Abstract
Background
Chemotherapy resistance is reported to correlate with up-regulation of anti-tumor agent transporter ABCB1 (p-gp) in epithelial ovarian cancer (EOC), but the results remain controversial. To reconcile the results, a systematic review followed by meta-analysis was performed to assess the association between high ABCB1 status or ABCB1 gene variants and overall survival (OS), progression free survival (PFS), and total response rate (TR) in patients with EOC.
Materials and Methods
Electronic searches were performed using Pubmed, EMBASE, Web of Science and Chinese Wanfang databases from January 1990 to February 2016. Summary hazard ratio (HR), risk ratio (RR) and 95% confidence intervals (CIs) were combined using fixed or random-effects models as appropriate.
Results
Thirty-eight retrospective studies of 8607 cases qualified for meta-analysis were identified. Our results suggested that ABCB1 over-expression was significantly associated with unfavorable OS (HR = 1.54; 95% CI, 1.25–1.90), PFS (HR = 1.49; 95% CI, 1.22–1.82) and TR (RR = 0.63; 95% CI, 0.54–0.75). After adjustment for age, clinical stage, residual disease, histological type and tumor grade, high ABCB1 status remained to be a significant risk factor for adverse OS and PFS. Patients with recurrent ABCB1 positivity suffered from poorer OS than those with primary ABCB1 positivity. However, stratified by chemotherapy regimen, inverse correlation between high ABCB1 status and poor OS, PFS and TR were only found in patients underwent platinum-based chemotherapy but not in patients received standard platinum/paclitaxel-based chemotherapy. No evidence was found for any association between ABCB1 gene polymorphisms and OS, PFS or TR.
Conclusion
High ABCB1 status is significantly associated with chemo-resistance and poor prognosis in patients with EOC. Large-scale, prospective studies are needed to assess the clinical value of ABCB1 expression in EOC more accurately.
Introduction
Ovarian cancer is the most lethal gynecological malignancy worldwide [1]. Epithelial ovarian cancer (EOC) accounts for over 90% of all ovarian cancers [2]. As its progression is usually asymptomatic and unpredictable, most (approximately 85%) patients were diagnosed at late stage, in whom cytoreductive surgery combined with platinum-based chemotherapy represents the current standard procedure [3]. Six cycles of taxane plus platinum chemotherapy represents the most widely used regimen for EOC; other platinum-based regimens included PAC (platinum + doxorubicin + cyclophosphamide) and PC (platinum + cyclophosphamide). Although this treatment procedure can achieve an overall response rate higher than 70% in patients with ovarian cancer, a great proportion (approximately 75%) of them suffer a recurrence within 2 to 3 years after initial treatment, which leads to poor outcomes [4]. The high recurrence rate is associated with the development of acquired chemo-resistance. Repeated chemotherapeutic stimulation induces biological changes in tumor cells allowing them to survive under chemotherapy. Identification of prognostic biomarkers predicting chemotherapy response was essentially necessary for individual therapy.
Insufficient accumulation of anti-cancer agents in tumor cells resulting from increased efflux and/or decreased influx is a critical mechanism of chemo-resistance [5,6]. ABCB1 (ATP binding cassette B1, also known as multidrug resistance protein 1, MDR1 or p-glycoprotein, p-gp) is a cellular surface protein, which transports a variety of chemotherapy drugs such as paclitaxel, docetaxel, doxorubicin and topotecan and plays a major role in cellular detoxification [7–10]. Functional experiments revealed that up-regulated ABCB1 expression impaired the sensitivity to cisplatin, paclitaxel, docetaxel and doxorubicin in ovarian cancer cell lines [11]. A substantial literature interrogated the relationship between ABCB1 status (expression or polymorphisms) and clinical outcomes such as overall survival (OS), progression free survival (PFS) and total response (including complete response and partial response according to Response Evaluation Criteria In Solid Tumors) to chemotherapy (TR) in EOC patients [12–55]. Although a great number of studies evaluated the effect of ABCB1 on EOC patients, the association between ABCB1 and EOC prognosis was still under debate. While certain amount of studies reported that high ABCB1 could predict unfavorable prognosis [23,28,30,31,34,35], quite a few did not find any significant association between ABCB1 and survival in patients with EOC [18,27,32]. The possible reasons for these inconsistent results might include (i) heterogeneities of the basic characteristics of the studies such as sample sizes, EOC subtypes, clinical stage, and populations, (ii) different intervention such as residual disease after surgery, time point of ABCB1 measurement and (iii) different methodology such as detection methods and procedures. Therefore, we performed a systematic and comprehensive meta-analysis to assess more precisely the correlation between ABCB1 status and EOC prognosis based on individual studies.
Methods
This meta-analysis was performed according to the PRISMA-statement for systematic review and meta-analysis of genetic association studies (S1 and S2 Checklists).
Search strategy
Thorough electronic search of Pubmed, Embase, Web of Science and Chinese Wanfang databases from January 1990 to Feb 2016 was conducted with the following key words: ‘ovarian cancer’, ‘ovarian carcinoma’ or ‘ovarian neoplasm’ and ‘ABCB1’ or ‘p-gp’. The search strategy was complemented by reviewing the similar articles and by examining the references of the retrieved literature. Additionally we examined the authors of each study to avoid overlap of cases. Only the most recent study was included when overlap occurred between studies.
Publication Selection and Quality Assessment
Two authors reviewed the studies independently to decide which were eligible for inclusion. The included studies had to meet the following criteria: 1) focused on epithelial ovarian carcinoma and evaluated correlation between ABCB1 status and prognosis of the patients; 2) published as full text in English or in Chinese; 3) analyzed ABCB1 status with practical and comparable method; 4) abided by the Hardy Weinberg Equilibrium (HWE) when performing gene associated analysis. The primary outcome was OS and the secondary outcome included PFS and TR. The methodology of each study was assessed independently by two authors according to the Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Each study was scored according to three aspects: selection, comparability and exposure. The maximum score of each aspect was 4, 2 and 3 respectively, adding up to a total score of 9. Higher score stood for better quality (S1 File).
Data Extraction and Statistical Methods
The data extracted from the included studies were authors, year of publication, countries, sizes of population, histology, clinical stage, follow-up time, treatment, tissue type, point of time when transporters are measured, assay method and corresponding scoring system, the results of survival analysis, the number of cases and controls, and response to chemotherapy. Two authors reviewed the literatures and extracted the data independently. If disagreement occurred, a third author was conferred with thus the issue be discussed altogether.
For time to event survival analysis, we assessed the effect of ABCB1 status on prognosis by hazard ratio (HR). For each study, the HR and its 95% confidence interval (CI) were retrieved. If these parameters were not available in studies, we used the software GetDATA Graph Digitizer 2.25 to extract the specific survival rates according to the Kaplan-Meier curves to calculate the HR by the methods described by Tierney et al [56]. For dichotomous outcomes such as TR, we measured the impact of ABCB1 status on the prognosis by risk ratio (RR). For each study, we extracted the number of patients who experienced the events of interest and the number of patients who were free from the events at the end point to calculate the RR and its 95% CI.
Meta-analysis was performed for OS, PFS and treatment response. For each endpoint, the analyses were stratified by ABCB1 detection methods, histology, clinical stage, geographical region of studies and chemotherapy regimens. Generally the individual HR and RR were pooled according to the method reported by DerSimonian, R et al. and Mantel, N et al. Fixed inverse variance model and random model were used to calculate pooled HRs and RRs of studies with class I-II heterogeneity (I2 <50%) and those with class III-IV hererogeneity (I2 ≥50%), respectively [57,58]. The heterogeneity between studies was assessed by I2 and p-value according to the method described by Higgins et al [59]. The effect of ABCB1 on prognosis was considered significant only when the 95% CI for HR and RR did not overlap with 1. Visual inspection of symmetry of funnel plots and Egger’s test were used to assess publication bias [60]. A two-sided P value < 0.05 was considered of statistical significance. All analyses were carried out using STATA14 (MP-Parallel Edition, College Station, Texas 77845 USA).
Results
Study characteristics
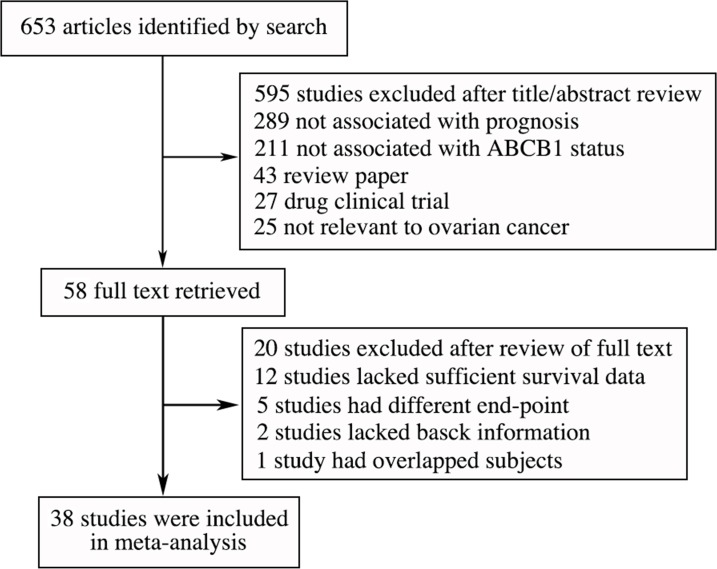
The procedure of study selection was presented in a flowchart (Fig 1). Initially, 653 articles related to the keywords were identified after removal of duplicates. Among these articles, 595 were excluded by review of title and abstract as they did not report on ABCB1, published as reviews or focused on ABCB1 cytological mechanisms and function. Full texts were retrieved of the remaining 58 studies and 20 of them were excluded after review of full text (S1 Table). Finally, 38 eligible studies involving 8607 cases were identified, including 23 studies for ABCB1 protein (p-gp) expression (Table 1), 9 for ABCB1 mRNA expression, and 7 for ABCB1 SNPs (Table 2).
Fig 1. Flow chart of literature search and selection.
Table 1. Characteristics of studies that identify ABCB1 protein (p-gp) expression in epithelial ovarian cancer.
| Detection method | First Author | Year | Country | No. of Cases | Stage | Histology | Anti-body | Score | Cut-off point | Tissue Type | Time point | Chemotherapy | End-point |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHC | Matsuo | 2014 | USA | 112 | I-IV | s | JSB1 | standard | 5% | FFPE | 1 | 2 | OS, PFS |
| Lu D | 2011 | China | 80 | I-IV | s,m,e | ZSGB | standard | 10% | FFPE | 1 | 1,2 | OS | |
| O'Tool | 2007 | Ireland | 77 | I-IV | s,m,c | JSB1 | standard | 10% | FFPE | 1 | 1,2 | OS, PFS | |
| Oda | 2007 | Japan | 53 | I-IV | s,e | JSB1 | standard | 10% | FFPE | 1 | 2 | OS, PFS | |
| Wei | 2007 | China | 42 | III | all | C219 | standard | 20% | FFPE | 1 | 2 | TR | |
| Yakirevich | 2006 | USA | 60 | I-IV | s | JSB1 | standard | 10% | FFPE | 1 | 2 | OS, TR | |
| Surowiak | 2006 | Poland | 43 | III-IV | all | C219 | standard | 20% | FFPE | 1,2 | 1 | OS, PFS, TR | |
| Obata | 2006 | Japan | 60 | III-IV | s,e | C-19 | standard | 5% | FFPE | 1 | 1,2 | TR | |
| Goto | 2006 | Japan | 21 | IIIC~IV | s | JSB1 | standard | 20% | FFPE | 1 | 2 | TR | |
| Raspollini | 2005 | Italy | 52 | III | s | MDR8 | standard | 30% | FFPE | 1 | 1 | TR | |
| Penson | 2004 | USA | 32 | II~IV | s,e,c | C219, c494 | standard | 20% | FFPE | 1,2 | 2 | OS, TR | |
| Ikeda | 2003 | Japan | 93 | II~IV | all | C-19 | standard | 10% | FFPE | 1 | 1,2 | OS | |
| Raspollini | 2003 | Italy | 83 | III | s | MDR8 | standard | 30% | FFPE | 1 | 1 | OS, PFS, TR | |
| Raspollini | 2002 | Italy | 22 | III | s,e | MDR8 | standard | 30% | FFPE | 1 | 1 | PFS, TR | |
| Itamochi | 2002 | Japan | 131 | III~IV | s,c | C494, C-20 | standard | 10% | FFPE | 1 | 1 | TR | |
| Zhang | 2001 | China | 27 | I-IV | all | C219 | standard | 10% | FFPE | 2 | 1 | TR | |
| Baekelandt | 2000 | Norway | 73 | III | all | JSB1 | standard | 10% | FFPE | 1 | 1 | OS, PFS, TR | |
| Yokoyama | 1999 | Japan | 22 | I-IV | all | LSAB | standard | 30% | FFPE | 1 | 1 | PFS, TR | |
| Arts | 1999 | Netherlands | 112 | I-IV | all | JSB1 | standard | 10% | FFPE | 1 | 1,2 | PFS, TR | |
| Khalifa | 1997 | Canada | 75 | I-IV | all | JSB1 | standard | 10% | FFPE | 1,2 | 1 | OS | |
| Van | 1995 | Netherlands | 89 | I-IV | all | JSB1 | standard | 5% | FFPE | 1 | 1 | PFS,TR | |
| Izquierdo | 1995 | Netherlands | 57 | III-IV | s,m,e | JSB1 | standard | 10% | FFPE | 1 | 1 | TR | |
| WB | Joncourt | 1998 | Switzerland | 39 | I-IV | all | Customized* | - | positive | Frozen | 1 | 1 | TR |
IHC: Immunohistochemistry; WB: Western blot
First Author: bolded when the result of ABCB1 association was significant
Histology: all = serous, mucinous, endometrial and clear cell carcinoma with or without mixed histology, s = serous ovarian cancer, m = mucinous ovarian cancer, e = endometrial ovarian cancer, c = clear cell ovarian cancer
Score: standard = score procedures were performed double-blindly by at least one pathologist according to standard scoring systems
Tissue Type: FFPE = formalin-fixed and paraffin-embedded
Time point: the time point of specimen obtained, 1 = specimens obtained during primary surgery, 2 = specimens obtained from recurrent tumor
Treatment: 1 = platinum-based chemotherapy, 2 taxane-containing chemotherapy
*The antibody used was a polyclonal rabbit antiserum raised against amino acids 1205–1224 of the human mdr protein. This peptide (ALDTESEKV- VQEALDKAREG) was made by Multiple Peptide Systems, Inc. (San Diego, CA).
Table 2. Characteristics of studies that identify ABCB1 mRNA expression and ABCB1 gene variants in epithelial ovarian cancer.
| First Author | Year | Country | No. of Cases | Stage | Histology | Detection method | Tissue Type | Time point | Chemotherapy | End-point | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABCB1 mRNA expression | Johnatty | 2013 | Australia | 143 | I-IV | s | PCR | frozen | 1 | 2 | OS, PFS |
| Lu L | 2007 | USA | 206 | I-IV | all | PCR | frozen | 1 | 1,2 | OS, PFS | |
| Materna | 2004 | Germany | 61 | I-IV | all | PCR | frozen | 1 | 1,2 | OS, PFS | |
| Nakayama | 2002 | Japan | 82 | I-IV | all | PCR | frozen | 1 | 1,2 | OS | |
| Galani | 2002 | Greece | 22 | III-IV | all | PCR | frozen | 1 | 1,2 | TR | |
| Kamazawa | 2002 | Japan | 27 | III-IV | s,e,c | PCR | frozen | 1 | 2 | TR | |
| Wang | 1998 | China | 27 | I-IV | all | PCR | frozen | 1 | 1 | TR | |
| Zhu L | 1997 | China | 32 | III | s,e,c | PCR | frozen | 1 | 1 | TR | |
| Kavallaris | 1996 | Australia | 53 | I-IV | all | PCR | frozen | 1 | 1 | PFS | |
| ABCB1 gene variants | Tecza K | 2015 | Poland | 126 | I-IV | all | ASA-PCR, multiplex-PCR,RFLP-PCR | blood | 1 | 2 | PFS |
| Johnatty | 2013 | Australia | 4450 | I-IV | all | Illumina Infinium iSelect BeadChip | blood | 1 | 2 | OS, PFS | |
| Tian | 2012 | USA | 511 | III-IV | all | Sequenom iPLEXTMGOLD Aassay and MALDI-TOF | blood | 1 | 2 | OS, PFS | |
| Prema | 2011 | USA | 445 | I-IV | all | Illumina Veracode Assay | blood | 1, 2 | 1 | PFS | |
| Bergmann | 2011 | Denmark | 119 | II-IV | all | Pyrosequencing and TaqMAN® predesigned SNP genotyping assays | FFPE | 1 | 1 | OS | |
| Grimm | 2010 | Austria | 106 | I-IV | all | Pyrosequencing | blood | 1 | 2 | OS, PFS | |
| Johnatty | 2008 | Australia | 309 | I-IV | all | MALDI-TOF | blood | 1 | 2 | OS, PFS |
First Author: bolded when the result of ABCB1 association was significant
Histology: all = serous, mucinous, endometrial and clear cell carcinoma with or without mixed histology, s = serous ovarian cancer, m = mucinous ovarian cancer, e = endometrial ovarian cancer, c = clear cell ovarian cancer
Tissue Type: FFPE = formalin-fixed and paraffin-embedded
Time point: the time point of specimen obtained, 1 = specimens obtained during primary surgery, 2 = specimens obtained from recurrent tumor
Treatment: 1 = platinum-based chemotherapy, 2 taxane-containing chemotherapy.
The number of studies addressing OS, PFS, and TR was 19, 19, and 21 respectively. In addition to the basic characteristics, 6 and 4 studies assessed the impact of ABCB1 on OS [20,23,29,37,45,48] and PFS [20,29,37,45], respectively, adjusted for age, clinical stage, residual disease, histological type and tumor grade by multivariate analysis (Tables 1 and 2). For p-gp intensity, IHC results were appraised by measuring the intensity of reaction and proportion of positive cells. For measurement of ABCB1 gene expression by PCR, most of the studies (8/9) used the median as the cut-point according to the distribution of each cohort. The distributions of ABCB1 genotype frequencies in all 7 studies did not depart from Hardy-Weinberg equilibrium. Most of the studies revealed no correlation between common ABCB1 variants and poor prognosis (6/7).
Meta-analysis of relationship between ABCB1 status and OS in EOC
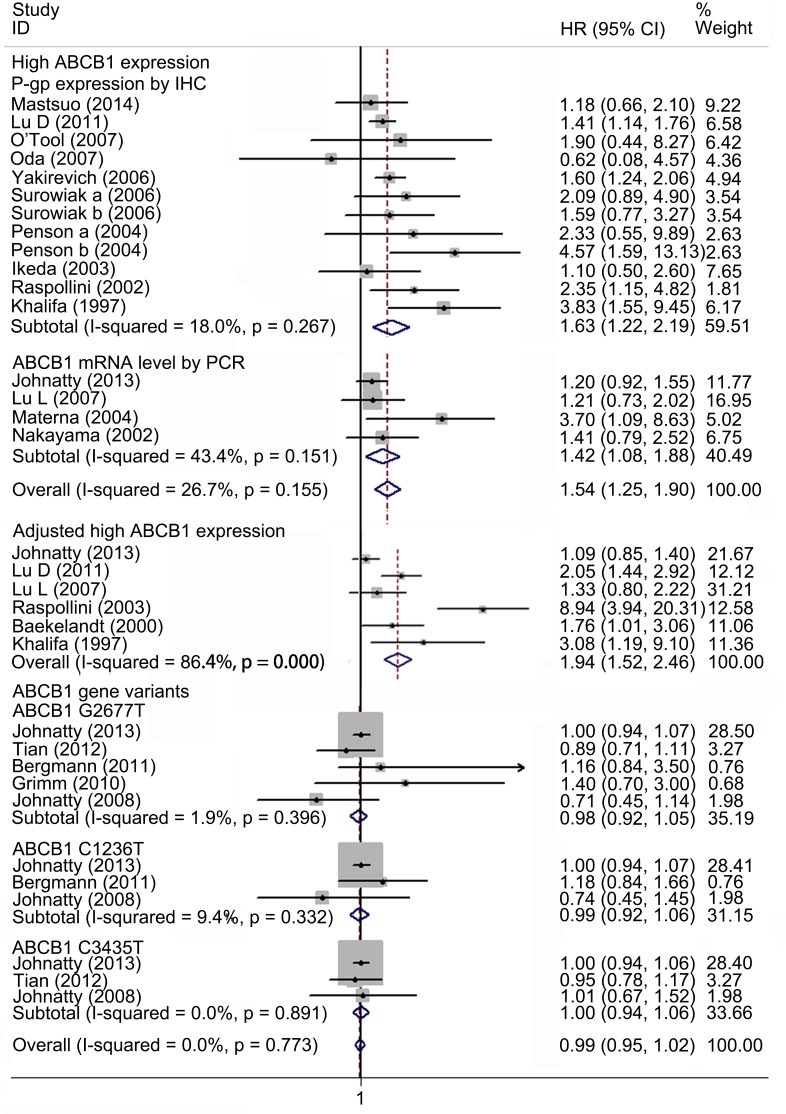
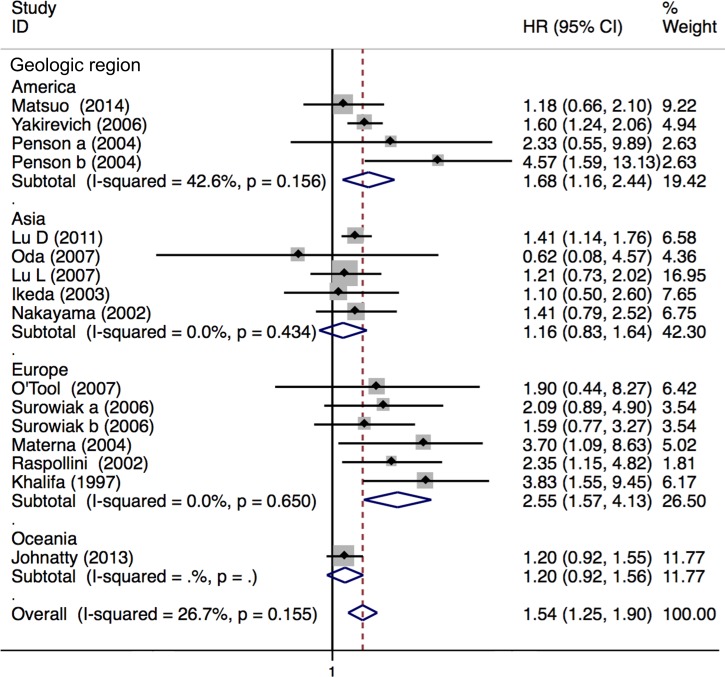
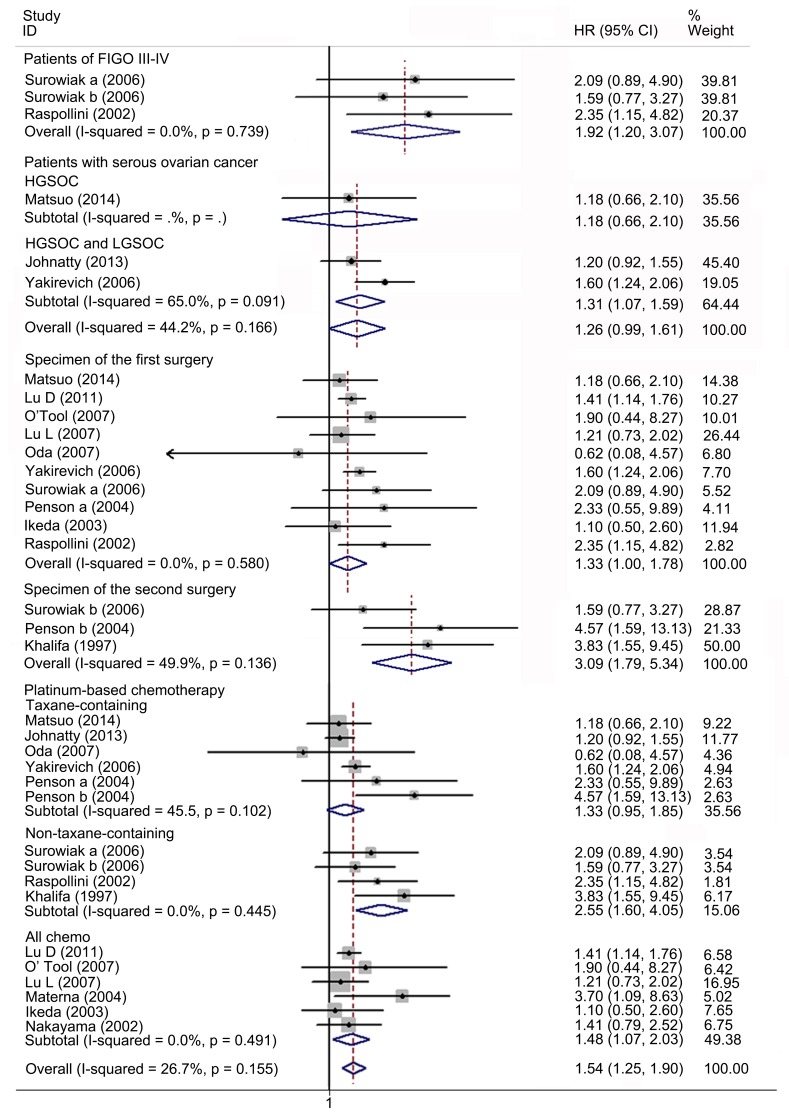
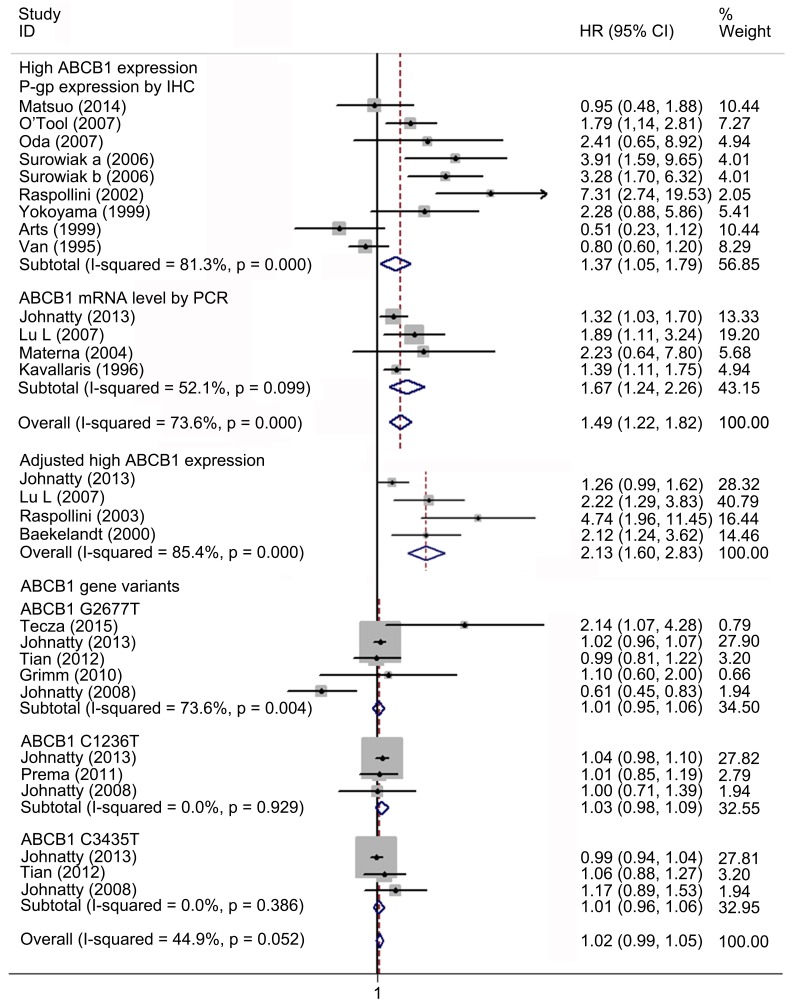
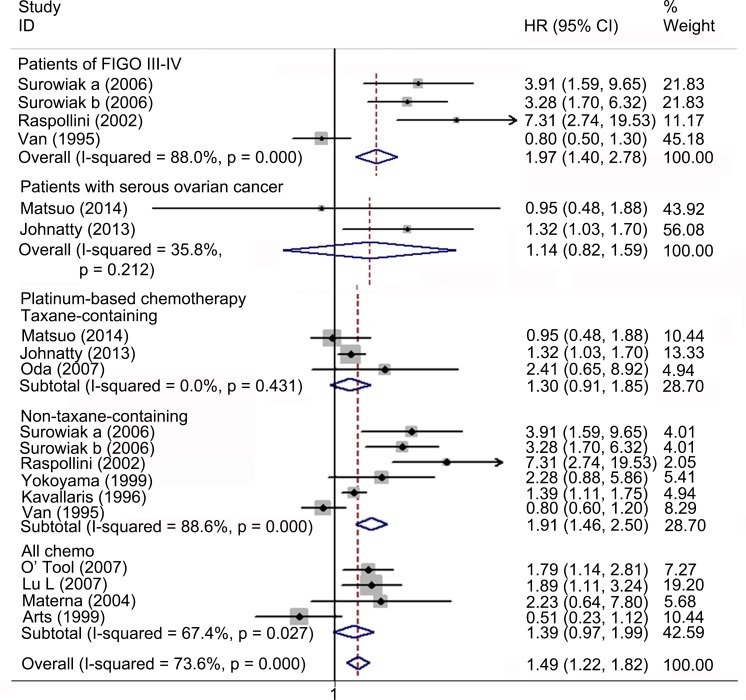
Nineteen studies with 6635 patients were included in the meta-analysis of OS. Fifteen studies with 1140 patients reported the association between high ABCB1 expression and OS. Overall, high ABCB1 expression was correlated with a poor OS (HR, 1.54; 95% CI: 1.25–1.90) in EOC. The effect of ABCB1 expression on OS remained significant in the IHC (HR, 1.63; 95% CI: 1.22–2.19), the PCR (HR, 1.42; 95% CI: 1.08–1.88), the FIGO III-IV (HR, 1.92; 95% CI: 1.20–3.07), the ABCB1 expression in recurrent tumor (HR, 3.09; 95% CI: 1.79–5.34), the platinum-based non-taxane-containing chemotherapy (HR, 2.55; 95% CI: 1.60–4.05), the America (HR, 1.68; 95% CI: 1.16–2.44) and the Europe (HR, 2.55; 95% CI: 1.57–4.13) subgroups. Similarly, when adjusted for residual disease (N = 6), age (N = 4), FIGO stage (N = 4), histological type (N = 2) and tumor grade (N = 1), high ABCB1 expression remained significantly associated with OS (HR, 1.94; 95% CI: 1.52–2.46). However, an inverse correlation was not found in the serous tumor, in the Asia, in the Oceania, in the patients with primary tumors, nor in the taxane-containing chemotherapy subgroups. We found that in the serous subgroup, the correlation between ABCB1 expression and OS became significant after excluding study specifically identifying high-grade serous ovarian cancer (HGSOC) (HR, 1.31; 95% CI: 1.07–1.59). For ABCB1 gene polymorphisms, although rs2235023, rs13237132, rs12334183, rs10264990, and rs4148732 were previously reported to be associated with ovarian cancer outcome, evidences from a study of more than 10000 cases suggested these candidate polymorphisms were not correlated with EOC prognosis [19]. Therefore we only evaluated the impact of rs2032582, rs1128503 and rs1045642 on prognosis. Five studies with 5495 patients reported association between these polymorphisms and OS in EOC [20,21,24,25,54]. No evidence of correlation between ABCB1 gene SNPs and OS was found (HR, 0.99; 95% CI: 0.95–1.02). The heterogeneity between studies in these analyses was acceptable (Figs 2–4 and Table 3).
Fig 2. Forest plots presenting HRs of EOC OS for high ABCB1 expression, adjusted results and ABCB1 gene variants.
HR = hazard ratio; EOC = epithelial ovarian cancer; OS = overall survival.
Fig 4. Forest plots of HRs of EOC OS for high ABCB1 expression stratified by geographical region.
HR = hazard ratio; EOC = epithelial ovarian cancer; OS = overall survival.
Table 3. Pooled HRs or RRs, heterogeneities and public cation biases of meta-analyses and subgroup analyses.
| No. of studies | HR (95%CI) or RR (95%CI)a | HR and RRP value | HeterogeneityP value | I2 value (%) | Bias P value | |
|---|---|---|---|---|---|---|
| OS | 19 | |||||
| High ABCB1 expression | 15 | 1.54 (1.25–1.90) | 0.000 | 0.267 | 18.0 | 0.078 |
| ABCB1 detected by IHC | 11 | 1.63 (1.22–2.19) | 0.000 | 0.151 | 43.4 | 0.206 |
| ABCB1 detected by by PCR | 4 | 1.42 (1.08–1.88) | 0.019 | 0.155 | 26.7 | 0.171 |
| FIGO III-IV | 2 | 1.92 (1.20–3.07) | 0.006 | 0.739 | 0.0 | - |
| Serous tumor | 3 | 1.26 (0.99–1.61) | 0.061 | 0.166 | 44.2 | 0.766 |
| ABCB1 expression in primary tumor | 10 | 1.33 (1.00–1.78) | 0.049 | 0.580 | 0.0 | 0.888 |
| ABCB1 expression in recurrent tumor | 3 | 3.09 (1.79–5.34) | 0.000 | 0.136 | 49.9 | 0.182 |
| Platinum-based chemotherapy | 14 | 1.54 (1.25–1.90) | 0.000 | 0.155 | 26.7 | 0.078 |
| Taxane-containing chemotherapyb | 5 | 1.33 (0.95–1.85) | 0.093 | 0.102 | 45.5 | 0.613 |
| Non-taxane-containing chemotherapyc | 3 | 2.55 (1.60–4.05) | 0.000 | 0.445 | 0.0 | 0.311 |
| All chemotherapyd | 6 | 1.48 (1.07–2.03) | 0.017 | 0.491 | 0.0 | 0.505 |
| Geographical region | ||||||
| America | 3 | 1.68 (1.16–2.44) | 0.006 | 0.156 | 42.6 | 0.957 |
| Asia | 5 | 1.16 (0.83–1.64) | 0.382 | 0.434 | 0.0 | 0.104 |
| Europe | 5 | 2.55 (1.57–4.13) | 0.000 | 0.650 | 0.0 | 0.327 |
| Oceania | 1 | 1.20 (0.92–1.56) | 0.171 | - | - | - |
| High ABCB1 expression adjusted for residual disease | 4 | 1.94 (1.52–2.46) | 0.000 | 0.000 | 86.4 | 0.080 |
| ABCB1 gene variants | 6 | 0.95 (0.99–1.02) | 0.989 | 0.053 | 54.1 | 0.748 |
| PFS | 19 | |||||
| High ABCB1 expression | 12 | 1.49 (1.22–1.82) | 0.000 | 0.000 | 73.6 | 0.146 |
| ABCB1 detected by IHC | 8 | 1.37 (1.05–1.79) | 0.042 | 0.000 | 81.3 | 0.122 |
| ABCB1 detected by by PCR | 4 | 1.67 (1.24–2.26) | 0.001 | 0.099 | 52.1 | 0.123 |
| FIGO III-IV | 3 | 1.97 (1.40–2.78) | 0.000 | 0.000 | 88.0 | 0.068 |
| Serous tumor | 2 | 1.14 (0.82–1.59) | 0.431 | 0.323 | 35.8 | - |
| Platinum-based chemotherapy | 12 | 1.49 (1.22–1.82) | 0.000 | 0.000 | 73.6 | 0.146 |
| Taxane-containing chemotherapyb | 3 | 1.30 (0.91–1.85) | 0.148 | 0.431 | 0.0 | 0.902 |
| Non-taxane-containing chemotherapyc | 5 | 1.91 (1.46–2.50) | 0.000 | 0.000 | 88.6 | 0.110 |
| All chemotherapyd | 4 | 1.39 (0.97–1.99) | 0.074 | 0.027 | 64.7 | 0.663 |
| Geographical region | ||||||
| America | 1 | 0.95 (0.48–1.88) | 0.883 | - | - | - |
| Asia | 3 | 2.04 (1.30–3.18) | 0.002 | 0.907 | 0.0 | 0.119 |
| Europe | 6 | 1.27 (0.92–1.75) | 0.141 | 0.000 | 87.6 | - |
| Oceania | 2 | 1.34 (1.10–1.62) | 0.003 | 0.732 | 0.0 | 0.199 |
| High ABCB1 expression adjusted for residual disease | 4 | 2.13 (1.60–2.83) | 0.000 | 0.000 | 85.4 | 0.003 |
| ABCB1 gene variants | 4 | 0.95 (0.81–1,12) | 0.555 | 0.003 | 78.4 | 0.582 |
| TR | 21 | |||||
| High ABCB1 expression | 21 | 0.63 (0.54–0.75) | 0.000 | 0.000 | 66.6 | 0.019 |
| ABCB1 detected by IHC | 16 | 0.63 (0.52–0.75) | 0.000 | 0.000 | 72.4 | 0.051 |
| ABCB1 detected by by PCR | 4 | 0.52 (0.33–0.80) | 0.003 | 0.214 | 33.0 | 0.464 |
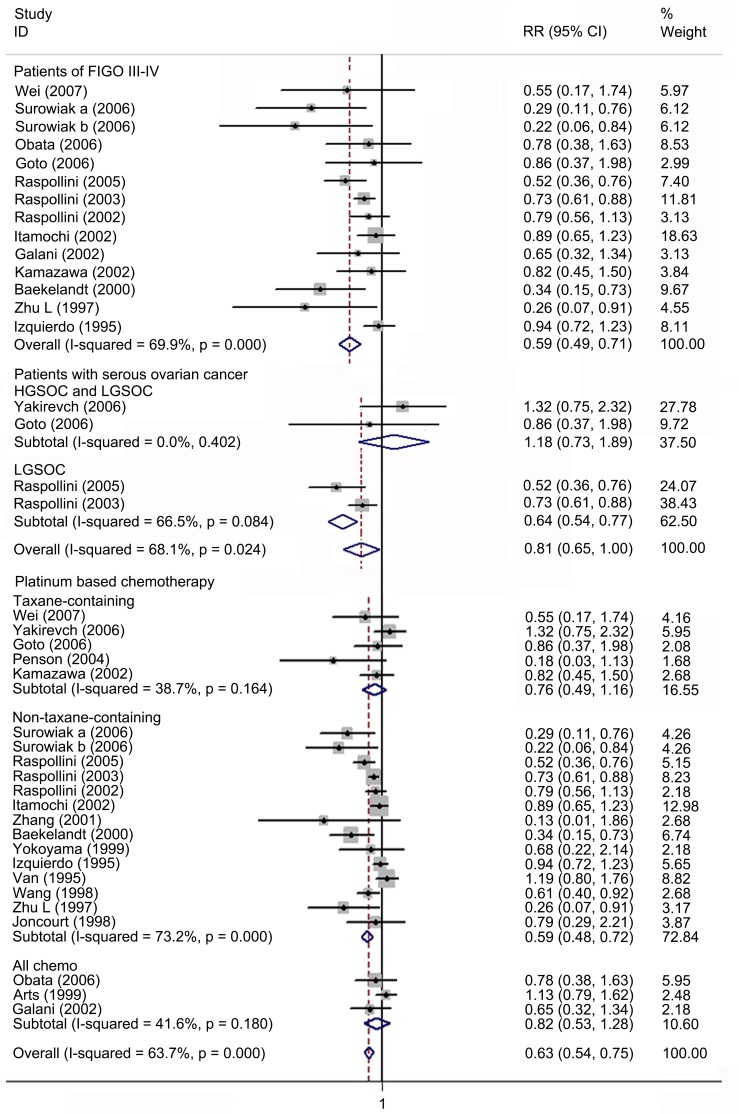
| FIGO III-IV | 13 | 0.57 (0.47–0.68) | 0.000 | 0.000 | 69.9 | 0.010 |
| Serous tumor | 4 | 0.72 (0.57–0.91) | 0.006 | 0.024 | 68.1 | 0.823 |
| Platinum-based chemotherapy | 21 | 0.63 (0.54–0.75) | 0.000 | 0.000 | 63.7 | 0.019 |
| Taxane-containing chemotherapyb | 5 | 0.76 (0.49–1.16) | 0.199 | 0.164 | 38.7 | 0.032 |
| Non-taxane-containing chemotherapyc | 13 | 0.59 (0.48–0.72) | 0.000 | 0.000 | 73.2 | 0.032 |
| All chemotherapyd | 3 | 0.82 (0.53–1.28) | 0.389 | 0.180 | 41.6 | 0.171 |
| Geographical region | ||||||
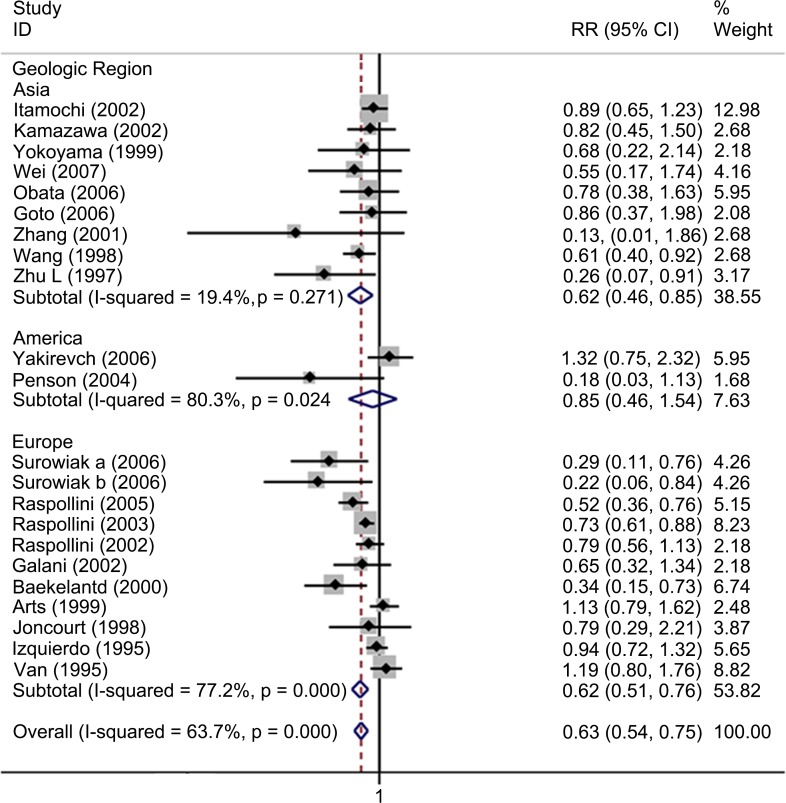
| America | 2 | 0.85 (0.46–1.54) | 0.587 | 0.024 | 80.3 | - |
| Asia | 9 | 0.62 (0.46–0.85) | 0.003 | 0.271 | 19.4 | 0.133 |
| Europe | 10 | 0.62 (0.51–0.76) | 0.000 | 0.000 | 77.2 | 0.547 |
a: HR and its 95%CI were used to assess OS and PFS, RR and its 95%CI were used to assess TR
b: PT (platinum + taxane) regimen
c: PAC (platinum + doxorubicin + cyclophosphamide), PA (platinum + doxorubicin) or PC (platinum + cyclophosphamide) regimen
d:Studies identified patients received PT, PAC, PA or PC regimen.
Fig 3. Forest plots of subgroup meta-analysis presenting HRs of EOC OS for high ABCB1 expression.
HR = hazard ratio; EOC = epithelial ovarian cancer; OS = overall survival.
Meta-analysis of the correlation between ABCB1 status and PFS in EOC
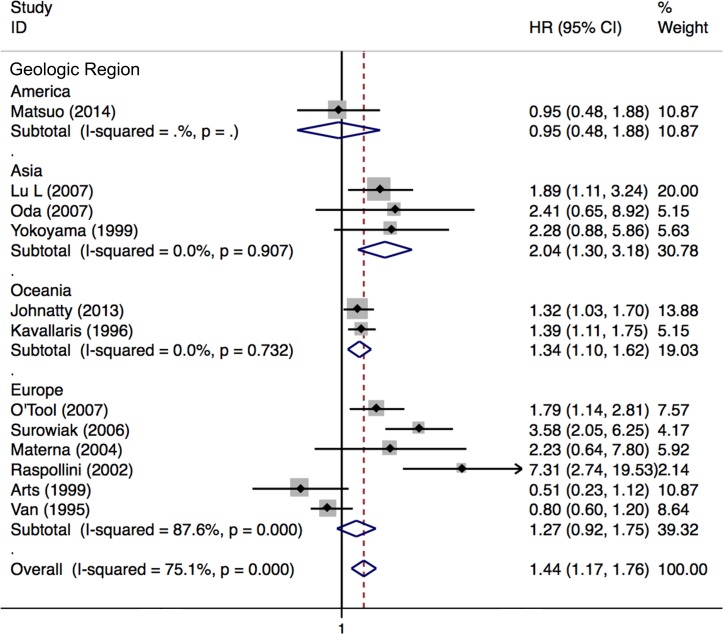
Nineteen studies with 7133 patients were included for meta-analysis of PFS. Fourteen studies with 1186 patients reported the association between high ABCB1 expression and PFS. Overall, high ABCB1 expression was associated with a poor PFS (HR 1.49; 95% CI: 1.22–1.82) in EOC. The effect of ABCB1 expression on PFS remained significant in the IHC (HR, 1.37; 95% CI: 1.05–1.79), the PCR (HR 1.67; 95% CI: 1.24–2.26), the FIGO III-IV (HR, 1.97; 95% CI: 1.40–2.78), the platinum-based non-taxane-containing chemotherapy (HR, 1.91; 95% CI: 1.46–2.50), the Asia (HR, 2.04; 95% CI: 1.30–3.18) and the Oceania (HR, 1.34; 95% CI: 1.10–1.62) subgroups. Similarly, when adjusted for residual disease (N = 4), age (N = 3), FIGO stage (N = 2), histological type (N = 1) and tumor grade (N = 1), high ABCB1 expression remained significantly associated with PFS (HR, 2.13; 95% CI: 1.60–2.83). However, such correlations were not found in the serous, the taxane-containing chemotherapy, the America and the Europe subgroups. Six studies with 5947 patients reported the association between rs2032582, rs1128503 or rs1045642 and PFS. No evidence of correlation between ABCB1 gene SNPs and PFS was found (HR, 1.02; 95% CI: 0.99–1.05). Significant heterogeneities were found in overall meta-analysis of high ABCB1 expression (I2 = 73.6%, P = 0.000), the IHC (I2 = 81.3%, P = 0.000), the PCR (I2 = 52.1%, P = 0.099), the rs2032582 (I2 = 73.6%, P = 0.004), the FIGO stage (I2 = 88.0%, P = 0.000), the platinum-based (I2 = 88.6%, P = 0.000), the all chemo (I2 = 64.7%, P = 0.027), the Europe (I2 = 87.6%, P = 0.000) subgroups and when adjusted for residual disease (I2 = 85.4%, P = 0.000) (Figs 5–7 and Table 3).
Fig 5. Forest plots presenting HRs of EOC PFS for high ABCB1 expression, adjusted results and ABCB1 gene variants.
HR = hazard ratio; EOC = epithelial ovarian cancer; PFS = progression free survival.
Fig 7. Forest plots of HRs of EOC PFS for high ABCB1 expression stratified by geographical region.
HR = hazard ratio; EOC = epithelial ovarian cancer; PFS = progression free survival.
Fig 6. Forest plots of subgroup meta-analysis presenting HRs of EOC PFS for high ABCB1 expression.
HR = hazard ratio; EOC = epithelial ovarian cancer; PFS = progression free survival.
Meta-analysis of the correlation between ABCB1 status and TR in EOC
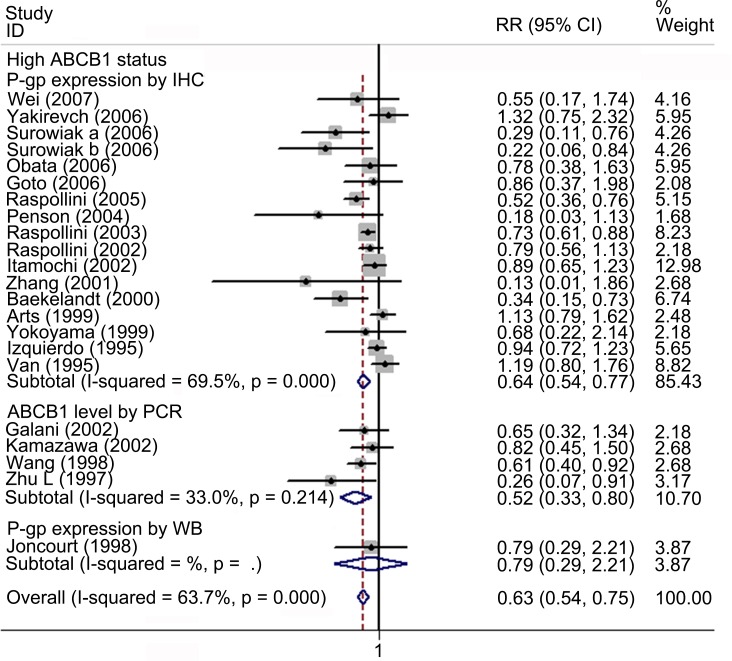
Twenty-one studies involving 966 patients were included for meta-analysis of TR. Overall, high ABCB1 expression was associated with a poor TR (RR, 0.63; 95% CI: 0.54–0.75) in EOC. The effect of ABCB1 expression on OS remained significant in the IHC (RR, 0.63; 95% CI: 0.52–0.75), the PCR (RR, 0.52; 95% CI: 0.33–0.80), the FIGO III-IV (RR, 0.57; 95% CI: 0.47–0.68), the platinum-based non-taxane-containing chemotherapy (RR, 0.59; 95% CI: 0.48–0.72), the Asia (RR, 0.62; 95% CI: 0.46–0.85) and the Europe (RR, 0.62; 95% CI: 0.51–0.76) subgroups. No evidence for correlations between ABCB1 expression and TR were found in the serous, the taxane-containing and the all chemo subgroups. We found that in the serous subgroup, the correlation between ABCB1 expression and TR became significant in studies specifically identified low-grade serous ovarian cancer (LGSOC) and remained non-significant in studies identified HGSOC. The heterogeneities of overall meta-analysis (I2 = 66.6%, P = 0.000), the IHC (I2 = 72.4%, P = 0.000), the FIGO stage (I2 = 69.9%, P = 0.000), the serous (I2 = 68.1%, P = 0.024), the platinum-based (I2 = 73.2%, P = 0.000), the America (I2 = 80.3%, P = 0.024) and the Europe (I2 = 77.2%, P = 0.000) subgroups were significant (Figs 8–10 and Table 3).
Fig 8. Forest plots presenting RRs of EOC TR for high ABCB1 expression.
RR = risk ratio; EOC = epithelial ovarian cancer; TR = total response rate.
Fig 10. Forest plots of RRs of EOC TR for high ABCB1 expression stratified by geographical region.
HR = hazard ratio; EOC = epithelial ovarian cancer; TR = total response rate.
Fig 9. Forest plots of subgroup meta-analysis presenting RRs of EOC TR for high ABCB1 expression.
RR = risk ratio; EOC = epithelial ovarian cancer; TR = total response rate.
Publication Bias
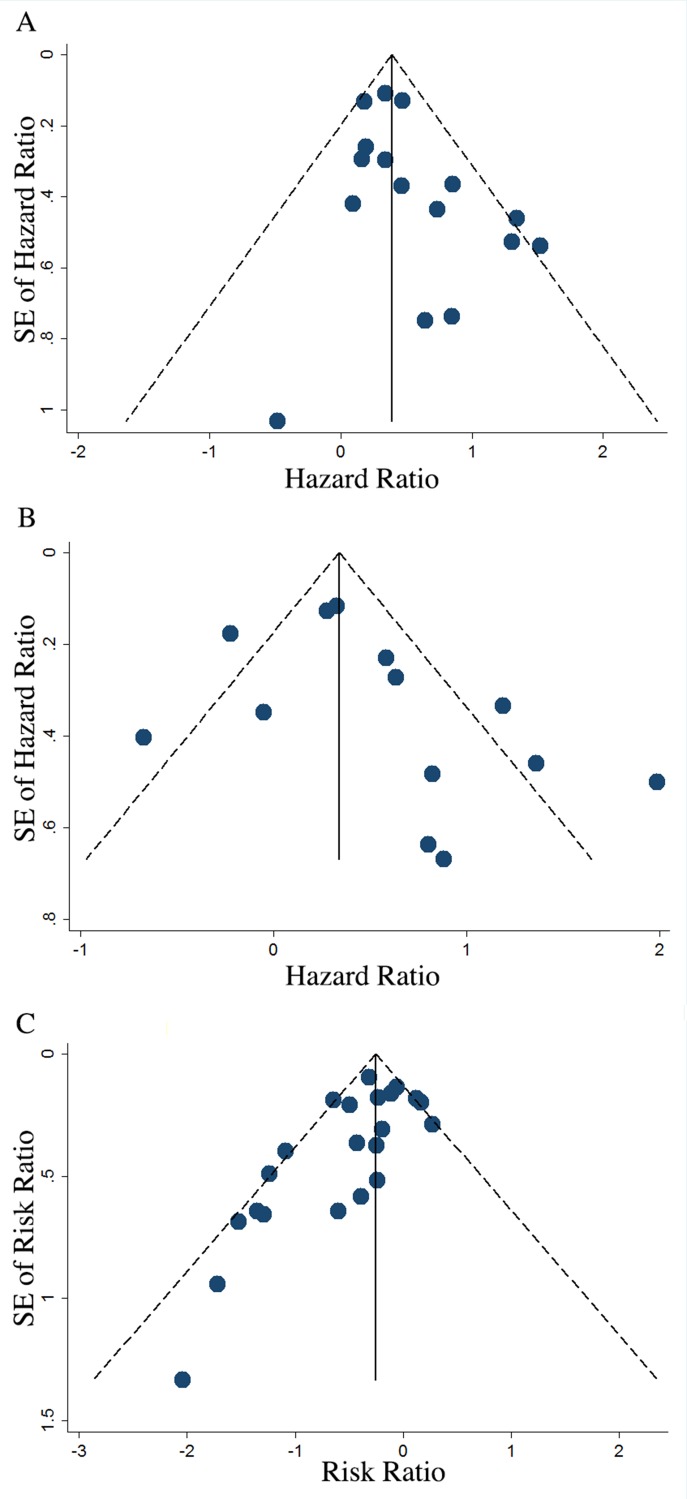
Publication bias was first assessed by visual inspection of funnel plots and then estimated with bias p-value from Egger’s test. While asymmetry of funnel plots was observed in overall meta-analysis of OS, PFS and TR, the results of egger’s test indicated that no publication bias existed in studies included for overall meta-analysis of OS, PFS and most subgroup meta-analysis. Publication bias was only found in studies for overall meta-analysis of TR (P = 0.019), analysis of PFS when adjusted for residual disease (P = 0.003), the FIGO stage (P = 0.010), the platinum-based (P = 0.032) and the taxane-containing (P = 0.032) subgroups of TR. After excluding certain studies to eliminate publication bias, the results of these analyses remained consistent to the original results (Fig 11 and Table 3).
Fig 11. Funnel plots presenting prognostic value of ABCB1 for OS, PFS and TR.
OS = overall survival; PFS = progression free survival; TR = total response rate. A, funnel plots showing the distribution of effect size and prevision of individual study estimate when evaluating the summary HR for OS. Egger’s test: P = 0.078. B, funnel plots showing the distribution of effect size and prevision of individual study estimate when evaluating the summary HR for PFS. Egger’s test: P = 0.146. C, funnel plots showing the distribution of effect size and prevision of individual study estimate when evaluating the summary RR for TR. Egger’s test: P = 0.019.
Discussion
Most EOC patients develop resistance to chemotherapy and eventually demise from the recurrent disease due to drug resistance. The development of chemo-resistance significantly impairs the survival in patients with EOC. The identification of predictive factors for chemo-resistance and poor survival might improve the individualized therapy for EOC. In the present study, we systematically assessed the impact of ABCB1, a transporter participating in efflux of a series of drugs, on chemotherapy response and survival in patients with EOC. We found that a high ABCB1 expression was significantly associated with decreased OS, PFS, and TR in EOC patients. These results are convincing because (i) the outcome definition was clear, (ii) the included studies were sub-grouped according to measuring methods to ensure the value of comparability, (iii) multivariate results further validated the association between ABCB1 and EOC prognosis and (iv) the number of studies reporting positive results or non-significant positive results were of greater quantity than the corresponding studies reporting negative results. Although heterogeneities were found in overall analyses for PFS and TR, we conducted subgroup analysis and determined histology and chemotherapy regimen as two main sources of heterogeneity between studies.
EOC consists of several histological subtypes, such as serous, mucinous, clear cell, endometrioid carcinomas. Different subtypes differ in genetic and biological features, clinical progression patterns, response to treatment, and prognosis. Therefore, it is meaningful to analyze the association between ABCB1 and prognosis in different ovarian cancer subtypes separately. However, in our subgroup analysis, we found only 6 out of 38 studies reported exclusively on serous ovarian carcinoma while the remaining reported on all subtypes as a whole. Since none of the studies reported independent data of any other subtypes other than serous ovarian carcinoma, we could not further determine what effects did other subtypes have on the significance association. Even though we find no evidence for correlation between ABCB1 expression and prognosis in patients with serous carcinoma, which accounts for 70% to 80% of all cases, the result is limited by small number of included studies (N = 6) therefore further validation is essential. Moreover, serous ovarian cancer can be classified into HGSOC and LGSOC according to the origins of tumorigensis, precursor lesions, genetic and molecular characteristics, morphologic features, clinical behaviors and chemotherapy responses [61,62]. HGSOC accounts for most ovarian cancer deaths as nearly 90% of the late stage HGSOC patients develop acquired resistance to platinum-based chemotherapy [63]. The mechanisms underlying acquired chemo-resistance in HGSOC include activation of AKT pathway, the reversion of BRCA1/BRCA2 germline mutations and ABCB1 overexpression [64]. Many argued that although decreased drug accumulation either by decreased influx, increased efflux or enhanced drug detoxification was identified as an important mechanism of drug resistance, most of these mechanisms were not clinically significant in HGSOC [65]. Similarly, we didn’t find any evidence for correlation between ABCB1 and prognosis of HGSOC patients. However, we did find unfavorable OS, PFS and TR accompanied by up-regulated ABCB1 in LGSOC patients, which is consistent with the fact that LGSOC is more resistant than HGSOC to initial chemotherapy (N = 2). Nevertheless, more studies were needed to validate the evolvement of ABCB1 in LGSOC chemo-resistance.
The regimen of platinum and taxane combination is the first-line treatment for EOC due to its superiority over other regimens regarding OS and adverse effects [66–68]. Although taxanes are substrates of ABCB1, our results did not reveal any evidence for a correlation between ABCB1 expression and prognosis in patients who received taxane-containing regimen. Instead, we found an adverse correlation between ABCB1 expression and prognosis in patients who received non-taxane-containing regimen, such as platinum combined with doxorubicin or cyclophosphamide. The biological effects resulted from up-regulated ABCB1 expression in the patients undergoing taxane-containing therapy might be compensated by other mechanisms that need further investigation. On one hand, it seemed that extensive use of taxanes successfully improved the general survival of EOC patients comparing to other drugs [69] and attenuated the sensitivity of ABCB1 as a prognosis indicator. On the other, since both cellular and clinical researches revealed that ABCB1 in ovarian cancer could be up-regulated by chemotherapy [70, 71], taxane induced alteration in ABCB1 through the course of treatment might also be a confounding factor to the measurement of survival analysis because ABCB1 expression was seldom obtained in matched analysis [72–74]. In pre-clinical studies, inhibitors targeting ABCB1 could reverse chemotherapy resistance to some extent [75,76]. However, the efficacies of these ABCB1 inhibitors were somehow discouraging in clinical trials for treatment of EOC patients with initial or acquired chemo-resistance [77]. Unselected patients might be one of the main reasons for that [78]. Our current results indicated that the patients undergoing non-taxane-containing chemotherapy rather than those undergoing taxane-containing chemotherapy might be benefited more from ABCB1 inhibition.
We observed an intimate relationship of ABCB1 expression in recurrent tumor with OS, but not that in primary tumor. Similarly, a whole-genome sequence analysis of a large cohort of sensitive, resistant and refractory ovarian carcinoma tissue specimens performed by Patch et al. revealed that ABCB1 was overexpressed in the recurrent and refractory tumors compared with the sensitive ones, which was caused by recurrent promoter fusion with SLC25A40 and led to acquired resistance to chemotherapy [79]. These findings suggest that the increase in ABCB1 induced by chemotherapy rather than the background ABCB1 expression is response for chemo-resistance and impaired survival and ABCB1 may play an essential role in the development of acquired drug resistance in ovarian cancer. However, the number of included studies for the ABCB1 in recurrent tumor was limited (N = 3) that restricts the reliability of this result.
To sum up, the present meta-analysis showed a significant negative correlation between ABCB1 expression level and prognosis in EOC patients and provided preliminary evidence highlighting this correlation in specific subgroups stratified by chemotherapy regimens, histological subtypes and tissues used for ABCB1 detection. Large-scale, prospective studies are needed to verify the clinical significance of ABCB1 expression in EOC.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This work was supported by National Natural Science Foundation of China (81272860, 81472443, 81572572).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by 81272860 National Natural Science Foundation of China http://www.nsfc.gov.cn ZW, 81472443 National Natural Science Foundation of China http://www.nsfc.gov.cn ZW, and 81572572 National Natural Science Foundation of China http://www.nsfc.gov.cn JC.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. 10.3322/caac.21254 . [DOI] [PubMed] [Google Scholar]
- 2.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA: a cancer journal for clinicians. 2011;61(3):183–203. 10.3322/caac.20113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RA, Manassaram-Baptiste D, Brooks D, Doroshenk M, Fedewa S, Saslow D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA: a cancer journal for clinicians. 2015;65(1):30–54. 10.3322/caac.21261 . [DOI] [PubMed] [Google Scholar]
- 4.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24 Suppl 6:vi24–32. 10.1093/annonc/mdt333 . [DOI] [PubMed] [Google Scholar]
- 5.Ali AY, Farrand L, Kim JY, Byun S, Suh JY, Lee HJ, et al. Molecular determinants of ovarian cancer chemoresistance: new insights into an old conundrum. Annals of the New York Academy of Sciences. 2012;1271:58–67. 10.1111/j.1749-6632.2012.06734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi MK, Kim DD. Platinum transporters and drug resistance. Archives of pharmacal research. 2006;29(12):1067–73. . [DOI] [PubMed] [Google Scholar]
- 7.Dalton WS, Durie BG, Alberts DS, Gerlach JH, Cress AE. Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein. Cancer research. 1986;46(10):5125–30. . [PubMed] [Google Scholar]
- 8.Wils P, Phung-Ba V, Warnery A, Lechardeur D, Raeissi S, Hidalgo IJ, et al. Polarized transport of docetaxel and vinblastine mediated by P-glycoprotein in human intestinal epithelial cell monolayers. Biochemical pharmacology. 1994;48(7):1528–30. . [DOI] [PubMed] [Google Scholar]
- 9.Webster L, Linsenmeyer M, Millward M, Morton C, Bishop J, Woodcock D. Measurement of cremophor EL following taxol: plasma levels sufficient to reverse drug exclusion mediated by the multidrug-resistant phenotype. Journal of the National Cancer Institute. 1993;85(20):1685–90. . [DOI] [PubMed] [Google Scholar]
- 10.Hendricks CB, Rowinsky EK, Grochow LB, Donehower RC, Kaufmann SH. Effect of P-glycoprotein expression on the accumulation and cytotoxicity of topotecan (SK&F 104864), a new camptothecin analogue. Cancer research. 1992;52(8):2268–78. . [PubMed] [Google Scholar]
- 11.Stordal B, Hamon M, McEneaney V, Roche S, Gillet JP, O'Leary JJ, et al. Resistance to paclitaxel in a cisplatin-resistant ovarian cancer cell line is mediated by P-glycoprotein. PloS one. 2012;7(7):e40717 10.1371/journal.pone.0040717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arao S, Suwa H, Mandai M, Tashiro H, Miyazaki K, Okamura H, et al. Expression of multidrug resistance gene and localization of P-glycoprotein in human primary ovarian cancer. Cancer research. 1994;54(5):1355–9. . [PubMed] [Google Scholar]
- 13.Izquierdo MA, van der Zee AG, Vermorken JB, van der Valk P, Belien JA, Giaccone G, et al. Drug resistance-associated marker Lrp for prediction of response to chemotherapy and prognoses in advanced ovarian carcinoma. Journal of the National Cancer Institute. 1995;87(16):1230–7. . [DOI] [PubMed] [Google Scholar]
- 14.van der Zee AG, Hollema H, Suurmeijer AJ, Krans M, Sluiter WJ, Willemse PH, et al. Value of P-glycoprotein, glutathione S-transferase pi, c-erbB-2, and p53 as prognostic factors in ovarian carcinomas. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1995;13(1):70–8. . [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama Y, Sato S, Fukushi Y, Sakamoto T, Futagami M, Saito Y. Significance of multi-drug-resistant proteins in predicting chemotherapy response and prognosis in epithelial ovarian cancer. The journal of obstetrics and gynaecology research. 1999;25(6):387–94. . [DOI] [PubMed] [Google Scholar]
- 16.Arts HJ, Katsaros D, de Vries EG, Massobrio M, Genta F, Danese S, et al. Drug resistance-associated markers P-glycoprotein, multidrug resistance-associated protein 1, multidrug resistance-associated protein 2, and lung resistance protein as prognostic factors in ovarian carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 1999;5(10):2798–805. . [PubMed] [Google Scholar]
- 17.Tecza K, Pamula-Pilat J, Kolosza Z, Radlak N, Grzybowska E. Genetic polymorphisms and gene-dosage effect in ovarian cancer risk and response to paclitaxel/cisplatin chemotherapy. Journal of experimental & clinical cancer research: CR. 2015;34:2 10.1186/s13046-015-0124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo K, Sheridan TB, Mabuchi S, Yoshino K, Hasegawa K, Studeman KD, et al. Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecologic oncology. 2014;133(3):473–9. 10.1016/j.ygyno.2014.03.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White KL, Vierkant RA, Fogarty ZC, Charbonneau B, Block MS, Pharoah PD, et al. Analysis of over 10,000 Cases finds no association between previously reported candidate polymorphisms and ovarian cancer outcome. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(5):987–92. 10.1158/1055-9965.EPI-13-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnatty SE, Beesley J, Gao B, Chen X, Lu Y, Law MH, et al. ABCB1 (MDR1) polymorphisms and ovarian cancer progression and survival: a comprehensive analysis from the Ovarian Cancer Association Consortium and The Cancer Genome Atlas. Gynecologic oncology. 2013;131(1):8–14. 10.1016/j.ygyno.2013.07.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian C, Ambrosone CB, Darcy KM, Krivak TC, Armstrong DK, Bookman MA, et al. Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes among women with advanced stage ovarian cancer treated with platinum and taxane-based chemotherapy: a Gynecologic Oncology Group study. Gynecologic oncology. 2012;124(3):575–81. 10.1016/j.ygyno.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Kim K, No JH, Jeon YT, Jeon HW, Kim YB. Prognostic value of biomarkers related to drug resistance in patients with advanced epithelial ovarian cancer. Anticancer research. 2012;32(2):589–94. . [PubMed] [Google Scholar]
- 23.Lu D, Shi HC, Wang ZX, Gu XW, Zeng YJ. Multidrug resistance-associated biomarkers PGP, GST-pi, Topo-II and LRP as prognostic factors in primary ovarian carcinoma. British journal of biomedical science. 2011;68(2):69–74. . [DOI] [PubMed] [Google Scholar]
- 24.Bergmann TK, Green H, Brasch-Andersen C, Mirza MR, Herrstedt J, Holund B, et al. Retrospective study of the impact of pharmacogenetic variants on paclitaxel toxicity and survival in patients with ovarian cancer. European journal of clinical pharmacology. 2011;67(7):693–700. 10.1007/s00228-011-1007-6 . [DOI] [PubMed] [Google Scholar]
- 25.Johnatty SE, Beesley J, Paul J, Fereday S, Spurdle AB, Webb PM, et al. ABCB1 (MDR 1) polymorphisms and progression-free survival among women with ovarian cancer following paclitaxel/carboplatin chemotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14(17):5594–601. 10.1158/1078-0432.CCR-08-0606 . [DOI] [PubMed] [Google Scholar]
- 26.Wei FH, Zhao XD, Zhang Y, He SR, Yang L. [The predictive factors for the response to platinum/paclitaxel based first-line adjuvant chemotherapy in advanced ovarian cancer]. Zhonghua yi xue za zhi. 2007;87(17):1187–9. . [PubMed] [Google Scholar]
- 27.Oda Y, Ohishi Y, Basaki Y, Kobayashi H, Hirakawa T, Wake N, et al. Prognostic implications of the nuclear localization of Y-box-binding protein-1 and CXCR4 expression in ovarian cancer: their correlation with activated Akt, LRP/MVP and P-glycoprotein expression. Cancer science. 2007;98(7):1020–6. 10.1111/j.1349-7006.2007.00492.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Toole SA, Sheppard BL, Laios A, O'Leary JJ, McGuinness EP, D'Arcy T, et al. Potential predictors of chemotherapy response in ovarian cancer—how do we define chemosensitivity? Gynecologic oncology. 2007;104(2):345–51. 10.1016/j.ygyno.2006.08.039 . [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Katsaros D, Wiley A, Rigault de la Longrais IA, Puopolo M, Yu H. Expression of MDR1 in epithelial ovarian cancer and its association with disease progression. Oncology research. 2007;16(8):395–403. . [DOI] [PubMed] [Google Scholar]
- 30.Yakirevich E, Sabo E, Naroditsky I, Sova Y, Lavie O, Resnick MB. Multidrug resistance-related phenotype and apoptosis-related protein expression in ovarian serous carcinomas. Gynecologic oncology. 2006;100(1):152–9. 10.1016/j.ygyno.2005.08.050 . [DOI] [PubMed] [Google Scholar]
- 31.Surowiak P, Materna V, Denkert C, Kaplenko I, Spaczynski M, Dietel M, et al. Significance of cyclooxygenase 2 and MDR1/P-glycoprotein coexpression in ovarian cancers. Cancer letters. 2006;235(2):272–80. 10.1016/j.canlet.2005.04.035 . [DOI] [PubMed] [Google Scholar]
- 32.Obata H, Yahata T, Quan J, Sekine M, Tanaka K. Association between single nucleotide polymorphisms of drug resistance-associated genes and response to chemotherapy in advanced ovarian cancer. Anticancer research. 2006;26(3B):2227–32. . [PubMed] [Google Scholar]
- 33.Goto T, Takano M, Sakamoto M, Kondo A, Hirata J, Kita T, et al. Gene expression profiles with cDNA microarray reveal RhoGDI as a predictive marker for paclitaxel resistance in ovarian cancers. Oncology reports. 2006;15(5):1265–71. . [PubMed] [Google Scholar]
- 34.Raspollini MR, Amunni G, Villanucci A, Boddi V, Taddei GL. Increased cyclooxygenase-2 (COX-2) and P-glycoprotein-170 (MDR1) expression is associated with chemotherapy resistance and poor prognosis. Analysis in ovarian carcinoma patients with low and high survival. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2005;15(2):255–60. 10.1111/j.1525-1438.2005.15212.x . [DOI] [PubMed] [Google Scholar]
- 35.Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF Jr., Goodman A, et al. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecologic oncology. 2004;93(1):98–106. 10.1016/j.ygyno.2003.11.053 . [DOI] [PubMed] [Google Scholar]
- 36.Materna V, Pleger J, Hoffmann U, Lage H. RNA expression of MDR1/P-glycoprotein, DNA-topoisomerase I, and MRP2 in ovarian carcinoma patients: correlation with chemotherapeutic response. Gynecologic oncology. 2004;94(1):152–60. 10.1016/j.ygyno.2004.03.035 . [DOI] [PubMed] [Google Scholar]
- 37.Raspollini MR, Amunni G, Villanucci A, Baroni G, Boddi V, Taddei GL. Prognostic value of P-glycoprotein and proliferative index in advanced low grade serous ovarian carcinomas. Journal of chemotherapy. 2003;15(4):380–6. 10.1179/joc.2003.15.4.380 . [DOI] [PubMed] [Google Scholar]
- 38.Ikeda K, Sakai K, Yamamoto R, Hareyama H, Tsumura N, Watari H, et al. Multivariate analysis for prognostic significance of histologic subtype, GST-pi, MDR-1, and p53 in stages II-IV ovarian cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2003;13(6):776–84. . [DOI] [PubMed] [Google Scholar]
- 39.Raspollini MR, Pinzani P, Pazzagli M, Baroni G, Taddei A, Amunni G, et al. Multidrug resistance in ovarian cancer: comparing an immunocytochemical study and ATP-tumor chemosensitivity assay. Journal of chemotherapy. 2002;14(5):518–25. 10.1179/joc.2002.14.5.518 . [DOI] [PubMed] [Google Scholar]
- 40.Nakayama K, Kanzaki A, Ogawa K, Miyazaki K, Neamati N, Takebayashi Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) as a cisplatin based chemoresistance marker in ovarian carcinoma: comparative analysis with expression of MDR1, MRP1, MRP2, LRP and BCRP. International journal of cancer Journal international du cancer. 2002;101(5):488–95. 10.1002/ijc.10608 . [DOI] [PubMed] [Google Scholar]
- 41.Kamazawa S, Kigawa J, Kanamori Y, Itamochi H, Sato S, Iba T, et al. Multidrug resistance gene-1 is a useful predictor of Paclitaxel-based chemotherapy for patients with ovarian cancer. Gynecologic oncology. 2002;86(2):171–6. . [DOI] [PubMed] [Google Scholar]
- 42.Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M, Terakawa N. Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstetrics and gynecology. 2002;100(2):281–7. . [DOI] [PubMed] [Google Scholar]
- 43.Galani E, Sgouros J, Petropoulou C, Janinis J, Aravantinos G, Dionysiou-Asteriou D, et al. Correlation of MDR-1, nm23-H1 and H Sema E gene expression with histopathological findings and clinical outcome in ovarian and breast cancer patients. Anticancer research. 2002;22(4):2275–80. 12174914. [PubMed] [Google Scholar]
- 44.Dabholkar M, Thornton K, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Increased mRNA levels of xeroderma pigmentosum complementation group B (XPB) and Cockayne's syndrome complementation group B (CSB) without increased mRNA levels of multidrug-resistance gene (MDR1) or metallothionein-II (MT-II) in platinum-resistant human ovarian cancer tissues. Biochemical pharmacology. 2000;60(11):1611–9. . [DOI] [PubMed] [Google Scholar]
- 45.Baekelandt MM, Holm R, Nesland JM, Trope CG, Kristensen GB. P-glycoprotein expression is a marker for chemotherapy resistance and prognosis in advanced ovarian cancer. Anticancer research. 2000;20(2B):1061–7. . [PubMed] [Google Scholar]
- 46.Wang S, Cai G. Clinical study of multi-drug resistance gene (MDR1) expression in primary ovarian cancer. Journal of Tongji Medical University = Tong ji yi ke da xue xue bao. 1998;18(1):58–60. . [DOI] [PubMed] [Google Scholar]
- 47.Zhu L, Lang J, Shen K. [The relationship between multi-drug resistance gene expression and drug resistance of ovarian carcinoma]. Zhonghua fu chan ke za zhi. 1997;32(8):462–6. . [PubMed] [Google Scholar]
- 48.Khalifa MA, Abdoh AA, Mannel RS, Walker JL, Angros LH, Min KW. P-glycoprotein as a prognostic indicator in pre- and postchemotherapy ovarian adenocarcinoma. International journal of gynecological pathology: official journal of the International Society of Gynecological Pathologists. 1997;16(1):69–75. . [DOI] [PubMed] [Google Scholar]
- 49.Coley HM. Drug resistance studies using fresh human ovarian carcinoma and soft tissue sarcoma samples. The Keio journal of medicine. 1997;46(3):142–7. . [DOI] [PubMed] [Google Scholar]
- 50.Codegoni AM, Broggini M, Pitelli MR, Pantarotto M, Torri V, Mangioni C, et al. Expression of genes of potential importance in the response to chemotherapy and DNA repair in patients with ovarian cancer. Gynecologic oncology. 1997;65(1):130–7. 10.1006/gyno.1996.4609 . [DOI] [PubMed] [Google Scholar]
- 51.Kavallaris M, Leary JA, Barrett JA, Friedlander ML. MDR1 and multidrug resistance-associated protein (MRP) gene expression in epithelial ovarian tumors. Cancer letters. 1996;102(1–2):7–16. . [DOI] [PubMed] [Google Scholar]
- 52.Veneroni S, Zaffaroni N, Daidone MG, Benini E, Villa R, Silvestrini R. Expression of P-glycoprotein and in vitro or in vivo resistance to doxorubicin and cisplatin in breast and ovarian cancers. European journal of cancer. 1994;30A(7):1002–7. . [DOI] [PubMed] [Google Scholar]
- 53.Holzmayer TA, Hilsenbeck S, Von Hoff DD, Roninson IB. Clinical correlates of MDR1 (P-glycoprotein) gene expression in ovarian and small-cell lung carcinomas. Journal of the National Cancer Institute. 1992;84(19):1486–91. . [DOI] [PubMed] [Google Scholar]
- 54.Grimm C, Polterauer S, Zeillinger R, Tong D, Heinze G, Wolf A, et al. Two multidrug-resistance (ABCB1) gene polymorphisms as prognostic parameters in women with ovarian cancer. Anticancer research. 2010;30(9):3487–91. . [PubMed] [Google Scholar]
- 55.Joncourt F, Buser K, Altermatt H, Bacchi M, Oberli A, Cerny T. Multiple drug resistance parameter expression in ovarian cancer. Gynecologic oncology. 1998;70(2):176–82. 10.1006/gyno.1998.5085 . [DOI] [PubMed] [Google Scholar]
- 56.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22(4):719–48. . [PubMed] [Google Scholar]
- 58.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. . [DOI] [PubMed] [Google Scholar]
- 59.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. 10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 60.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horiuchi A, Itoh K, Shimizu M, Nakai I, Yamazaki T, Kimura K, et al. Toward understanding the natural history of ovarian carcinoma development: a clinicopathological approach. Gynecologic oncology. 2003;88(3):309–17. . [DOI] [PubMed] [Google Scholar]
- 62.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. The American journal of pathology. 2004;164(5):1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramalingam P. Morphologic, Immunophenotypic, and Molecular Features of Epithelial Ovarian Cancer. Oncology (Williston Park). 2016;30(2):166–76. . [PubMed] [Google Scholar]
- 64.Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC, Jr., Beral V, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nature reviews Cancer. 2015;15(11):668–79. 10.1038/nrc4019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–88. 10.1016/S0140-6736(13)62146-7 . [DOI] [PubMed] [Google Scholar]
- 66.Harries M, Gore M. Part II: chemotherapy for epithelial ovarian cancer-treatment of recurrent disease. The Lancet Oncology. 2002;3(9):537–45. . [DOI] [PubMed] [Google Scholar]
- 67.Harries M, Gore M. Part I: chemotherapy for epithelial ovarian cancer-treatment at first diagnosis. The Lancet Oncology. 2002;3(9):529–36. . [DOI] [PubMed] [Google Scholar]
- 68.Covens A, Carey M, Bryson P, Verma S, Fung Kee Fung M, Johnston M. Systematic review of first-line chemotherapy for newly diagnosed postoperative patients with stage II, III, or IV epithelial ovarian cancer. Gynecologic oncology. 2002;85(1):71–80. 10.1006/gyno.2001.6552 . [DOI] [PubMed] [Google Scholar]
- 69.Jean S, Li J, Katsaros D, Wubbenhorst B, Maxwell KN, Fishbein L, et al. Paclitaxel is necessary for improved survival in epithelial ovarian cancers with homologous recombination gene mutations. Oncotarget. 2016. 10.18632/oncotarget.9373 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia YZ, Yang L, Xue GM, Zhang C, Guo C, Yang YW, et al. Combining GRP78 suppression and MK2206-induced Akt inhibition decreases doxorubicin-induced P-glycoprotein expression and mitigates chemoresistance in human osteosarcoma. Oncotarget. 2016. 10.18632/oncotarget.10890 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu KJ, He JH, Su XD, Sim HM, Xie JD, Chen XG, et al. Saracatinib (AZD0530) is a potent modulator of ABCB1-mediated multidrug resistance in vitro and in vivo. International journal of cancer Journal international du cancer. 2013;132(1):224–35. 10.1002/ijc.27649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zajchowski DA, Karlan BY, Shawver LK. Treatment-related protein biomarker expression differs between primary and recurrent ovarian carcinomas. Molecular cancer therapeutics. 2012;11(2):492–502. 10.1158/1535-7163.MCT-11-0746 . [DOI] [PubMed] [Google Scholar]
- 73.Hille S, Rein DT, Riffelmann M, Neumann R, Sartorius J, Pfutzner A, et al. Anticancer drugs induce mdr1 gene expression in recurrent ovarian cancer. Anti-cancer drugs. 2006;17(9):1041–4. 10.1097/01.cad.0000231480.07654.b5 . [DOI] [PubMed] [Google Scholar]
- 74.Schondorf T, Neumann R, Benz C, Becker M, Riffelmann M, Gohring UJ, et al. Cisplatin, doxorubicin and paclitaxel induce mdr1 gene transcription in ovarian cancer cell lines. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2003;161:111–6. . [DOI] [PubMed] [Google Scholar]
- 75.Ludwig JA, Szakacs G, Martin SE, Chu BF, Cardarelli C, Sauna ZE, et al. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer research. 2006;66(9):4808–15. 10.1158/0008-5472.CAN-05-3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu KJ, He JH, Su XD, Sim HM, Xie JD, Chen XG, et al. Saracatinib (AZD0530) is a potent modulator of ABCB1-mediated multidrug resistance in vitro and in vivo. International journal of cancer Journal international du cancer. 2013;132(1):224–35. 10.1002/ijc.27649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shukla S, Ohnuma S, Ambudkar SV. Improving cancer chemotherapy with modulators of ABC drug transporters. Current drug targets. 2011;12(5):621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu M, Ocana A, Tannock IF. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: why has it failed to provide clinical benefit? Cancer metastasis reviews. 2013;32(1–2):211–27. 10.1007/s10555-012-9402-8 . [DOI] [PubMed] [Google Scholar]
- 79.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–94. 10.1038/nature14410 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.