Abstract
Nosocomial infection constitutes a major public health problem worldwide. Increasing antibiotic resistance of pathogens associated with nosocomial infections has also become a major therapeutic challenge for physicians. Thus, development of alternative treatment protocols, such as the use of probiotics, matters. The aim of this research was to determine the antagonistic properties of Lactobacillus plantarum and Lb. fermentum isolated from the faeces of healthy infants against nonfermentative bacteria causing nosocomial infections. One hundred five samples of nosocomial infections were collected and processed for bacterial isolation and antimicrobial susceptibility testing following standard bacteriologic techniques. The antibiotic susceptibility test was performed by the disk diffusion method, and antagonistic effect of Lactobacillus strains was investigated by well diffusion method. Of 105 samples, a total of 29 bacterial strains were identified as nonfermentative bacteria, including 17 Acinetobacter baumannii and 12 Pseudomonas aeruginosa. A. baumannii showed high resistance to tested antibiotics except ampicillin/sulbactam, and P. aeruginosa showed resistance to ampicillin/sulbactam and gentamicin and sensitive to amikacin and meropenem. Lb. plantarum had antagonistic properties against both A. baumannii and P. aeruginosa strains. Lb. plantarum had considerable effects on preventing the growth of A. baumannii and P. aeruginosa strains. However, further research is needed to better understanding of these effects on P. aeruginosa.
Keywords: Antimicrobial resistance, Lactobacillus, nonfermentative bacteria, nosocomial, probiotics
Introduction
Nosocomial infections are one of the major problems in hospitals throughout the world. The term “nosocomial” applies to any infections that develop in a patient during a stay in a hospital or other clinical facilities which were not present at the time of admission. It may become clinically apparent either during the hospitalization or after discharge [1]. Nosocomial infections cause morbidity and mortality, functional disability, emotional suffering and economic burden among hospitalized patients [2], [3]. Infections such as surgical wound, respiratory, bloodstream and urinary tract infections are the most common types of nosocomial infections that occur in hospital settings [4]. Nonfermentative bacteria such as Acinetobacter baumannii and Pseudomonas aeruginosa play an important role in causing nosocomial infections [5].
Probiotics are microorganisms that are believed to provide health benefits for the digestive system when consumed [6]. A number of Lactobacillus species, Bifidobacterium sp., Saccharomyces boulardii and some other microbes have been proposed as and are used as probiotic strains, i.e. live microorganisms as a food supplement to benefit health, such as exerting an antagonistic effect on the gastroenteric pathogens Clostridium difficile, Campylobacter jejuni, Helicobacter pylori and rotavirus, neutralizing food mutagens produced in the colon and lowering serum cholesterol [7]. Various health and nutritional effects of lactic acid bacteria have been described, including improvement of the quality of human and animal foods, metabolic stimulation of the synthesis of vitamins and enzymes, stabilization of the intestinal microflora, competence with intestinal pathogens, host innate immune boost, production of antimicrobials, reduction of the risk of colon cancer by neutralizing carcinogens and suppression of tumors by modulating the probiotic strains [8], [9]. Probiotic properties are not seen in all strains but rather are seen only in certain species, depending on the strain [10]. Most bacteria have developed antibiotic resistance; investing in alternative treatments such as probiotics may help solve this problem.
The aim of this research was to study the effect of Lb. plantarum and Lb. rhamnosus isolated from the faeces of healthy infants in reducing the rates of nonfermentative bacteria causing nosocomial infection.
Material and Methods
In total 105 bacterial samples were collected from Valiasr Hospital Laboratory, Tehran University of Medical Sciences, Tehran, Iran. These samples were collected from the patients in nosocomial infection epidemics. All the samples were primarily investigated for morphologic and biochemical characteristics, including Gram stain, motility, catalase, oxidation and fermentation, grown at 42°C, indole and esculin test. The isolated strains were transferred into tryptic soy broth after adding 15% glycerol in a 1.5 mL microtube and stored at −20°C. Antimicrobial susceptibility testing was performed by the agar diffusion method on Mueller-Hinton agar as recommended by the United States Clinical and Laboratory Standards Institute (CLSI) [11].
Lactobacillus strains
Lb. plantarum 34-5 and Lb. fermentum 89-1 were isolated from the faeces of healthy newborns. Identification of these two strains was performed with 16S rDNA gene sequencing. In brief, genomic DNA was extracted according to a previously described method [8]. The PCR primer sequences were as follows: forward primer 5′-CTCGTTGCGGGACTTAA-3′, and reverse primer 5′-GCAGCAGTAGGGAATCTTC-3′ (Bioneer, Korea). The reaction mixture consisted of 3 pmol primers, 1.5 mM MgCl2, 0.2 mM dNTPs, 2 μL of genomic DNA, 5 μL 10× PCR buffer and 1.5 U of Taq DNA polymerase (Sinaclon, Iran) in a final volume of 50 μL. The PCR program started with an initial denaturation at 94°C for 2 minutes, followed by 30 cycles of 94°C for 30 seconds, 53°C for 1 minute and 72°C for 1 minute [12]. PCR products were separated by agarose gel electrophoresis (1.5% w/v) and visualized by staining with ethidium bromide. The PCR products of strains were sent to a sequencing company (Bioneer, Korea), and the 16S rDNA sequences were compared to known sequences in GenBank using the Basic Local Alignment Search Tool (BLAST; http://www.ncbi.nlm.nih.gov/blast).
Antagonistic test
To test the antagonistic effect of Lactobacillus, the nonfermentative isolates were first cultured on MacConkey and then on nutrient agar. The Lactobacillus strains were inoculated into de Man, Rogosa and Sharpe (MRS) broth and incubated in anaerobic jar at 37°C for 24 hours. On the surface of nutrient agar plate, holes 5 mm in diameter and depth were created under sterile conditions using a Pasteur pipette. Then, using a sterile swab, nonfermentative bacteria of 1/10 McFarland dilutions were inoculated into the surface of nutrient agar. The MRS broth containing Lactobacillus was centrifuged at 6000 rpm for 10 minutes. Supernatant was filtered with a bacteriologic filter. Then 100 μL of solution of each of lactobacilli was poured into a separate well. The media were kept in the refrigerator for 2 hours until the liquid was absorbed, then transferred into the incubator and incubated for 14 to 15 hours at 37°C. After incubation, the diameter of the inhibition zones (mm) around the well was measured using a ruler. The antagonistic effect of lactobacillus against nonfermentative isolates was interpreted on the bases of inhibitory growth zones as follows: negative (−), <11 mm; medium (+), 11 to 16 mm; strong (++), 17 to 22 mm; and very strong (+++), >22 mm [9]. Standard Lb. rhamnosus GG, obtained from the Department of Microbiology, School of Health, Tehran University of Medical Sciences, was used as a control.
Statistical analysis
Data of each assay were analysed by one-way analysis of variance by SAS 9.2 software (SAS Institute, Cary, NC, USA). Comparison among treatment means was performed using Duncan’s new multiple range test. Differences were considered significant at P < 0.05.
Results
Of 105 samples, a total of 29 were identified as nonfermentative bacteria, including 17 A. baumannii and 12 P. aeruginosa. Table 1 shows the antimicrobial susceptibility patterns of A. baumannii. High resistance to tested antibiotics was seen except ampicillin/sulbactam, and 100% resistance to cotrimoxazole was observed. P. aeruginosa showed resistance to ampicillin/sulbactam and gentamicin and was sensitive to amikacin and meropenem (Table 2, Table 3). Lb. plantarum and Lb. rhamnosus had antagonistic properties on both A. baumannii and P. aeruginosa (Table 4). All the tested Lactobacillus strains had a significant antagonistic effect on A. baumannii. Lb. plantarum 34-5 had a more powerful effect compared to the other lactobacilli (Table 4). All the tested lactobacilli isolates had a significant antagonistic effect on P. aeruginosa. Lb. plantarum 34-5 had more powerful effect compared to the other lactobacilli (Table 5). The inhibition zones of A. baumannii and P. aeruginosa isolates by lactobacilli are shown in Fig. 1, Fig. 2.
Table 1.
Antimicrobial resistance patterns of Acinetobacter baumannii isolates
| Antibiotic | Resistant abundance, n (%) | Intermediate abundance, n (%) | Sensitive abundance, n (%) |
|---|---|---|---|
| Amikacin, 30 μg | 13 (76.47) | 1 (5.88) | 3 (17.64) |
| Ciprofloxacin, 5 μg | 16 (94.11) | 1 (5.88) | — |
| Piperacillin/tazobactam, 100/10 μg | 14 (82.35) | 2 (11.76) | 1 (5.88) |
| Cotrimoxazole, 25 μg | 17 (100) | — | — |
| Meropenem, 10 μg | 15 (88.23) | — | 2 (11.76) |
| Ampicillin/sulbactam, 10/10 μg | 8 (47.05) | 1 (5.88) | 8 (47.05) |
| Ceftriaxone, 30 μg | 17 (100) | — | — |
| Ceftazidime, 30 μg | 15 (88.23) | — | 2 (11.76) |
Table 2.
Antimicrobial resistance patterns of Pseudomonas aeruginosa isolates
| Antibiotic | Resistant abundance, n (%) | Intermediate abundance, n (%) | Sensitive abundance, n (%) |
|---|---|---|---|
| Amikacin, 30 μg | 2 (16.66) | 3 (25) | 7 (58.33) |
| Ciprofloxacin, 5 μg | 5 (41.66) | 2 (16.66) | 5 (41.66) |
| Piperacillin/tazobactam, 100/10 μg | 6 (50) | — | 6 (50) |
| Gentamycin, 10 μg | 9 (75) | — | 3 (25) |
| Meropenem, 10 μg | 6 (50) | — | 6 (50) |
| Ampicillin/sulbactam, 10/10 μg | 11 (91.66) | — | 1 (8.33) |
| Ceftriaxone, 30 μg | 7 (58.33) | 1 (8.33) | 4 (33.33) |
| Ceftazidime, 30 μg | 7 (58.33) | — | 5 (41.66) |
Table 3.
Inhibition zone in susceptibility testing of Acinetobacter baumannii and Pseudomonas aeruginosa (mm)
| Isolate | Ceftazidime 30 μg | Ceftriaxone 30 μg | Ampicillin/sulbactam 10/10 μg | Meropenem 10 μg | Gentamicin 10 μg | Cotrimoxazole 25 μg | Piperacillin/tazobactam 100/10 μg | Ciprofloxacin 5 μg | Amikacin 30 μg |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 | 7 | 16 | 9 | NA | 8 | 12 | 10 | 13 |
| 2 | 10 | 8 | 17 | 9 | NA | 8 | 11 | 10 | 12 |
| 3 | 12 | 8 | 17 | 11 | NA | 7 | 12 | 11 | 12 |
| 4 | 12 | 6 | 10 | 12 | NA | 9 | 18 | 9 | 9 |
| 5 | 11 | 5 | 9 | 9 | NA | 8 | 13 | 11 | 13 |
| 6 | 10 | 6 | 16 | 11 | NA | 9 | 12 | 13 | 14 |
| 7 | 18 | 6 | 17 | 6 | NA | 10 | 19 | 10 | 15 |
| 8 | 13 | 7 | 17 | 6 | NA | 5 | 21 | 12 | 11 |
| 9 | 10 | 5 | 10 | 6 | NA | 9 | 14 | 10 | 17 |
| 10 | 11 | 6 | 9 | 10 | NA | 6 | 15 | 8 | 18 |
| 11 | 10 | 7 | 7 | 9 | NA | 5 | 12 | 10 | 14 |
| 12 | 10 | 8 | 9 | 8 | NA | 5 | 11 | 11 | 12 |
| 13 | 19 | 6 | 11 | 7 | NA | 8 | 14 | 16 | 13 |
| 14 | 9 | 5 | 8 | 8 | NA | 7 | 13 | 11 | 11 |
| 15 | 12 | 5 | 16 | 16 | NA | 7 | 12 | 12 | 10 |
| 16 | 13 | 6 | 18 | 17 | NA | 5 | 11 | 10 | 18 |
| 17 | 11 | 9 | 7 | 9 | NA | 6 | 14 | 10 | 9 |
| 18 | 14 | 12 | 8 | 15 | 11 | NA | 14 | 17 | 18 |
| 19 | 13 | 10 | 9 | 13 | 10 | NA | 13 | 13 | 19 |
| 20 | 13 | 10 | 8 | 20 | 8 | NA | 14 | 14 | 12 |
| 21 | 19 | 15 | 7 | 13 | 8 | NA | 21 | 21 | 21 |
| 22 | 19 | 21 | 6 | 19 | 10 | NA | 21 | 22 | 19 |
| 23 | 18 | 21 | 15 | 22 | 15 | NA | 22 | 18 | 20 |
| 24 | 12 | 10 | 7 | 14 | 9 | NA | 23 | 15 | 15 |
| 25 | 11 | 11 | 9 | 14 | 8 | NA | 14 | 21 | 18 |
| 26 | 19 | 13 | 10 | 19 | 7 | NA | 13 | 14 | 15 |
| 27 | 13 | 22 | 9 | 20 | 17 | NA | 13 | 22 | 14 |
| 28 | 11 | 21 | 8 | 21 | 17 | NA | 21 | 14 | 16 |
| 29 | 11 | 11 | 8 | 13 | 9 | NA | 22 | 22 | 18 |
NA, not applicable.
Table 4.
Antagonistic effect (inhibition zone) of Lactobacillus fermentum 89-1, Lb. plantarum 34-5 and Lb. rhamnosus GG against Acinetobacter baumannii isolates
| Lactobacillus genus | Antagonistic effect (inhibition zone)a by abundance, n (%) |
|||
|---|---|---|---|---|
| − | + | ++ | +++ | |
| plantarum 34-5 | — | 7 (41.17) | 10 (58.82) | — |
| fermentum 89-1 | — | 14 (82.35) | 3 (17.64) | — |
| rhamnosus GG | — | 15 (88.23) | 2 (11.76) | — |
Inhibitory growth zones were interpreted as follows: negative (−), <11 mm; medium (+), 11–16 mm; strong (++), 17–22 mm; and very strong (+++), >22 mm.
Table 5.
Antagonistic effect (inhibition zone) of Lactobacillus fermentum 89-1, Lb. plantarum 34-5 and Lb. rhamnosus GG against Pseudomonas aeruginosa isolates
| Lactobacillus genus | Antagonistic effect (inhibition zone)a by abundance, n (%) |
|||
|---|---|---|---|---|
| − | + | ++ | +++ | |
| plantarum 34-5 | 1 (8.33) | — | 10 (83.33) | 1 (8.33) |
| fermentum 89-1 | 1 (8.33) | 9 (75) | 2 (16.66) | — |
| rhamnosus GG | 1 (8.33) | 3 (25) | 8 (66.66) | — |
Inhibitory growth zones were interpreted as follows: negative (−), <11 mm; medium (+), 11–16 mm; strong (++), 17–22 mm; and very strong (+++), >22 mm.
Fig. 1.
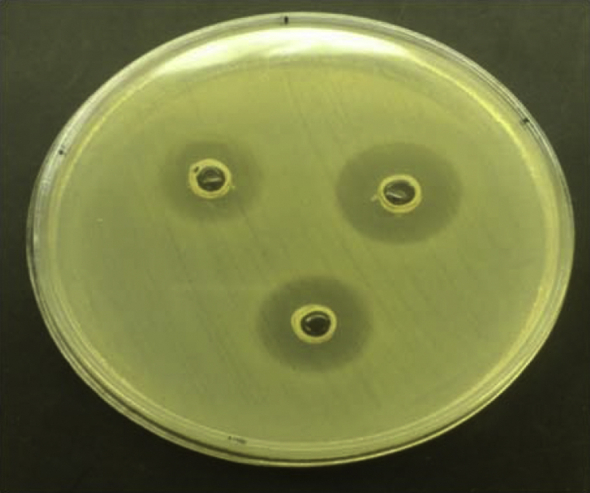
Inhibition zone of Acinetobacter baumannii caused by Lactobacillus spp. (Top left) Lb. fermentum 89-1 (16 mm). (Top right) Inhibition zone of A. baumannii caused by Lb. plantarum 34-5 (19 mm). (Bottom) Lb. rhamnosus GG (17 mm).
Fig. 2.
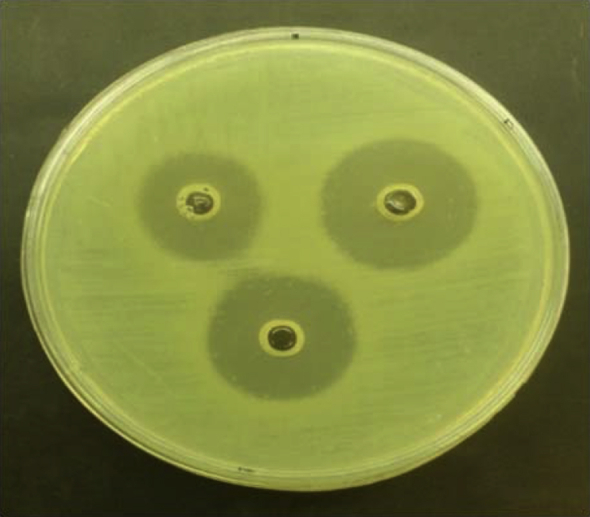
Inhibition zone of P. aeruginosa caused by Lactobacillus spp. (Top left) Lb. fermentum 89-1 (20 mm). (Top right) Inhibition zone of P. aeruginosa caused by Lb. plantarum 34-5 (24 mm). (Bottom) Lb. rhamnosus GG (22 mm).
Discussion
Eradication and treatment of infections caused by P. aeruginosa and A. baumannii are difficult because of their high resistance to antibiotics and disinfectants [13], [14]. These bacteria can cause serious infections in hospitalized patients because they can grow under a variety of different conditions and have acquired widespread antibiotic resistance. They therefore result in important nosocomial infections that impose high costs on healthcare [15]. Lactobacilli are harmless microorganisms capable of producing acid secretion, bacteriocins and other by-products that can neutralize some pathogens, can regulate the inflammatory response of the immune system and can be used in the treatment of gastrointestinal disorders [16], [17].
Our results showed that lactobacilli had an inhibition growth effect on both A. baumannii and P. aeruginosa. These results are in agreement with previously reported findings that different strains of lactobacilli inhibit the growth of bacteria such as Staphylococcus aureus, Escherichia coli, P. aeruginosa, Klebsiella pneumoniae, and Burkholderia cepacia [18], [19]. Others have reported that lactobacilli had an inhibitory effect on the growth of both Gram-negative and Gram-positive bacteria [20]. In addition, there is also in vitro report of probiotics against pathogenic bacteria [21]. Several previous studies have shown that probiotic production factors other than lactic acid, such as bacteriocins, proteinase, peroxidase and exopolysaccharide, can exert antibacterial effects [18]. Some studies have reported that lactobacilli such as Lb. plantarum, Lb. paracasei, Lb. fermentum, Lb. bokash and Lb. boots isolated from the faeces of infants had inhibitory activity against food-contaminated bacteria such as E. coli, S. aureus, Yersinia enterocolitica and Bacillus cereus [9], [22]. A previous study demonstrated the antibacterial effect of Lactobacillus isolated from breast milk against the gastrointestinal pathogenic bacteria E. coli, Shigella, Pseudomonas and Salmonella [22]. Another study found that strains of lactobacilli lower the effect of production of elastase and biofilm formation [15].
Conclusion
The lactobacilli had good effects on preventing the growth of A. baumannii and P. aeruginosa. These results are in agreement with other published reports from different countries that indicate that infection control efforts may be achieved with probiotic bacteria. We believe that more attention should be paid to these areas, particularly to create a standardized approach.
Acknowledgements
Supported in part by Tehran University of Medical Sciences (contract 28577). We also thank Dr. A. R. Abdolahi, head of Valiasr Laboratory, and our colleagues, S. A. Dehghan, S. Adina and E. Mohammadnejad. We also thank Dr. Abdolahi, head of Valiasr Laboratory, and our colleagues, Miss Dehghan, Adina and Mr. Mohammadnejad.
Conflict of Interest
None declared.
References
- 1.Ducel G., Fabry J., Nicolle L. 2nd ed. World Health Organization; Geneva: 2002. Prevention of hospital acquired infections: a practical guide. [Google Scholar]
- 2.Kamat U., Ferreira A., Savio R., Motghare D. Antimicrobial resistance among nosocomial isolates in a teaching hospital in Goa. Indian J Community Med. 2008;33:89–92. doi: 10.4103/0970-0218.40875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endalafer N., Gebre-Selassie S., Kotiso B. Nosocomial bacterial infections in a tertiary hospital in Ethiopia. J Infect Prev. 2011;12:38–43. [Google Scholar]
- 4.Graves N. Personal Social Services Research Unit, University of Kent; 2001. The cost of hospital acquired infections. Unit costs of health and social care; pp. 25–30. [Google Scholar]
- 5.Ferrara A.M. Potentially multidrug-resistant non-fermentative Gram-negative pathogens causing nosocomial pneumonia. Int J Antimicrob Agents. 2006;27:183–195. doi: 10.1016/j.ijantimicag.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Kotzampassi K., Giamarellos-Bourboulis E.J. Probiotics for infectious diseases: more drugs, less dietary supplementation. Int J Antimicrob Agents. 2012;40:288–296. doi: 10.1016/j.ijantimicag.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Gu R.X., Yang Z.Q., Li Z.H., Chen S.L., Luo Z.L. Probiotic properties of lactic acid bacteria isolated from stool samples of longevous people in regions of Hotan, Xinjiang and Bama, Guangxi, China. Anaerobe. 2008;14:313–317. doi: 10.1016/j.anaerobe.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Davoodabadi A., Soltan Dallal M.M., Foroushani A.R., Douraghi M., Harati F.A. Antibacterial activity of Lactobacillus spp. isolated from the feces of healthy infants against enteropathogenic bacteria. Anaerobe. 2015;34:53–58. doi: 10.1016/j.anaerobe.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Tsai C.C., Lin P.P., Hsieh Y.M. Three Lactobacillus strains from healthy infant stool inhibit enterotoxigenic Escherichia coli grown in vitro. Anaerobe. 2008;14:61–67. doi: 10.1016/j.anaerobe.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Servin A.L., Coconnier M.H. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol. 2003;17:741–754. doi: 10.1016/s1521-6918(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute (CLSI) Clinical and Laboratory Standards Institute; Wayne, PA: 2012. Performance standards for antimicrobial disk susceptibility testing: approved standard. M100-S22 Vol. 32 No. 3. [Google Scholar]
- 12.Yun J.H., Lee K.B., Sung Y.K., Kim E.B., Lee H.G., Choi Y.J. Isolation and characterization of potential probiotic lactobacilli from pig feces. J Basic Microbiol. 2009;49:220–226. doi: 10.1002/jobm.200800119. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlowsky J.A., Draghi D.C., Jones M.E., Thornsberry C., Friedland I.R., Sahm D.F. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrobial Agents Chemother. 2003;47:1681–1688. doi: 10.1128/AAC.47.5.1681-1688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdez J., Peral M., Rachid M., Santana M., Perdigon G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect. 2005;11:472–479. doi: 10.1111/j.1469-0691.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 16.Emami A., Hashemizadeh Z., Nouei A.R. Investigating the antibacterial activity of L. casei and L. acidophilus against common agents of nosocomial infections. J Qazvin Univ Med Sci. 2010;14:31–37. [Google Scholar]
- 17.Soltan Dallal M.M., Mirak S., Azarsa M., Rahbar M., Yazdi M.K.S. Evaluation of antimicrobial activity of Lactobacillus plantarum and ruteri on Burkholderia cepacia isolated from nosocomial infections. Pajoohandeh J. 2013;18:202–207. [Google Scholar]
- 18.Coconnier M.H., Lievin V., Hemery E., Servin A.L. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573–4580. doi: 10.1128/aem.64.11.4573-4580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi M., Taguchi H., Yamaguchi H., Osaki T., Komatsu A., Kamiya S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol. 2004;41:219–226. doi: 10.1016/j.femsim.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Servin A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Arici M., Bilgin B., Sagdic O., Ozdemir C. Some characteristics of Lactobacillus isolates from infant faeces. Food Microbiol. 2004;21:19–24. [Google Scholar]
- 22.Jara S., Sanchez M., Vera R., Cofre J., Castro E. The inhibitory activity of Lactobacillus spp. isolated from breast milk on gastrointestinal pathogenic bacteria of nosocomial origin. Anaerobe. 2011;17:474–477. doi: 10.1016/j.anaerobe.2011.07.008. [DOI] [PubMed] [Google Scholar]


