Abstract
The aim of our study was to evaluate the seasonal variations and whether short-term exposure to environmental risk factors, such as climate and air pollution, is associated with PTB-related hospital admissions in human immunodeficiency virus (HIV)-infected patients in Spain during the era of combined antiretroviral therapy (cART). A retrospective study was carried out using data from the Minimum Basic Data Set (MBDS) and the State Meteorological Agency (AEMET) of Spain. The primary outcome variable was hospital admissions with PTB diagnosis. The environmental risk factors evaluated were season, temperature, humidity, NO2, SO2, O3, PM10, and CO. Overall, HIV-infected patients had a lower frequency of PTB-related hospital admissions in summer (22.8%) and autumn (22.4%), but higher values in winter (26.6%) and spring (28.2%). Using a Bayesian temporal model, PTB-related hospital admissions were less frequent in summer-autumn and more abundant in winter-spring during the first years of follow-up. During the later years of follow-up, the seasonal trends continued resulting in the lowest values in autumn and the highest in spring. When considering short-term exposure to environmental risk factors, lower temperatures at 1 week (odds ratio (OR) = 1.03; p = 0.008), 1.5 weeks (OR = 1.03; p<0.001), 2 weeks (OR = 1.04; p<0.001), and 3 weeks (OR = 1.03; p<0.001) prior to PTB admission. In addition, higher concentration of NO2 at the time of admission were significantly associated with higher likelihoods of PTB-related hospital admission in HIV-infected patients when 1.5 weeks (OR = 1.1; p = 0.044) and 2 weeks (OR = 1.21; p<0.001) were used as controls. Finally, higher concentration of SO2 at 1.5 weeks prior to PTB admission was significantly associated with a higher likelihood of PTB-related hospital admissions (OR = 0.92; p = 0.029). In conclusion, our data suggest an apparent seasonal variation in hospital admissions of HIV-infected patients with a PTB diagnosis (summer/autumn vs. winter/spring), as well as a link to short-term exposure to environmental risk factors, such as temperature and ambient NO2 and SO2.
Introduction
Tuberculosis remains one of the world’s deadliest communicable diseases. Despite notable progress in the last decades, tuberculosis is still a public health concern in most of the countries within the WHO European Region [1]. Human immunodeficiency virus (HIV) infection is the most important risk factor for developing tuberculosis in patients already infected with Mycobacterium tuberculosis [2,3], and tuberculosis is the most common acquired immunodeficiency syndrome (AIDS)-defining condition worldwide [4]. Globally in 2014, 9.6 million people developed tuberculosis and around 1.5 million died from the disease. At least one-third of people living with HIV worldwide were infected by tuberculosis. There were around 1.2 million new cases of tuberculosis amongst people who were HIV-positive and about four hundred thousand people died of HIV-associated tuberculosis [5]. Among European Union/European Economic Area countries, Spain had one of the highest incidences of AIDS (from 1994 to 2009) and tuberculosis (from 1995 to 2009) [6,7].
Mycobacterium tuberculosis is the causative agent of pulmonary tuberculosis (PTB). There are several possible outcomes for a person exposed to Mycobacterium tuberculosis bacilli [8]: i) the infection may be immediately destroyed by the host's innate immune response; ii) a proportion of individuals develop active PTB due to an inability to control the initial infection and mount a protective response; iii) in the majority of persons a clinically latent infection will be established, and approximately 5–10% of these will experience reactivation of the infection causing active tuberculosis.
Seasonal variation is a considerable factor in regard to PTB, but the specific effects it has on the epidemic is not entirely clear since several studies have identified incidence peaks in winter, spring, and summer [9–11]. Furthermore, the interpretation of seasonality in relation to PTB risk is complicated due to the large number of factors to consider such as environmental factors (temperature, humidity, sunlight), social factors (crowding and person-to-person contact), and delays in the diagnosis and treatment of tuberculosis particularly in winter [12,13]. These factors seem to affect both primary infection and reoccurrence, although on the latter to a lesser degree. The link between vitamin D deficiency and impaired host defenses against Mycobacterium tuberculosis may be a factor since seasonal variability of PTB seems to mirror the seasonal fluctuation of vitamin D levels [13–15]. Vitamin D levels depend on the conversion rate of pro-vitamin D3 to pre-vitamin D3 by sunlight, which is affected by seasonal characteristics (cloud cover, hours of daylight, outdoor activities, exposed skin surface area, etc.), latitude, radiation, etc. [12,15]. Thus, the conversion to pre-vitamin D3 tends to drop in autumn until spring, and the highest conversion rates are found in the summer [12].
PTB is a disease that may be influenced by environmental factors [16]. Hospital admissions in colder months are significantly higher than in warmer months [17,18], however the occurrence of extremely high temperatures during the summer has resulted in a significant increase in the number of tuberculosis cases [19]. Moreover, air pollution is a substantial cause of morbidity and mortality worldwide [20]. Several studies suggest an association between long-term exposure to ambient air pollution and tuberculosis [21–25], and one study found a significant association between short-term ambient air pollution exposure and tuberculosis risk [17]. However, to our knowledge, no epidemiologic studies analyzing environmental factors and PTB incidence in HIV-infected patients has been conducted.
Biologically, environmental factors could be involved in the pathogenesis of tuberculosis through an impact on immune function [16,26,27] and thereby increase susceptibility to developing active PTB. Increased levels of air pollutants have been related to impaired lung function via oxidative stress, which may produce inflammation of the airways, decrease macrophage function, and increase susceptibility to pathogens [16,26]. Moreover, diesel exhaust particles suppress the expression of proinflammatory mediators during tuberculosis infection, inducing a hyporesponsive cellular state, which is a possible mechanism by which air pollutants alter antimicrobial immunity [28]. Also of concern, a weakened immune system caused by HIV infection may promote tuberculosis reactivation [29].
The aim of our study was to evaluate the seasonal variations of hospital admissions with a PTB diagnosis and to determine whether short-term exposure to environmental risk factors (climatological factors and air pollution levels) is related to PTB-related hospital admission in HIV-infected patients in Spain during the era of combined antiretroviral therapy (cART).
Materials and Methods
Study population
We performed a retrospective analysis using data of all HIV positive patients aged 16 years and older with a hospital discharge and PTB diagnosis in Spanish hospitals from 1 January 1997 to 31 December 2012. Patients without postal code information in the MBDS were excluded. At hospital discharge, out of 45,427 patients with PTB and postal code in the MBDS, 5,712 were infected with HIV. We did not have postal code for 41% of the records. However, 69% of patients with postal code were distributed in a uniform manner throughout Spain (see S1 Fig).
Data from patients with a diagnosis of PTB were obtained from the Spanish Minimum Basic Data Set (MBDS) provided by the Ministry of Health Social Services and Equality (MSSSI). The MBDS is a clinical and administrative database containing information obtained and recorded at time of hospital discharge, with an estimated coverage of 97.7% of total hospital admissions to public hospitals in Spain [30]. The National Health System (NHS) provides free medical care to 99.5% of the Spanish population, although those persons not covered by the NHS can be attended to at the public hospitals.
The MBDS provided the encrypted patient identification number, sex, date of birth, dates of hospital admission and discharge, patients’ residential postal code, medical institutions providing the services, the diagnosis and procedure codes according to the International Classification of Diseases, 9th ed, Clinical Modification (ICD-9-CM), and outcome at discharge. The CMBD includes up to 14 discharge diagnoses and up to 20 procedures performed during the hospital stay. The Spanish MSSSI sets standards for record-keeping and performs periodic audits [30].
Ethics statement
This study involves the use of patient medical data from the Spanish MBDS, which is hosted by the MSSSI. The MBDS is regulated by law that explains how institutions are required to utilize health-related personal data. As described in detail previously [31], the data were treated with full confidentiality according to Spanish legislation. We requested the databases by filling, signing and sending a questionnaire with a Confidentiality Commitment. The MSSSI evaluated the protocol of our investigation and considered it to meet all ethical aspects according to Spanish legislation. Given the anonymous and mandatory nature of the dataset, it was not necessary to obtain informed consent. Additionally, our study was approved by the Research Ethic Committee (Comité de Ética de la Investigación y de Bienestar Animal) of the Instituto de Salud Carlos III (Madrid, Spain).
Environmental data
We did not have any data on the exposure levels experienced by individuals. However, exposure to climatic and pollutant factors was obtained as a surrogate using the nearest meteorological station to his or her postal code at the time of hospitalization. Environmental data were provided by the State Meteorological Agency (AEMET) (http://www.aemet.es/). For each station, AEMET provided daily data for temperature, humidity, sulfur dioxide (SO2), carbon monoxide (CO), nitrogen dioxide (NO2), ozone (O3), particulate matter up to 10 μm in size (PM10), and station geolocation (latitude, longitude, and altitude).
Outcome variables
The outcome variable analyzed in this study was the first PTB-related hospital admission. A hospitalization was defined as a discharge record in the MBDS. The index episode of a patient was defined as the first hospital discharge encoded in MBDS with a PTB diagnosis (ICD-9-CM code 011). Hospital readmissions were not counted as new episode of PTB.
Statistical analysis
In order to evaluate the seasonal effect on PTB-related hospital admissions, the dates of hospital admission were divided into 4 seasons: spring (March-May), summer (June-August), autumn (September-November) and winter (December-February). A model for seasonal variation was obtained assuming that the sums were independent Gaussian with precision Gamma = (1; 0,001) [32]. The effect was adapted automatically to higher cases in the winter and lower cases in the summer. This specification of the model allows time-varying disease onsets, which is not possible using simple sums of sine and cosine component summer. Seasonal effects were evaluated using a Bayesian model with Poisson distribution [32]. Significance of seasonal effects was calculated based on deviance information criterion.
As described in detail previously [31], a case-crossover design (CCD) was used to evaluate the effect of each environmental factor (temperature, humidity, NO2, SO2, O3, PM10, and CO) on the PTB-related hospital admissions. In the CCD, each individual experiencing a health event serves as his or her own reference. In other words, individuals act as their own control [33]. In air pollution epidemiology, CCD is the most suitable study design for studying the effects on health outcomes of varying short-term exposure [34]. In the case of PTB, 4 different short time periods before hospital admission were considered as control periods (1 week, 1.5 week, 2 weeks and 3 weeks), in order to compare the environmental exposure of individual patients at the time of presentation with a PTB-related hospital admission (baseline) [35]. For each time period, we looked at an average value for each environmental factor over a 3-day period (on the day of PTB-related hospital admission and the 2 days immediately prior to hospital admission) in order to mitigate for any single day with an extreme level. Finally, conditional logistic regression (CLR) was used to evaluate the association between environmental factors and hospital admissions. The odds ratio (OR) and its 95% confidence interval were calculated by exact method. In a CCD for each factor, the odds of an event with respect to an increase in the average level of the environmental factor around the date of hospitalization (case at hospital admission) were compared to the change in the factor when PTB hospitalization did not occur (control time at 1 week, 1.5 weeks, 2 weeks, and 3 weeks before hospital admission). In the basic inference, for each case the exposure status during admission (encoded as “1”) and control time (encoded as “0”) are compared, and only subjects with different levels of exposure are informative [34]. In our study, OR values higher than 1 indicate an association of the analyzed factor with higher risk when it is increased at the time of hospital admission or decreased at timepoints before hospital admission; whereas OR values lower than one indicate the analyzed factor is associated with greater risk when it is increased at the control time or decreased at the time of hospital admission. All environmental factors, except temperature, were log-transformed because they varied greatly across dates of measurements. Results of single- and multi- environmental factors model (temperature + humidity + NO2 + SO2 + O3 + PM10 + CO) were presented. The multi-environmental factors analysis for air pollution and PTB association was considered because it may account for possible mutual confounding between environmental factors.
Statistical analysis was performed using the R statistical package version 3.1.1 (GNU General Public License) [36]. All tests were two-tailed with p-values <0.05 considered significant.
Results
Characteristics of study population
A total of 45,427 patients had a PTB diagnosis in the MBDS between 1997 and 2012, of which 5,712 were HIV positive. Table 1 shows the clinical and epidemiological characteristics of the patients included in the study. The median age was 37.9 years and 80.1% were male. The most frequent comorbidities were mild liver disease (31.9%), chronic pulmonary disease (4.9%), and cancer (3.7%).
Table 1. Summary of epidemiological and clinical characteristics of HIV-infected patients with a diagnosis of pulmonary tuberculosis from 1997 to 2012 in Spain.
| Description | HIV-infected patients |
|---|---|
| No. of patients | 5,712 |
| Males | 4,577 (80.1%) |
| Age (years) | 37.96 (37.74; 38.18) |
| Comorbidities | |
| Myocardial Infarction | 19 (0.3%) |
| Congestive Heart Failure | 21 (0.4%) |
| Peripheral Vascular Disease | 7 (0.1%) |
| Cerebrovascular Disease | 43 (0.8%) |
| Dementia | 30 (0.5%) |
| Chronic Pulmonary Disease | 279 (4.9%) |
| Connective Tissue Disease-Rheumatic Disease | 3 (0.1%) |
| Peptic Ulcer Disease | 18 (0.3%) |
| Mild Liver Disease | 1,824 (31.9%) |
| Diabetes without complications | 87 (1.5%) |
| Diabetes with complications | 2 (0%) |
| Paraplegia and Hemiplegia | 44 (0.8%) |
| Renal Disease | 74 (1.3%) |
| Cancer | 212 (3.7%) |
| Moderate or Severe Liver Disease | 86 (1.5%) |
| Metastatic Carcinoma | 33 (0.6%) |
Values are expressed as absolute number (percentage) and mean (95% of confidence interval).
Abbreviations: HIV, human immunodeficiency virus.
Effect of season on PTB-related hospital admission
The annual distribution and seasonal distribution of hospital admissions with PTB diagnoses for the study period are shown in Table 2. The 1997–1999 period had the largest number, but then it decreased during the rest of the study period. Overall, HIV-infected patients had lower frequencies of PTB-related hospital admissions in summer (22.8%) and autumn (22.4%), and higher values in winter (26.6%) and spring (28.2%) (Table 2).
Table 2. Summary of annual distribution and seasonal distribution of pulmonary tuberculosis diagnoses between 1997 and 2012 in Spain among HIV-infected patients.
| Seasonal distribution | |||||
|---|---|---|---|---|---|
| Year | Annual | Summer | Autumn | Winter | Spring |
| 1997 | 619 (10.8%) | 132 (21.3%) | 123 (19.9%) | 205 (33.1%) | 159 (25.7%) |
| 1998 | 685 (12%) | 151 (22%) | 121 (17.7%) | 209 (30.5%) | 204 (29.8%) |
| 1999 | 670 (11.7%) | 153 (22.8%) | 158 (23.6%) | 166 (24.8%) | 193 (28.8%) |
| 2000 | 500 (8.8%) | 111 (22.2%) | 81 (16.2%) | 157 (31.4%) | 151 (30.2%) |
| 2001 | 446 (7.8%) | 96 (21.5%) | 100 (22.4%) | 118 (26.5%) | 132 (29.6%) |
| 2002 | 360 (6.3%) | 77 (21.4%) | 88 (24.4%) | 88 (24.4%) | 107 (29.7%) |
| 2003 | 300 (5.3%) | 68 (22.7%) | 67 (22.3%) | 84 (28%) | 81 (27%) |
| 2004 | 302 (5.3%) | 65 (21.5%) | 64 (21.2%) | 82 (27.2%) | 91 (30.1%) |
| 2005 | 311 (5.4%) | 83 (26.7%) | 77 (24.8%) | 79 (25.4%) | 72 (23.2%) |
| 2006 | 190 (3.3%) | 52 (27.4%) | 44 (23.2%) | 36 (18.9%) | 58 (30.5%) |
| 2007 | 293 (5.1%) | 87 (29.7%) | 59 (20.1%) | 66 (22.5%) | 81 (27.6%) |
| 2008 | 252 (4.4%) | 64 (25.4%) | 60 (23.8%) | 71 (28.2%) | 57 (22.6%) |
| 2009 | 284 (5%) | 68 (23.9%) | 63 (22.2%) | 76 (26.8%) | 77 (27.1%) |
| 2010 | 110 (1.9%) | 30 (27.3%) | 26 (23.6%) | 25 (22.7%) | 29 (26.4%) |
| 2011 | 210 (3.7%) | 51 (24.3%) | 46 (21.9%) | 50 (23.8%) | 63 (30%) |
| 2012 | 180 (3.2%) | 37 (20.6%) | 44 (24.4%) | 54 (30%) | 45 (25%) |
| 1997–2012 | |||||
| Median | 5.3% | 22.4% | 26.6% | 28.2% | 22.8% |
| 25th percentile | 4.2% | 20.9% | 24.3% | 26.2% | 21.5% |
| 75th percentile | 8.0% | 23.7% | 28.6% | 29.8% | 25.7% |
| Interquartile range | 3.8% | 2.8% | 4.3% | 3.6% | 4.2% |
| Minimum | 1.9% | 16.2% | 18.9% | 22.6% | 20.6% |
| Maximum | 12.0% | 24.8% | 33.1% | 30.5% | 29.7% |
| Range | 10.1% | 8.6% | 14.2% | 7.9% | 9.1% |
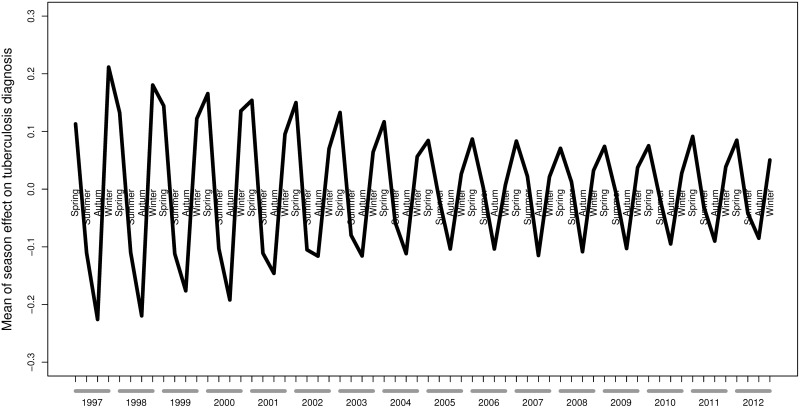
When seasonal effects were evaluated using a Bayesian model (Fig 1), PTB-related hospital admissions were less frequent in summer-autumn and more abundant in winter-spring during the first years of follow-up, with a transition to the lowest values in autumn and the highest values in spring during the later years of follow-up.
Fig 1. Mean of the seasonal effect on pulmonary tuberculosis diagnosis in HIV-infected patients from Spain between 1997 and 2012.
Effects of short-term exposure to environmental risk factors on PTB-related hospital admissions
When we used a single environmental factor model, we found significant associations of temperature, humidity, NO2, SO2, O3, and PM10 with PTB-related hospital admissions (Table 3). Also, we found significant associations of temperature, NO2, and SO2 with PTB-related hospital admission when we used a multi-environmental factor model (Table 3). Both temperature and NO2 concentrations showed significant OR values >1 (Table 3). Thus, lower temperatures at 1 week (OR = 1.03; p = 0.008), 1.5 weeks (OR = 1.03; p<0.001), 2 weeks (OR = 1.04; p<0.001), and 3 weeks (OR = 1.03; p<0.001) prior to PTB admission were significantly associated with higher likelihoods of PTB-related hospital admission in HIV-infected patients, as was higher concentrations of NO2 at the time of admission when 1.5 weeks (OR = 1.1; p = 0.044) and 2 weeks (OR = 1.21; p<0.001) were used as controls. Concentrations of SO2 showed significant OR values <1 (Table 3); specifically, higher concentrations of SO2 at 1.5 weeks prior to PTB admission were significantly associated with higher likelihoods of PTB-related hospital admissions (OR = 0.92; p = 0.029). Note that associations of humidity, O3, and PM10 with PTB-related hospital admission were lost in the multi-environmental factor model. We did not find any significant association between CO concentrations and risk of PTB hospitalization.
Table 3. Summary of associations between short-term exposure of environmental factors and hospital admissions in HIV-infected patients with pulmonary tuberculosis when compared with levels at 1 week, 1.5 weeks, 2 weeks, and 3 weeks before hospital admission.
| Single-factor analysis | Multi-factor analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| 1 week | ||||
| Temperature (°C) | 1.03 (1.01; 1.04) | 0.008 | 1.03 (1.01; 1.04) | 0.008 |
| Humidity (%) | 0.98 (0.95; 1.01) | 0.159 | 0.98 (0.95; 1.01) | 0.15 |
| NO2 (μg/m3) | 1.05 (0.97; 1.15) | 0.221 | 1.09 (0.99; 1.19) | 0.086 |
| SO2 (μg/m3) | 0.97 (0.9; 1.04) | 0.417 | 0.95 (0.88; 1.03) | 0.224 |
| O3 (μg/m3) | 1 (0.91; 1.09) | 0.924 | 0.97 (0.88; 1.08) | 0.620 |
| PM10 (μg/m3) | 0.98 (0.91; 1.06) | 0.668 | 0.98 (0.9; 1.06) | 0.586 |
| CO (μg/m3) | 1 (0.92; 1.09) | 0.939 | 1 (0.92; 1.1) | 0.974 |
| 1.5 weeks | ||||
| Temperature (°C) | 1.03 (1.01; 1.04) | 0.001 | 1.03 (1.02; 1.05) | <0.001 |
| Humidity (%) | 0.99 (0.96; 1.03) | 0.725 | 1 (0.97; 1.03) | 0.827 |
| NO2 (μg/m3) | 1.01 (0.93; 1.1) | 0.824 | 1.1 (1; 1.21) | 0.044 |
| SO2 (μg/m3) | 0.93 (0.87; 0.99) | 0.025 | 0.92 (0.86; 0.99) | 0.029 |
| O3 (μg/m3) | 1.06 (0.97; 1.17) | 0.183 | 1.02 (0.92; 1.12) | 0.754 |
| PM10 (μg/m3) | 0.95 (0.88; 1.02) | 0.149 | 0.95 (0.88; 1.03) | 0.197 |
| CO (μg/m3) | 0.91 (0.84; 0.98) | 0.017 | 0.92 (0.85; 1) | 0.063 |
| 2 weeks | ||||
| Temperature (°C) | 1.03 (1.02; 1.05) | <0.001 | 1.04 (1.03; 1.05) | <0.001 |
| Humidity (%) | 0.98 (0.95; 1.01) | 0.252 | 0.98 (0.95; 1.01) | 0.141 |
| NO2 (μg/m3) | 1.19 (1.1; 1.29) | <0.001 | 1.21 (1.1; 1.33) | <0.001 |
| SO2 (μg/m3) | 1.03 (0.97; 1.1) | 0.307 | 0.98 (0.91; 1.05) | 0.500 |
| O3 (μg/m3) | 0.96 (0.88; 1.05) | 0.395 | 0.99 (0.89; 1.09) | 0.790 |
| PM10 (μg/m3) | 1.09 (1.01; 1.17) | 0.018 | 1.03 (0.95; 1.11) | 0.511 |
| CO (μg/m3) | 1.05 (0.97; 1.13) | 0.234 | 1 (0.92; 1.09) | 0.931 |
| 3 weeks | ||||
| Temperature (°C) | 1.03 (1.02; 1.04) | <0.001 | 1.03 (1.02; 1.05) | <0.001 |
| Humidity (%) | 0.97 (0.94; 1) | 0.037 | 0.97 (0.94; 1) | 0.058 |
| NO2 (μg/m3) | 1 (0.92; 1.08) | 0.937 | 1.07 (0.98; 1.17) | 0.149 |
| SO2 (μg/m3) | 0.94 (0.87; 1) | 0.050 | 0.93 (0.87; 1) | 0.064 |
| O3 (μg/m3) | 1.1 (1; 1.2) | 0.039 | 1.03 (0.93; 1.14) | 0.554 |
| PM10 (μg/m3) | 0.97 (0.9; 1.05) | 0.467 | 0.97 (0.9; 1.06) | 0.542 |
| CO (μg/m3) | 0.93 (0.86; 1.01) | 0.069 | 0.96 (0.88; 1.04) | 0.313 |
Abbreviations: NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone; PM10, particulate matter up to 10 μm in size; CO: carbon monoxide; OR, odds ratio; 95% CI, 95% of confidence interval; HIV, human immunodeficiency virus.
Discussion
To our knowledge, this is the first report analyzing the influence of seasonality and short-term exposure to climatological factors and air pollution on PTB epidemiology in HIV-infected patients.
In our study, a seasonal effect was observed since the highest frequencies of PTB-related hospital admissions were found in winter-spring. Vitamin D levels may be affected by seasonality [10,11,37], which affects the risk of developing tuberculosis [38]. Sunlight exposure is related to vitamin D production. Specifically, the highest incidence of tuberculosis has been found in areas where ultraviolet exposure is reduced and vitamin D deficiency is more prevalent [11], and low sunlight exposure has been associated with an increased incidence of tuberculosis several weeks later [10,14]. The consensus reached among nutritionists is that the serum 25 (OH) D concentration is quite low or absent above latitude 33°N in winter [39]. Spain lies between 45°N and 55°N, and although the production of pre-vitamin D3 in the skin is higher than in other farther northern European countries, vitamin D levels may also be affected by seasonality [39]. Spain is a European country of the Mediterranean basin with mild winters with colder temperatures, rain and a significant reduction in sunshine exposure, especially in the northern regions, which might contribute to a higher incidence of tuberculosis [40]. Thus, it may be plausible that the reduced exposure to sunshine in the winter and decreased vitamin D production may result in impaired host defense against tuberculosis, and may explain the winter peak (likely new PTB cases) and spring peak (likely PTB reactivations) in our study. Similarly, seasonal changes of neuroendocrine function (glucocorticoid and melatonin levels), which may influence the immune response, have been proposed to contribute to seasonality of infectious diseases [12].
Lower temperatures were a significant risk factor for PTB-related hospital admission in our results. Hospital admissions in colder months are significantly higher than in warmer months [17,18]. Moreover, it has been postulated that climatic conditions during the cold months may facilitate tuberculosis transmission, particularly in overcrowded and poorly ventilated conditions [41,42]. Another factor contributing to seasonal patterns may be other respiratory infections that are more prevalent during the winter months, such as influenza, respiratory syncytial virus or bacterial pneumonia [43]. These infections may impair a person’s immunity during the winter and might lead to the development or reactivation of PTB disease in the spring [43].
Most hospital admissions during the first years of follow-up occurred in the winter, but a transition to peaks of incidence in the spring was found during the later years of follow-up. This seasonal variation might be due to activation of latent Mycobacterium tuberculosis infection due to late winter nadirs in vitamin D, but we cannot rule out that it may be due to an increased transmission of tuberculosis due to wintertime indoor crowding. We do not have a clear explanation for this finding, but it might be possible that vitamin D fluctuations explain the seasonality differences in HIV-infected patients. In addition to “classic” risk factors for vitamin D deficiency, cART may affect vitamin D levels [44]. Vitamin D deficiency is a very common disorder in HIV-positive subjects on cART from the Iberian Peninsula [45–47]. In Spain cART was introduced through the national health system in 1996, and since then the percentage of patients on cART has been steadily increasing [48]. Our hypothesis is that this increase in patients on cART could have resulted in a higher percentage of patients with low vitamin D levels, which may be partly responsible. On the other hand, it should not be ruled out that a possible diagnostic delay due to tuberculosis symptoms being similar to other respiratory infections prevalent in winter [49] could result in PTB hospitalizations in the spring.
We also found that higher concentrations of atmospheric pollutants were significant risk factors for PTB-related hospital admission. Specifically, higher concentrations of NO2 and SO2 prior to hospital admission were a significant risk factor for hospital admissions. However, no association with CO, O3, and PM10 was found, unlike what has been seen in other studies on the general population [17,21–25,50]. This fact could be due to the limited sample size of our group of HIV-infected patients. Another potential confounding factor may be immune system dysfunction in HIV-infected patients, who have varying degrees of immune system damage according to the stage of their disease and their response to antiretroviral therapy. Of note, among new HIV diagnoses in Spain from 2003–2011, around 30–40% had CD4+ values <200 cells /mm3 in the first test after HIV diagnosis [51,52]. In addition, a large number of HIV patients are intravenous drug users, who usually have limited access to cART and low treatment adherence [53,54].
Tuberculosis is a chronic infection which takes from weeks to months for manifestation of the disease [27]. Previous studies have evaluated the impact of long-term exposure to ambient air pollution on PTB risk [21,24,25], but there is little information about short-term exposure to ambient air pollution and PTB risk [17]. We think short-term exposure to air pollution may play an important role in the exacerbation and pathogenesis of PTB, just as in other chronic respiratory diseases and infections [16]. In fact, short-term exposure to ambient air pollution in adult patients has been associated with lower lung function [55], increases in blood markers of inflammation, decreases in blood markers of coagulation [56], and DNA methylation changes at CpG sites residing in genes involved in inflammation and oxidative stress response [57]. On the other hand, it also reduces early lung immune responses to mycobacteria infection (i.e. it reduces the total immune cell number and causes a significant decline in the recruitment of polymorphonuclear leukocytes as measured in bronchoalveolar lavage fluid, O2 (-) generation, and secretion of TNFα, and IL-6) in an animal model [58].
The ambient air pollution is a persistent public health problem, and millions of people worldwide die each year from causes directly related to air pollution [20,59]. Given the large number of HIV-infected patients with PTB and who are often exposed to high levels of air contaminants, the association between air pollution and PTB may be considered a major public health concern. Thus, interventions to improve air quality could contribute to TB control in patients infected with HIV and in the general population. Moreover, the health services of cities with high levels of air pollution should be alerted to the possible increase in short-term risk of hospital admission due to PTB in the HIV positive population.
Several aspects of the study must be taken into account for a correct interpretation of the results. Firstly, this study had a retrospective design and we had no access to patient clinical data, which would be necessary to fully interpret the impact on PTB-related hospital admission. Secondly, MBDS data are anonymous and it is not possible to identify whether a patient has been hospitalized at different hospitals within the same calendar year. This may have caused a slight overestimation in our results, because we may have considered disease exacerbations or remissions to be new patients. Thirdly, we had no access to detailed information about the microclimate and indoor conditions where each patient was living. Instead of this metric, we defined individual exposure levels based on the closest climatological and air pollution monitoring stations, which can be a good approximation to the actual ambient air conditions for each patient. Fourthly, we had no access to detailed information about indoor air pollution, which is a major risk factor for tuberculosis, particularly from low-income and middle-income countries [60]. However, indoor air pollution is a minor risk factor for tuberculosis in high-income countries [60], and it should not have affected our results.
Conclusions
In conclusion, our data suggest an apparent seasonal variation in hospital admissions of HIV-infected patients with a PTB diagnosis (summer/autumn vs. winter/spring), as well as a link to short-term exposure to environmental risk factors, such as temperature and ambient NO2 and SO2. These findings lend further support to the potential role of seasonality and environmental factors in assessing the risk of PTB in patients with HIV infection, and we hope these findings help inform the direction of future research that may contribute to global tuberculosis control.
Supporting Information
Distribution of HIV positive patients aged 16 years and older with a hospital discharge, PTB diagnosis, and postal code in Spanish hospitals from 1 January 1997 to 31 December 2012.
(DOCX)
Acknowledgments
We wish to thank: a) the Spanish Ministry of Health and Social Policy for providing the records of the Minimum Basic Data Set (MBDS); b) the State Meteorological Agency (Agencia Estatal de Metereología-AEMET) for providing daily environmental data.
Data Availability
All relevant data are in the paper. Raw data belong to the Minimum Basic Data Set (MBDS) of the Ministry of Health and Social Services and Equality (MSSSI) of Spain. All interested researchers may access the data by a request to MSSSI. These data may be delivered to researchers by a request in a standard form signed, which downloadable in this link (www.msssi.gob.es/…/SolicitudCMBDdocs/Formulario_Peticion_Datos_CMBD). The standard form signed must be sent by email to icmbd@msssi.es.
Funding Statement
This work has been supported by a grant from “Instituto de Salud Carlos III” (Ref. PI14CIII/00011 to SR and PI12/00019 to AAM) (http://www.isciii.es).
References
- 1.European Centre for Disease Prevention and Control/WHO Regional Office for Europe (2014) Tuberculosis surveillance and monitoring in Europe 2014. Stockholm, Sweden: European Centre for Disease Prevention and Control. [Google Scholar]
- 2.Girardi E, Sabin CA, d'Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, et al. (2005) Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 41: 1772–1782. 10.1086/498315 [DOI] [PubMed] [Google Scholar]
- 3.Muga R, Ferreros I, Langohr K, de Olalla PG, Del Romero J, Quintana M, et al. (2007) Changes in the incidence of tuberculosis in a cohort of HIV-seroconverters before and after the introduction of HAART. AIDS 21: 2521–2527. 10.1097/QAD.0b013e3282f1c933 [DOI] [PubMed] [Google Scholar]
- 4.STOP TB Partnership (2011) The global plan to stop TB 2006–2015; Available at: http://www.stoptb.org/. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 5.WHO (2015) Global Tuberculosis Report: 2015. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 6.ECDC/WHO (2011) HIV/AIDS surveillance in Europe 2011. Available at: http://ecdc.europa.eu/en/publications/Publications/20121130-Annual-HIV-Surveillance-Report.pdf. Stockholm, Sweden: European Centre for Disease Prevention and Control. [Google Scholar]
- 7.ECDC/WHO (2013) Tuberculosis surveillance and monitoring in Europe 2013. Available at: http://www.ecdc.europa.eu/en/publications/Publications/Tuberculosis-surveillance-monitoring-2013.pdf. Stockholm, Sweden: European Centre for Disease Prevention and Control. [Google Scholar]
- 8.Flynn JL, Chan J (2001) Tuberculosis: latency and reactivation. Infect Immun 69: 4195–4201. 10.1128/IAI.69.7.4195-4201.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Duan Q, Wang J, Zhang Z, Jiang G (2014) Seasonal variation of newly notified pulmonary tuberculosis cases from 2004 to 2013 in Wuhan, China. PLoS ONE 9: e108369 10.1371/journal.pone.0108369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh GC, Hawthorne G, Turner AM, Kunst H, Dedicoat M (2013) Tuberculosis incidence correlates with sunshine: an ecological 28-year time series study. PLoS ONE 8: e57752 10.1371/journal.pone.0057752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maclachlan JH, Lavender CJ, Cowie BC (2012) Effect of latitude on seasonality of tuberculosis, Australia, 2002–2011. Emerging Infectious Diseases 18: 1879–1881. 10.3201/eid1811.120456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fares A (2013) Factors influencing the seasonal patterns of infectious diseases. Int J Prev Med 4: 128–132. [PMC free article] [PubMed] [Google Scholar]
- 13.Fares A (2011) Seasonality of tuberculosis. J Glob Infect Dis 3: 46–55. 10.4103/0974-777X.77296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wingfield T, Schumacher SG, Sandhu G, Tovar MA, Zevallos K, Baldwin MR, et al. (2014) The seasonality of tuberculosis, sunlight, vitamin D, and household crowding. Journal of Infectious Diseases 210: 774–783. 10.1093/infdis/jiu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos LG, Pires GN, Azeredo Bittencourt LR, Tufik S, Andersen ML (2012) Chronobiology: relevance for tuberculosis. Tuberculosis (Edinb) 92: 293–300. [DOI] [PubMed] [Google Scholar]
- 16.Laumbach RJ, Kipen HM (2012) Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. J Allergy Clin Immunol 129: 3–11; quiz 12–13. 10.1016/j.jaci.2011.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shilova MV, Glumnaia TV (2004) [Influence of seasonal and environmental factors on the incidence of tuberculosis]. Probl Tuberk Bolezn Legk: 17–22. [PubMed] [Google Scholar]
- 18.Atun RA, Samyshkin YA, Drobniewski F, Kuznetsov SI, Fedorin IM, Coker RJ (2005) Seasonal variation and hospital utilization for tuberculosis in Russia: hospitals as social care institutions. European Journal of Public Health 15: 350–354. 10.1093/eurpub/cki018 [DOI] [PubMed] [Google Scholar]
- 19.Onozuka D, Hagihara A (2014) The association of extreme temperatures and the incidence of tuberculosis in Japan. International Journal of Biometeorology. [DOI] [PubMed] [Google Scholar]
- 20.WHO (2011) Air Quality and Health Fact Sheet N313. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 21.Smith GS, Schoenbach VJ, Richardson DB, Gammon MD (2014) Particulate air pollution and susceptibility to the development of pulmonary tuberculosis disease in North Carolina: an ecological study. Int J Environ Health Res 24: 103–112. 10.1080/09603123.2013.800959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwai K, Mizuno S, Miyasaka Y, Mori T (2005) Correlation between suspended particles in the environmental air and causes of disease among inhabitants: cross-sectional studies using the vital statistics and air pollution data in Japan. Environ Res 99: 106–117. 10.1016/j.envres.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 23.Tremblay GA (2007) Historical statistics support a hypothesis linking tuberculosis and air pollution caused by coal. Int J Tuberc Lung Dis 11: 722–732. [PubMed] [Google Scholar]
- 24.Jassal MS, Bakman I, Jones B (2013) Correlation of ambient pollution levels and heavily-trafficked roadway proximity on the prevalence of smear-positive tuberculosis. Public Health 127: 268–274. 10.1016/j.puhe.2012.12.030 [DOI] [PubMed] [Google Scholar]
- 25.Smith GS, Van Den Eeden SK, Garcia C, Shan J, Baxter R, Herring AH, et al. (2016) Air Pollution and Pulmonary Tuberculosis: A Nested Case-Control Study among Members of a Northern California Health Plan. Environ Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer RN, Diaz-Sanchez D, Jaspers I (2012) Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. Journal of Allergy and Clinical Immunology 129: 14–24; quiz 25–16. 10.1016/j.jaci.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn SD, Zumla AI (2011) Tuberculosis. Lancet 378: 57–72. 10.1016/S0140-6736(10)62173-3 [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Song Y, Kipen HM, Laumbach RJ, Zhang J, Strickland PA, et al. (2012) Suppression of the NF-kappaB pathway by diesel exhaust particles impairs human antimycobacterial immunity. Journal of Immunology 188: 2778–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronacher K, Joosten SA, van Crevel R, Dockrell HM, Walzl G, Ottenhoff TH (2015) Acquired immunodeficiencies and tuberculosis: focus on HIV/AIDS and diabetes mellitus. Immunol Rev 264: 121–137. 10.1111/imr.12257 [DOI] [PubMed] [Google Scholar]
- 30.Subdirección General de Desarrollo (2001) Instituto Nacional de Salud. Ministerio de Sanidad y Consumo [http://www.ingesa.msc.es/estadEstudios/documPublica/CMBD-2001.htm] Conjunto Mínimo Básico de Datos Hospitales de Insalud 2001.
- 31.Alvaro-Meca A, Palomares-Sancho I, Diaz A, Resino R, De Miguel AG, Resino S (2015) Pneumocystis pneumonia in HIV-positive patients in Spain: epidemiology and environmental risk factors. Journal of the International AIDS Society 18: 19906 10.7448/IAS.18.1.19906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rue H, Martino S, Lindgren F, Simpson D, Riebler A (2014) INLA: Functions which allow to perform full Bayesian analysis of latent Gaussian models using Integrated Nested Laplace Approximaxion. R package version 00–1389624686.
- 33.Maclure M (1991) The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 133: 144–153. [DOI] [PubMed] [Google Scholar]
- 34.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP (2006) The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of Hepatology 45: 529–538. 10.1016/j.jhep.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 35.Jaakkola JJ (2003) Case-crossover design in air pollution epidemiology. European Respiratory Journal Supplement 40: 81s–85s. [DOI] [PubMed] [Google Scholar]
- 36.The R Core Team (2011) R: A Language and Environment for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing. [Google Scholar]
- 37.Martineau AR, Nhamoyebonde S, Oni T, Rangaka MX, Marais S, Bangani N, et al. (2011) Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proceedings of the National Academy of Sciences of the United States of America 108: 19013–19017. 10.1073/pnas.1111825108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nnoaham KE, Clarke A (2008) Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. International Journal of Epidemiology 37: 113–119. 10.1093/ije/dym247 [DOI] [PubMed] [Google Scholar]
- 39.Wacker M, Holick MF (2013) Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol 5: 51–108. 10.4161/derm.24494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Barroso D, Rodriguez-Valin E, Ramis R, Cano R (2013) Spatio-temporal analysis of tuberculosis in Spain, 2008–2010. International Journal of Tuberculosis and Lung Disease 17: 745–751. 10.5588/ijtld.12.0702 [DOI] [PubMed] [Google Scholar]
- 41.Rios M, Garcia JM, Sanchez JA, Perez D (2000) A statistical analysis of the seasonality in pulmonary tuberculosis. European Journal of Epidemiology 16: 483–488. [DOI] [PubMed] [Google Scholar]
- 42.Thorpe LE, Frieden TR, Laserson KF, Wells C, Khatri GR (2004) Seasonality of tuberculosis in India: is it real and what does it tell us? Lancet 364: 1613–1614. 10.1016/S0140-6736(04)17316-9 [DOI] [PubMed] [Google Scholar]
- 43.Dangor Z, Izu A, Moore DP, Nunes MC, Solomon F, Beylis N, et al. (2014) Temporal association in hospitalizations for tuberculosis, invasive pneumococcal disease and influenza virus illness in South African children. PLoS One 9: e91464 10.1371/journal.pone.0091464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffin AT, Arnold FW (2012) Review of metabolic, immunologic, and virologic consequences of suboptimal vitamin D levels in HIV infection. AIDS Patient Care and STDs 26: 516–525. 10.1089/apc.2012.0145 [DOI] [PubMed] [Google Scholar]
- 45.Portilla J, Moreno-Perez O, Serna-Candel C, Escoin C, Alfayate R, Reus S, et al. (2014) Vitamin D insufficiency and subclinical atherosclerosis in non-diabetic males living with HIV. Journal of the International AIDS Society 17: 18945 10.7448/IAS.17.1.18945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzman-Fulgencio M, Garcia-Alvarez M, Berenguer J, Jimenez-Sousa MA, Cosin J, Pineda-Tenor D, et al. (2014) Vitamin D deficiency is associated with severity of liver disease in HIV/HCV coinfected patients. Journal of Infection 68: 176–184. 10.1016/j.jinf.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 47.Boura M, Sutre AF, Badura R, Zagalo A, Afonso C, Caldeira L, et al. (2014) Hypovitaminosis D in HIV-infected patients in Lisbon: a link with antiretroviral treatment. Journal of the International AIDS Society 17: 19826 10.7448/IAS.17.4.19826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grupo de Trabajo de la Encuesta Hospitalaria. [Working Group of the Hospital Survey] (June 2014) Encuesta hospitalaria de pacientes con VIH/sida. Resultados 2013. Análisis de la evolución 2000–2013. [Hospital survey of patients with HIV/AIDS. Results 2013. Analysis of the development from 2000 to 2013]. Madrid, Spain: Centro Nacional de Epidemiología/Subdirección General de Promoción de la salud y Epidemiología—Plan Nacional sobre el Sida. 1–33; Available at: http://www.msssi.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/InformeVIHSida_Junio2014.pdf p.
- 49.Rodger A, Jaffar S, Paynter S, Hayward A, Carless J, Maguire H (2003) Delay in the diagnosis of pulmonary tuberculosis, London, 1998–2000: analysis of surveillance data. BMJ (Clinical Research Ed) 326: 909–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hwang SS, Kang S, Lee JY, Lee JS, Kim HJ, Han SK, et al. (2014) Impact of outdoor air pollution on the incidence of tuberculosis in the Seoul metropolitan area, South Korea. Korean Journal of Internal Medicine 29: 183–190. 10.3904/kjim.2014.29.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliva J, Galindo S, Vives N, Arrillaga A, Izquierdo A, Nicolau A, et al. (2010) [Delayed diagnosis of HIV infection in Spain]. Enfermedades Infecciosas y Microbiologia Clinica 28: 583–589. 10.1016/j.eimc.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 52.Oliva J, Diez M, Galindo S, Cevallos C, Izquierdo A, Cereijo J, et al. (2014) Predictors of advanced disease and late presentation in new HIV diagnoses reported to the surveillance system in Spain. Gaceta Sanitaria 28: 116–122. [DOI] [PubMed] [Google Scholar]
- 53.Vallecillo G, Sanvisens A, Martinez E, Torrens M, Bolao F, Tor J, et al. (2010) Use of highly active antiretroviral therapy is increasing in HIV positive severe drug users. Curr HIV Res 8: 641–648. [DOI] [PubMed] [Google Scholar]
- 54.Kavasery R, Galai N, Astemborski J, Lucas GM, Celentano DD, Kirk GD, et al. (2009) Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr 50: 360–366. 10.1097/QAI.0b013e318198a800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, et al. (2013) Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med 188: 1351–1357. 10.1164/rccm.201308-1414OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hildebrandt K, Ruckerl R, Koenig W, Schneider A, Pitz M, Heinrich J, et al. (2009) Short-term effects of air pollution: a panel study of blood markers in patients with chronic pulmonary disease. Part Fibre Toxicol 6: 25 10.1186/1743-8977-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang R, Jones MJ, Sava F, Kobor MS, Carlsten C (2014) Short-term diesel exhaust inhalation in a controlled human crossover study is associated with changes in DNA methylation of circulating mononuclear cells in asthmatics. Part Fibre Toxicol 11: 71 10.1186/s12989-014-0071-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delfosse VC, Tasat DR, Gioffre AK (2015) In vivo short-term exposure to residual oil fly ash impairs pulmonary innate immune response against environmental mycobacterium infection. Environ Toxicol 30: 589–596. 10.1002/tox.21936 [DOI] [PubMed] [Google Scholar]
- 59.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.WHO (2009) Global health risks: Mortality and burden of disease attributable to selected major risks. Geneva, Switzerland: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of HIV positive patients aged 16 years and older with a hospital discharge, PTB diagnosis, and postal code in Spanish hospitals from 1 January 1997 to 31 December 2012.
(DOCX)
Data Availability Statement
All relevant data are in the paper. Raw data belong to the Minimum Basic Data Set (MBDS) of the Ministry of Health and Social Services and Equality (MSSSI) of Spain. All interested researchers may access the data by a request to MSSSI. These data may be delivered to researchers by a request in a standard form signed, which downloadable in this link (www.msssi.gob.es/…/SolicitudCMBDdocs/Formulario_Peticion_Datos_CMBD). The standard form signed must be sent by email to icmbd@msssi.es.