Abstract
A supramolecular polymeric system that shows an unusual “racemate-rules” chiroptical property, an effect opposite to the well-known “majority-rules”, has been utilized for accurate determination of malic acid enantiopurity at high ee values.
The development of asymmetric synthetic methods demands accurate analytical techniques for quantification of product enantiopurity. Recently, optical protocols based on synthetic chemical receptors that give NMR, fluorescence, UV-Vis absorption and circular dichroism responses to the enantiomeric excess of chiral analytes have attracted significant attention.1 Those receptors are either chiral and differentiate between enantiomers via formation of diastereomeric complexes with different stabilities and/or spectroscopic properties,2 or achiral/dynamically racemic receptors that preferentially form one helicity over the other upon binding an enantiomer.3 These optical methods have advantages over routine chromatographic techniques in analytical time, cost-effectiveness, and amenability to high throughput analysis.4 Although rapid, a drawback of the optical methods is the generally higher error in analyzing ee values relative to HPLC. While HPLC is commonly reported to have errors near or lower than 1%, most optical methods have errors around 5%. This is particularly vexing when seeking to distinguish subtle ee differences on the high end of the ee scale, such as between 95 and 100%.
Our group,1b and others,3a have been interested in developing achiral sensors (hosts) that show circular dichroism (CD) induction upon binding chiral analytes (guests). In most of these reported systems, a linear relationship between the induced CD intensity and the ee of the chiral guest was observed,5 although small deviation from linearity occasionally occurred with a host/guest stoichiometry higher than 1:1.6 To improve the error in determining high ee values, we recently explored polymeric systems.7 Polymers allow the possibility for non-linear CD-ee relationships.1a Of particular interest is the majority rules effect (MRE) which states that the enantiomer present in the majority dictates the helical sense of the polymer, to which the minority enantiomer adjusts.8 In MRE systems, the CD-ee curve shows the steepest slope at 0% ee. We reported an exploitation of Yashima’s MRE helical polymer9 for sensing high ee of amino acids. This was achieved by adding an equal amount of the minor enantiomer to the high ee analytical sample, thus moving the ee point from around 100% to around 0%, within the region where the CD intensity is the most sensitive to ee changes. Drawbacks, however, are the required prior knowledge of sample concentration and oxidative instability of this particular polymer.7
Recently, Jiang and coworkers have reported a series of supramolecular polymeric system built from perylenebisimide (PBI) scaffolds decorated with molecular recognition entities.10 One particular set of receptors contains boronic acids that can bind sugars and α-hydroxycarboxylic acids10a,11 (Scheme 1). These structures form supramolecular stacks, analogous to those reported by Meijer.8 Most of the reported polymeric systems most commonly give either linear or MRE9 responses, implying either no cooperative binding of enantiomers or cooperative binding of the same enantiomer along the polymer chain, respectively. However, we found an unusual response opposite to the often observed MRE, i.e. the response of the CD signals generated by enantiomeric mixtures near zero values were lower than would be expected for a linear CD-ee or MRE plot.10a
Scheme 1.
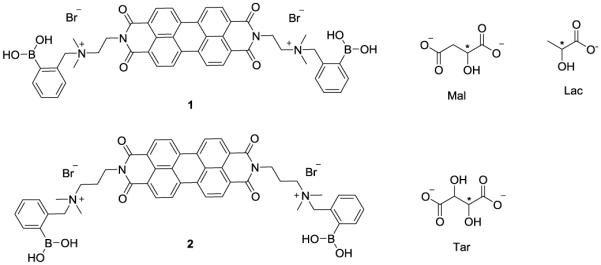
PBI hosts 1 and 2, and α-hydroxycarboxylate guests malate (Mal), lactate (Lac) and tartrate (Tar).
There is no a priori thermodynamic reason that the enantiomer present in excess should always cooperatively influence the binding of the subsequent enantiomer to be of the same handedness, thereby dictating the helical pitch of the polymer. Instead, it could be that a particular polymer cooperatively binds opposite enantiomers (a racemic mix) until the solution is so depleted in enantiomers that the polymer can only bind those of the same handedness, which would occur with solutions of higher ee values. In such a case, the CD response to changes in ee values around zero would be flat because preferential binding of a racemate would not introduce a pitch to the polymer. A helical pitch, and therefore a CD signal, would only arise at the high ends of ee values. This is a possible explanation for the “lower than linear” CD-ee relationship we observed. Although still poorly understood, the abovementioned hypothesis could explain the disappearance of this effect (restoration of the linear CD-ee relationship) when the chiral guest was used at lower concentrations10a so that the host was no longer able to select the guest due to its limited availability. We term this as a Racemate Rules Effect (RRE).
The RRE would lead to a large dynamic response to ee in the range of values where lower errors are most beneficial to ee determination, i.e. high ee values. To support this claim, we explored different systems based on boronic acid-appended PBI hosts and α-hydroxycarboxylate guests, to seek out a linear CD-ee response, a MRE, and a RRE, to compare the accuracy of determining guest ee in these systems. Reported PBI 1 and a new PBI 2 were used as the hosts and lactate (Lac), tartrate (Tar) and malate (Mal) used as the guests. These guests were found to induce strong CD signals from the PBI chromophore upon host-guest binding. Notably, the analyte concentration required for the chiroptical response was much lower for Tar and Mal than for Lac. We attributed this effect to simultaneous binding of a guest molecule to two PBI hosts in the cases of Tar and Mal. For Tar this divalent binding presumably occurred via two boronate ester linkages, and for Mal via one boronate ester linkage and one salt bridge between a Mal carboxylate group and a PBI quaternary ammonium group. Preliminary molecular mechanics modelling studies support the compatibility of these divalent binding motifs with PBI stacking (Fig. S1 and S2). In most cases, linearity in the CD vs ee values plot was found. For example, Figure 1(a) shows a typical response, in this case with receptor 1 and Lac. Figure 1(b), generated using 2 and Tar, shows a classic MRE response. Yet, Figure 1(c) shows the desired response, a RRE. This response arises from the use of receptor 1 binding Mal. Having found the RRE, we then set out to discover how to improve the dynamic range and cooperative effect of binding racemates.
Fig. 1.
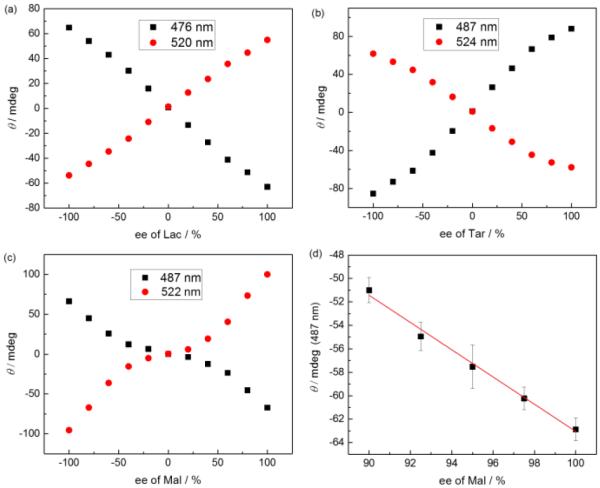
CD-ee relationship of 1/Lac (a), 2/Tar (b) and 1/Mal (c) assemblies in pH 5.0 acetate buffer containing 2.5% (v%) DMSO. (d) CD response of 1 to ee of Mal (at 487 nm) in the high ee region. [1] = [2] = 50 μ M, [L-Lac] + [D-Lac] = 50 mM, [L-Tar] + [D-Tar] = 30 μ M, [L-Mal] + [D-Mal] = 0.3 mM.
We attempted to increase the RRE of the 1/Mal system by changing the solution pH (Fig. 2a), and adding osmolytes (Fig. 2c and 2d) which are known to affect intramolecular interactions.12 Of all attempts, the extent of RRE remained the same or even weakened. Hence the best conditions (pH 5.0, with 50 mM acetate buffer) were employed for evaluation of the potency of the 1/Mal system for ee determination.
Fig. 2.
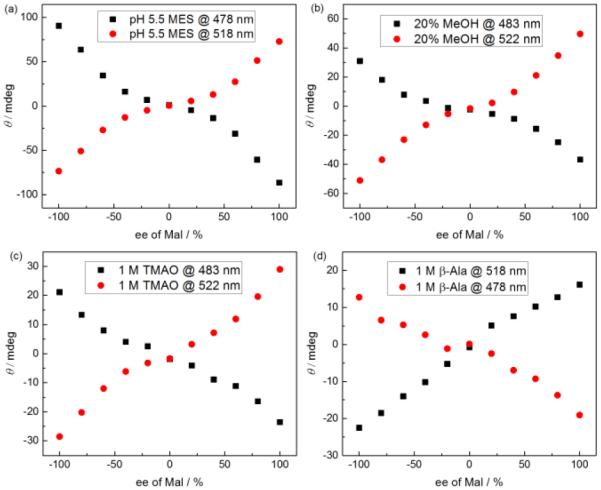
CD intensity of 1 versus ee of Mal under different conditions: (a) at pH 5.5; (b) with 20% (vol%) MeOH at pH 5.0; (c) in the presence of TMAO (1 M) at pH 5.0; (d) in the presence of β-Ala (1 M) at pH 5.0. [1] = 50 μM, [L-Mal] + [D-Mal] = 0.3 mM.
Blind “unknown” samples with ee ranging from 92% to 100% were mixed with the perylenebisimide sensors and the CD spectra of the solutions were obtained, from which the ee values were determined based on the CD-ee calibration curves for the respective host-guest pair. The experimentally determined ee values were compared with their “actual” values on the basis of the amount of enantiomers used in sample preparation. As shown in Table 1, the RRE-active 1/Mal system gave a low average absolute error of 0.2% for Mal ee determination, which is even lower than the error of ee determination by HPLC methods (~1%). In contrast, the MRE-active 2/Tar had a higher average absolute error of 2.2% and an average error of 1.6% for the 1/Lac system that featured a linear CD-ee relationship. This demonstrates the superiority of using RRE-active systems for determination of high ee, compared with linear and MRE systems.
Table 1.
ee (%) Determination of Mal based on CD signals of 1/Mal complex at 487 nm
| Actual ee | 92.0 | 96.0 | 98.0 | −94.0 | −96.0 | −98.0 |
| Calcd. ee | 91.9 | 96.5 | 98.0 | −93.5 | −96.0 | −98.3 |
| Error | −0.1 | 0.5 | 0.0 | 0.5 | 0.0 | −0.3 |
| Average Error |
0.2 | |||||
In summary, we report a RRE on supramolecular polymers, and show that it gives improved errors in ee determination. By exploiting a RRE, we remove the necessity of having to add solutions of the opposite enantiomer to high ee value unknown samples to dial the CD response to a range that is highly sensitive to small changes in CD. This example of a RRE response is unique to malate and compound 1, but it is highly reproducible and the optical responses are very stable. We thus propose that the use of supramolecular polymers could present a general platform for constructing RRE systems, that would create analytical tools for determining ee values with very low errors, even lower than those of HPLC, while retaining all the benefit of parallel analysis and speed. Having demonstrated the idea, the important next step is to understand the fundamental design principles for creating RRE polymers, such that they can be designed and their full benefit can be realized.
Supplementary Material
Table 2.
ee (%) Determination of Lac based on CD signals of 1/Lac complex at 476 nm
| Actual ee | 92.0 | 96.0 | 98.0 | 94.0 | 96.0 | 98.0 |
| Calcd. ee | 94.6 | 94.9 | 96.7 | 91.3 | 95.7 | 96.6 |
| Error | 2.6 | −1.1 | −1.3 | 2.7 | 0.3 | 1.4 |
| Average Error |
1.6 | |||||
Table 3.
ee (%) Determination of Tar based on ICD signals of 2/Tar complex at 487 nm
| Actual ee | 92.0 | 96.0 | 98.0 | 94.0 | 96.0 | 98.0 |
| Calcd. ee | 93.8 | 100.7 | 95.6 | 92.1 | 95.8 | 95.9 |
| Error | 1.8 | 4.7 | −2.4 | 1.9 | 0.2 | 2.1 |
| Average Error |
2.2 | |||||
Acknowledgement
The project was financially supported by the NIH (R01GM077437) and the Welch Regents Chair (F-0046). XXC thanks the China Scholarship Council for a PhD studentship.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.(a) Chen Z, Wang Q, Wu X, Li Z, Jiang YB. Chem. Soc. Rev. 2015;44:4249–4263. doi: 10.1039/c4cs00531g. [DOI] [PubMed] [Google Scholar]; (b) Jo HH, Lin C-Y, Anslyn EV. Acc. Chem. Res. 2014;47:2212–2221. doi: 10.1021/ar500147x. [DOI] [PubMed] [Google Scholar]
- 2.(a) Zhang X, Yin J, Yoon J. Chem. Rev. 2014;114:4918–4959. doi: 10.1021/cr400568b. [DOI] [PubMed] [Google Scholar]; (b) Pu L. Acc. Chem. Res. 2011;45:150–163. doi: 10.1021/ar200048d. [DOI] [PubMed] [Google Scholar]
- 3.(a) Wolf C, Bentley KW. Chem. Soc. Rev. 2013;42:5408–5424. doi: 10.1039/c3cs35498a. [DOI] [PubMed] [Google Scholar]; (b) Berova N, Pescitelli G, Petrovic AG, Proni G. Chem. Commun. 2009:5958–5980. doi: 10.1039/b909582a. [DOI] [PubMed] [Google Scholar]
- 4.Leung D, Kang SO, Anslyn EV. Chem. Soc. Rev. 2012;41:448. doi: 10.1039/c1cs15135e. [DOI] [PubMed] [Google Scholar]
- 5.(a) Bentley KW, Wolf C. J. Org. Chem. 2014;79:6517–6531. doi: 10.1021/jo500959y. [DOI] [PubMed] [Google Scholar]; (b) You L, Pescitelli G, Anslyn EV, Di Bari L. J. Am. Chem. Soc. 2012;134:7117–7125. doi: 10.1021/ja301252h. [DOI] [PubMed] [Google Scholar]; (c) Joyce LA, Maynor MS, Dragna JM, da Cruz GM, Lynch VM, Canary JW, Anslyn EV. J. Am. Chem. Soc. 2011;133:13746–13752. doi: 10.1021/ja205775g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Bentley KW, Zhang P, Wolf C. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1501162. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dragna JM, Pescitelli G, Tran L, Lynch VM, Anslyn EV, Di Bari L. J. Am. Chem. Soc. 2012;134:4398–4407. doi: 10.1021/ja211768v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seifert HM, Jiang Y-B, Anslyn EV. Chem. Commun. 2014;50:15330–15332. doi: 10.1039/c4cc07927b. [DOI] [PubMed] [Google Scholar]
- 8.van Gestel J, Palmans ARA, Titulaer B, Vekemans JAJM, Meijer EW. J. Am. Chem. Soc. 2005;127:5490–5494. doi: 10.1021/ja0501666. [DOI] [PubMed] [Google Scholar]
- 9.Nonokawa R, Yashima E. J. Am. Chem. Soc. 2003;125:1278–1283. doi: 10.1021/ja028348c. [DOI] [PubMed] [Google Scholar]
- 10.(a) Wu X, Chen X-X, Song B-N, Huang Y-J, Li Z, Chen Z, James TD, Jiang Y-B. Chem. Eur. J. 2014;20:11793–11799. doi: 10.1002/chem.201402627. [DOI] [PubMed] [Google Scholar]; (b) Chen X-X, Wu X, Zhang P, Zhang M, Song B-N, Huang Y-J, Li Z, Jiang Y-B. Chem. Commun. 2015;51:13630–13633. doi: 10.1039/c5cc03495g. [DOI] [PubMed] [Google Scholar]
- 11.Bull SD, Davidson MG, van den Elsen JMH, Fossey JS, Jenkins ATA, Jiang Y-B, Kubo Y, Marken F, Sakurai K, Zhao J, James TD. Acc. Chem. Res. 2012;46:312–326. doi: 10.1021/ar300130w. [DOI] [PubMed] [Google Scholar]
- 12.Bolen DW, Baskakov IV. J. Mol. Biol. 2001;310:955–963. doi: 10.1006/jmbi.2001.4819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


