA agents and should be more suitable for RNA research and development. Further research is necessary to critically assess the utility of recombinant ncRNAs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the grant number R01GM113888 from the National Institute of General Medical Sciences, National Institutes of Health.
References
- [1].Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet 2006; 15 Spec No 1:R17-29; PMID:16651366; http://dx.doi.org/ 10.1093/hmg/ddl046 [DOI] [PubMed] [Google Scholar]
- [2].Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev 2003; 67:657-85; PMID:14665679; http://dx.doi.org/ 10.1128/MMBR.67.4.657-685.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215-33; PMID:19167326; http://dx.doi.org/ 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther 2011; 18:1121-6; PMID:21633392; http://dx.doi.org/ 10.1038/gt.2011.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shah MY, Calin GA. MicroRNAs as therapeutic targets in human cancers. Wiley Interdiscip Rev RNA 2014; 5:537-48; PMID:24687772; http://dx.doi.org/ 10.1002/wrna.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ho PY, Yu AM. Bioengineering of noncoding RNAs for research agents and therapeutics. Wiley Interdiscip Rev RNA 2016; 7:186-97; PMID:26763749; http://dx.doi.org/ 10.1002/wrna.1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet 2012; 3:120; PMID:22783274; http://dx.doi.org/ 10.3389/fgene.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Adams D, Cauquil C, Theaudin M, Rousseau A, Algalarrondo V, Slama MS. Current and future treatment of amyloid neuropathies. Expert Rev Neurother 2014; 14:1437-51; PMID:25416603; http://dx.doi.org/ 10.1586/14737175.2014.983905 [DOI] [PubMed] [Google Scholar]
- [9].Beckert B, Masquida B. Synthesis of RNA by in vitro transcription. Methods Mol Biol 2011; 703:29-41; PMID:21125481; http://dx.doi.org/ 10.1007/978-1-59745-248-9_3 [DOI] [PubMed] [Google Scholar]
- [10].Pranchevicius MC, Vieira TR. Production of recombinant immunotherapeutics for anticancer treatment: the role of bioengineering. Bioengineered 2013; 4:305-12; PMID:23644447; http://dx.doi.org/ 10.4161/bioe.24666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Balabanova L, Golotin V, Podvolotskaya A, Rasskazov V. Genetically modified proteins: functional improvement and chimeragenesis. Bioengineered 2015; 6:262-74; PMID:26211369; http://dx.doi.org/ 10.1080/21655979.2015.1075674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov 2008; 7:21-39; PMID:18097458; http://dx.doi.org/ 10.1038/nrd2399 [DOI] [PubMed] [Google Scholar]
- [13].Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, Hirose T, Kraszewski A, Itakura K, Riggs AD. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A 1979; 76:106-10; PMID:85300; http://dx.doi.org/ 10.1073/pnas.76.1.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yu AM, Tian Y, Tu MJ, Ho PY, Jilek JL. MicroRNA pharmacoepigenetics: posttranscriptional regulation mechanisms behind variable drug disposition and strategy to develop more effective therapy. Drug Metab Dispos 2016; 44:308-19; PMID:26566807; http://dx.doi.org/ 10.1124/dmd.115.067470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Deng Y, Wang CC, Choy KW, Du Q, Chen J, Wang Q, Li L, Chung TK, Tang T. Therapeutic potentials of gene silencing by RNA interference: principles, challenges, and new strategies. Gene 2014; 538:217-27; PMID:24406620; http://dx.doi.org/ 10.1016/j.gene.2013.12.019 [DOI] [PubMed] [Google Scholar]
- [16].Davidson BL, McCray PB Jr. Current prospects for RNA interference-based therapies. Nat Rev Genet 2011; 12:329-40; PMID:21499294; http://dx.doi.org/ 10.1038/nrg2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kubowicz P, Zelaszczyk D, Pekala E. RNAi in clinical studies. Curr Med Chem 2013; 20:1801-16; PMID:23432579; http://dx.doi.org/ 10.2174/09298673113209990118 [DOI] [PubMed] [Google Scholar]
- [18].Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 2014; 15:509-24; PMID:25027649; http://dx.doi.org/ 10.1038/nrm3838 [DOI] [PubMed] [Google Scholar]
- [19].Ozcan G, Ozpolat B, Coleman RL, Sood AK, Lopez-Berestein G. Preclinical and clinical development of siRNA-based therapeutics. Adv Drug Deliv Rev 2015; 87:108-19; PMID:25666164; http://dx.doi.org/ 10.1016/j.addr.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res 2010; 70:7027-30; PMID:20807816; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res 2012; 110:496-507; PMID:22302756; http://dx.doi.org/ 10.1161/CIRCRESAHA.111.247916 [DOI] [PubMed] [Google Scholar]
- [22].Huang L, Jin J, Deighan P, Kiner E, McReynolds L, Lieberman J. Efficient and specific gene knockdown by small interfering RNAs produced in bacteria. Nat Biotechnol 2013; 31:350-6; PMID:23475073; http://dx.doi.org/ 10.1038/nbt.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jin J, Cid M, Poole CB, McReynolds LA. Protein mediated miRNA detection and siRNA enrichment using p19. Biotechniques 2010; 48:xvii-xxiii; PMID:20569217; http://dx.doi.org/ 10.2144/000113364 [DOI] [PubMed] [Google Scholar]
- [24].Qiu W, Park JW, Scholthof HB. Tombusvirus P19-mediated suppression of virus-induced gene silencing is controlled by genetic and dosage features that influence pathogenicity. Mol Plant Microbe Interact 2002; 15:269-80; PMID:11952130; http://dx.doi.org/ 10.1094/MPMI.2002.15.3.269 [DOI] [PubMed] [Google Scholar]
- [25].Silhavy D, Molnar A, Lucioli A, Szittya G, Hornyik C, Tavazza M, Burgyán J. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J 2002; 21:3070-80; PMID:12065420; http://dx.doi.org/ 10.1093/emboj/cdf312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pitulle C, Dsouza L, Fox GE. A low molecular weight artificial RNA of unique size with multiple probe target regions. Syst Appl Microbiol 1997; 20:133-6; PMID:11540055; http://dx.doi.org/ 10.1016/S0723-2020(97)80057-4 [DOI] [PubMed] [Google Scholar]
- [27].Pitulle C, Hedenstierna KO, Fox GE. A novel approach for monitoring genetically engineered microorganisms by using artificial, stable RNAs. Appl Environ Microbiol 1995; 61:3661-6; PMID:7487004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pitulle C, Hedenstierna KO, Fox GE. Useful properties of restriction enzymes that recognize interrupted palindromes. Biotechniques 1996; 21:619-20, 22; PMID:8891210 [DOI] [PubMed] [Google Scholar]
- [29].Liu Y, Stepanov VG, Strych U, Willson RC, Jackson GW, Fox GE. DNAzyme-mediated recovery of small recombinant RNAs from a 5S rRNA-derived chimera expressed in Escherichia coli. BMC Biotechnol 2010; 10:85; PMID:21134283; http://dx.doi.org/ 10.1186/1472-6750-10-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang X, Potty AS, Jackson GW, Stepanov V, Tang A, Liu Y, Kourentzi K, Strych U, Fox GE, Willson RC. Engineered 5S ribosomal RNAs displaying aptamers recognizing vascular endothelial growth factor and malachite green. J Mol Recognit 2009; 22:154-61; PMID:19195013; http://dx.doi.org/ 10.1002/jmr.917 [DOI] [PubMed] [Google Scholar]
- [31].Stepanov VG, Fox GE. In vivo production of small recombinant RNAs embedded in a 5S rRNA-derived protective scaffold. Methods Mol Biol 2015; 1316:45-65; PMID:25967052; http://dx.doi.org/ 10.1007/978-1-4939-2730-2_5 [DOI] [PubMed] [Google Scholar]
- [32].Ponchon L, Dardel F. Recombinant RNA technology: the tRNA scaffold. Nat Methods 2007; 4:571-6; PMID:17558412; http://dx.doi.org/ 10.1038/nmeth1058 [DOI] [PubMed] [Google Scholar]
- [33].Ponchon L, Beauvais G, Nonin-Lecomte S, Dardel F. A generic protocol for the expression and purification of recombinant RNA in Escherichia coli using a tRNA scaffold. Nat Protoc 2009; 4:947-59; PMID:19478810; http://dx.doi.org/ 10.1038/nprot.2009.67 [DOI] [PubMed] [Google Scholar]
- [34].Li MM, Addepalli B, Tu MJ, Chen QX, Wang WP, Limbach PA, LaSalle JM, Zeng S, Huang M, Yu AM. Chimeric MicroRNA-1291 biosynthesized efficiently in escherichia coli is effective to reduce target gene expression in human carcinoma cells and improve chemosensitivity. Drug Metab Dispos 2015; 43:1129-36; PMID:25934574; http://dx.doi.org/ 10.1124/dmd.115.064493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li MM, Wang WP, Wu WJ, Huang M, Yu AM. Rapid production of novel pre-microRNA agent hsa-mir-27b in escherichia coli using recombinant RNA technology for functional studies in mammalian cells. Drug Metab Dispos 2014; 42:1791-5; PMID:25161167; http://dx.doi.org/ 10.1124/dmd.114.060145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nelissen FH, Leunissen EH, van de Laar L, Tessari M, Heus HA, Wijmenga SS. Fast production of homogeneous recombinant RNA–towards large-scale production of RNA. Nucleic Acids Res 2012; 40:e102; PMID:22457065; http://dx.doi.org/ 10.1093/nar/gks292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Paige JS, Nguyen-Duc T, Song W, Jaffrey SR. Fluorescence imaging of cellular metabolites with RNA. Science 2012; 335:1194; PMID:22403384; http://dx.doi.org/ 10.1126/science.1218298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Paige JS, Wu KY, Jaffrey SR. RNA mimics of green fluorescent protein. Science 2011; 333:642-6; PMID:21798953; http://dx.doi.org/ 10.1126/science.1207339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ponchon L, Catala M, Seijo B, El Khouri M, Dardel F, Nonin-Lecomte S, Tisné C. Co-expression of RNA-protein complexes in Escherichia coli and applications to RNA biology. Nucleic Acids Res 2013; 41:e150; PMID:23804766; http://dx.doi.org/ 10.1093/nar/gkt576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen QX, Wang WP, Zeng S, Urayama S, Yu AM. A general approach to high-yield biosynthesis of chimeric RNAs bearing various types of functional small RNAs for broad applications. Nucleic Acids Res 2015; 43:3857-69; PMID:25800741; http://dx.doi.org/ 10.1093/nar/gkv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pereira PA, Tomas JF, Queiroz JA, Figueiras AR, Sousa F. Recombinant pre-miR-29b for Alzheimer s disease therapeutics. Sci Rep 2016; 6:19946; PMID:26818210; http://dx.doi.org/ 10.1038/srep19946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pereira P, Pedro AQ, Tomas J, Maia CJ, Queiroz JA, Figueiras A, Sousa F. Advances in time course extracellular production of human pre-miR-29b from Rhodovulum sulfidophilum. Appl Microbiol Biotechnol 2016; 100:3723-34; PMID:26860940; http://dx.doi.org/ 10.1007/s00253-016-7350-x [DOI] [PubMed] [Google Scholar]
- [43].Wang WP, Ho PY, Chen QX, Addepalli B, Limbach PA, Li MM, Wu WJ, Jilek JL, Qiu JX, Zhang HJ, et al.. Bioengineering novel chimeric microRNA-34a for prodrug cancer therapy: high-yield expression and purification, and structural and functional characterization. J Pharmacol Exp Ther 2015; 354:131-41; PMID:26022002; http://dx.doi.org/ 10.1124/jpet.115.225631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhao Y, Tu MJ, Yu YF, Wang WP, Chen QX, Qiu JX, Yu AX, Yu AM. Combination therapy with bioengineered miR-34a prodrug and doxorubicin synergistically suppresses osteosarcoma growth. Biochem Pharmacol 2015; 98:602-13; PMID:26518752; http://dx.doi.org/ 10.1016/j.bcp.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao Y, Tu MJ, Wang WP, Qiu JX, Yu AX, Yu AM. Genetically engineered miR-34a prodrug suppresses orthotopic osteosarcoma tumor growth via the induction of apoptosis and cell cycle arrest. Sci Rep 2016; 6:26611; PMID:27216562; http://dx.doi.org/ 10.1038/srep26611 [DOI] [PMC free article] [PubMed] [Google Scholar]


