Abstract
Background
The prognostic significance of vascular endothelial growth factor C (VEGF-C) expression in breast cancer (BC) patients remains controversial. Therefore, this meta-analysis was performed to determine the prognostic significance of VEGF-C expression in BC patients.
Materials and Methods
Several electronic databases were searched from January 1991 to August 2016. The pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to evaluate the prognostic significance of VEGF-C expression for disease free survival (DFS) and overall survival (OS).
Results
The present meta analysis totally included 21 eligible studies and 2828 patients with BC. The combined HRs were 1.87(95% CI 1.25–2.79, P = 0.001) for DFS and 1.96(95% CI 1.15–3.31, P = 0.001) for OS. The pooled HRs of non-Asian subgroup were 2.04(95%CI 1.36–3.05, P = 0.001) for DFS and 2.61(95%CI 1.51–4.52, P = 0.001) for OS, which were significantly higher than that of Asian subgroup. The funnel plot for publication bias was symmetrical. The further Egger's test and Begg's test did not detect significant publication bias (all P>0.05).
Conclusions
The present meta analysis strongly supported the prognostic role of VEGF-C expression for DFS and OS in BC patients, especially for patients in non-Asian countries. Furthermore, stratification by VEGF-C expression may help to optimize the treatments and the integrated managements for BC patients.
Introduction
Breast cancer (BC) is the most common malignant tumor and the leading cause of cancer death in females worldwide, resulting in 14% of the total cancer deaths in 2008 [1]. Breast cancer is a complicated tumor with different clinical characteristics and poor prognosis. Several clinical and pathological features, including HER-2 status, histological grade, and hormone receptor status, have been used to predict the treatment response and clinical prognosis in BC patients[2–3]. However, these factors are insufficient to accurately predict poor prognosis for BC patients. Therefore, it is necessary to find a reliable prognostic factor to predict the clinical prognosis for BC patients.
As a member of VEGF family, vascular endothelial growth factor C (VEGF-C) is an important influence factor for angiogenesis and lymphangiogenesis in tumors. It has been reported that VEGF-C is commonly expressed in breast cancer[4].So far, the relationship between VEGF-C expression and prognosis of BC patients was still contradictory in various original studies [5–25]. Several studies reported that high VEGF-C expression had a significant correlation with poor survival in BC patients [17, 19, 24]. On the contrary, some studies reported that high VEGF-C expression had a significant association with favorable survival for BC patients [13, 22]. More interestingly, four relevant meta analyses provided two opposite opinions on relationship between high VEGF-C expression and clinical prognosis in BC patients [26–29]. The contradiction among these original studies and meta analyses seriously hindered the clinical utility of VEGF-C expression in BC patients. Therefore, we performed this meta-analysis to further clarify the prognostic significance and clinical value of VEGF-C expression for BC patients.
Materials and Methods
Literature search strategy
Several electronic databases, including EMBASE, PubMed, Web of Knowledge, and Cochrane Library, were searched from January 1991 to August 2016. We performed literature search by combining MeSH term and text word in PubMed databases with the follow terms: "VEGF-C" or "vascular endothelial growth factor C " and "breast" or " mammary " and "cancer" or "carcinoma" or "tumor" and "survival" or "outcome" or "prognosis" or "prognostic". The search strategies for EMBASE and other databases were similar but were adapted correspondingly. Expanded search of hyponym was performed in the retrieval process. In addition, we performed a manual search according to the references of the relevant articles to supplement eligible studies. If necessary, we even contacted the corresponding author to get necessary information. The search was restricted to human studies, but there were no restrictions on language or publication time. All clinical investigation and data achievement were performed according to the principles of Declaration of Helsinki.
Criteria for inclusion and exclusion
The inclusion criteria of eligible studies were as follows: (1) proven pathological diagnosis of BC in humans; (2) enough information of overall survival such as hazard ratio (HR) and 95% confidence interval (CI). Studies not directly providing hazard ratio and 95% confidence interval were included if survival information were available from curves or tables for statistical estimation of HR. Articles published in Chinese were included in this meta-analysis as English literature. For multiple studies from the same population, only the most recently published study was included in this meta-analysis.
The following studies were excluded: (1) laboratory studies; (2) non-human experiments; (3) reviews, letters, case reports, and conference abstracts without original data; (4) lack of the necessary survival data.
Quality assessment of studies
Two reviewers (Zhiqiao Zhang and Hongfeng Tang) independently evaluated the quality of the studies included in the present meta analysis by using Newcastle-Ottawa Quality Assessment Scale (NOS). The NOS comprises assessments of patient selection, study comparability, follow-up, and outcome of interest. The total scores were used to assess the study quality. Disagreements between two reviewers were resolved through consensus with another reviewer (Guanying Luo).
Data extraction
Two investigators (Zhiqiao Zhang and Hongfeng Tang) independently extracted the following data from the original studies: surname of the first author, detection method of VEGF-C expression, patient number, region, publication year, disease stage, clinical parameters, and survival outcome data (HRs and 95%CIs). The information were extracted and recorded by using a standardized form. All eligible studies were coded as surname of the first author + publish year in the standardized form. Study authors were contacted to get key information if necessary. Disagreements between two investigators were resolved by discussion. If necessary, a third investigator (Guanying Luo) helped to reach a consensus.
Statistical analysis
The statistical analysis was performed according to the suggestions of the Meta-Analysis of Observational Studies in Epidemiology group (MOOSE)[30]. The hazard ratios (HRs) and 95% CIs were used to summary outcome of overall survival. We directly obtained pooled HRs and 95% CIs if the statistical data were reported in the study. While HRs and 95% CIs were not directly reported in the studies, survival information was extracted from Kaplan-Meier curve and used to estimate HR. The heterogeneity was evaluated using I2 statistic, which was defined according to the Cochrane Handbook [31]: 0% to 40%, negligible heterogeneity; 30% to 60%, moderate heterogeneity; 50% to 90%, substantial heterogeneity; 75% to 100%, considerable heterogeneity. The subsequently meta-analysis was performed using random effect model with DerSimonian and Laird method[32], which applying the inverse of variance as a weighing factor. Meta-regression analyses with REstricted Maximum Likelihood (REML) method and subgroup analyses were performed to explore the sources of heterogeneity. Funnel plot, Begg's test[33], and Egger's test[34] were employed to evaluate publication bias. P value<0.05 was considered statistically significant. The statistical analyses were performed by STATA version 12.0 software (Stata Corporation, College Station, Texas, USA).
Results
Search Results
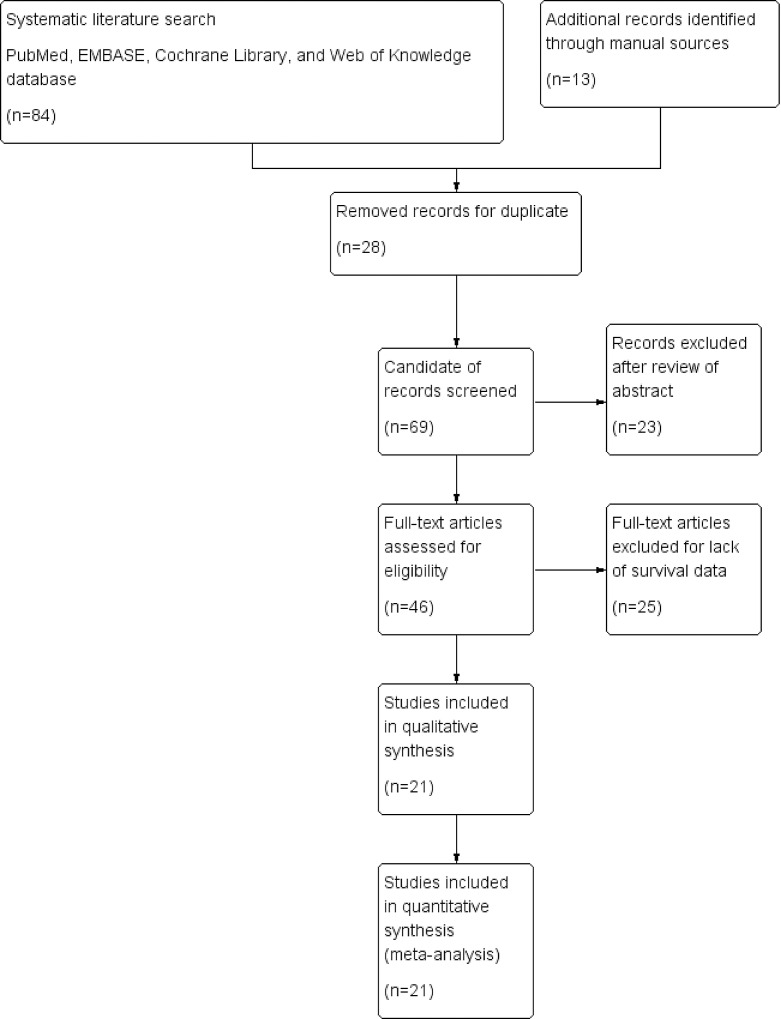
The initial search found a total of 97 articles (with 28 duplicate articles). After reviewing the abstracts, 23 irrelevant articles were excluded according to the criteria for inclusion and exclusion. Reviewers identified 46 potential studies for full-text review and 25 articles were eliminated due to inadequate survival data. Finally, 21 eligible studies were included in the present meta-analysis [5–25]. The details of search process were summarized in Fig 1. Quality assessment of 21 involved studies were assessed by using the Newcastle-Ottawa Scale (NOS).
Fig 1. Flowchart of study selection in present meta-analysis.
Study selection and characteristics
The characteristic of the 21 included studies were summarized in Table 1. The publication time of the included studies ranged from 2001 to 2015.The patient number of 21 studies ranged from 61 to 377, with a mean sample size of 135. The mean time of follow-up period ranged from 32 to 135 months. The NOS scores of 21 included studies varied from 7 to 8, with a mean value of 7.1. VEGF-C expression was measured in surgical tumor tissues. All studies provided enough information of survival data and/or survival curve.
Table 1. Characteristics of studies included in present meta analysis.
| Cutoff | Total | Positive | Age | History | Survival | Data | NOS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Method | Level | Number | (%) | (range) | Type | Stage | Therapy | Outcome | Extraction | Value |
| Zhang 2006 | China | PCR | Median | 61 | 67.4 | 52.3(28–75) | BC | NR | S | DFS | Curve | 7 |
| Linardou 2012 | Greece | PCR | 75.0% | 167 | 24.9 | 50(22–78) | BC | NR | S+C | DFS/OS | Curve | 8 |
| Watanabe 2005 | Japan | IHC | Score≥2+ | 87 | 43.7 | 53.5±14.4 | BC | NR | S+C | DFS/OS | Curve | 8 |
| Yang 2001 | China | IHC | Median | 107 | 50 | 52(33–77) | BC | NR | S+C | DFS | Curve | 7 |
| Zhao 2012 | China | IHC | Score≥4 | 78 | 47.4 | 54(29–75) | BC | I-III | S+C | DFS/OS | Curve | 7 |
| Cao 2003 | China | IHC | Score≥3 | 66 | 66.6 | 49(29–77) | BC | I-III | S | OS | Curve | 7 |
| Li 2009 | China | IHC | Score≥2+ | 117 | 58.1 | 51(29–74) | BC | I-III | S+C | DFS | Curve | 7 |
| Liu 2013 | China | IHC | Score≥1+ | 116 | 50.9 | 52(32–77) | BC | I-III | S | DFS/OS | Curve | 7 |
| Bando 2006 | Japan | ELISA | Score≥0.326 | 193 | 54.4 | 54(30–86) | BC | NR | S+C | DFS/OS | Reported | 7 |
| Gisterel 2010 | Poland | PCR | ≥1784 | 377 | NR | 57(29–83) | BC | I-III | S+C | DFS | Reported | 7 |
| Mohammed 2007 | England | IHC | Median | 177 | 37 | 57(32–70) | BC | I-II | S | DFS/OS | Reported | 7 |
| Tsutsui 2010 | Japan | IHC | Score≥5 | 242 | 78 | 58(23–86) | BC | I-III | S+C | DFS | Reported | 7 |
| Gu 2008 | China | IHC | Score≥2+ | 61 | 70.5 | 58(29–90) | BC | I-III | S+C | DFS/OS | Reported | 7 |
| Kinoshita 2001 | Japan | IHC | ≥10% | 98 | 39.8 | 55(30–86) | BC | I-IV | S+C | DFS | Reported | 7 |
| Mylona 2007 | Greece | IHC | ≥10% | 177 | 48 | 57(25–86) | BC | I-III | S | DFS/OS | Reported | 7 |
| Nakamura 2006 | Japan | IHC | Score≥5 | 113 | 82.3 | NR | BC | I-III | S+C | OS | Reported | 7 |
| Nakamura 2003 | Japan | IHC | ≥10% | 103 | 83.7 | 51(24–87) | BC | I-III | S | DFS | Reported | 7 |
| Ni 2013 | China | IHC | NR | 75 | 64 | NR | BC | I-III | S+C | OS | Reported | 7 |
| Yang 2015 | China | IHC | ≥10% | 218 | 62.3 | NR | BC | I-III | S | OS | Reported | 7 |
| Zhang 2008 | China | IHC | Score≥2+ | 70 | 42.9 | 49(30–77) | BC | I-III | S | DFS/OS | Reported | 7 |
| Zhou 2004 | China | IHC | ≥25% | 125 | 47.8 | 48(23–78) | BC | I-III | S | OS | Reported | 7 |
Note: NR, not reported; OS, overall survival; DFS, disease free survival; S, surgery; C, chemotherapy; NOS, Newcastle-Ottawa Quality Assessment Scale. Age was presented as median (range); IHC, immunohistochemistry; ELISA, enzyme linked immunosorbent assay; PCR, polymerase chain reaction; BC, breast cancer.
Prognostic significance of high VEGF-C expression in BC patients
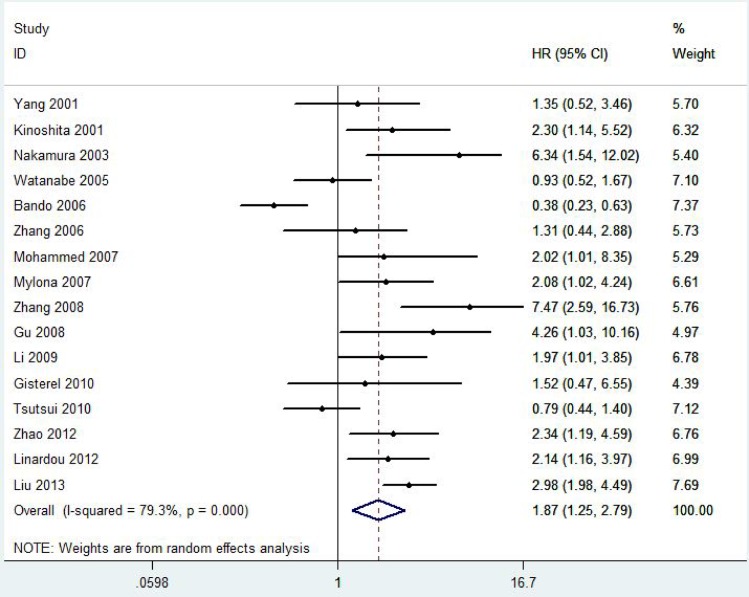
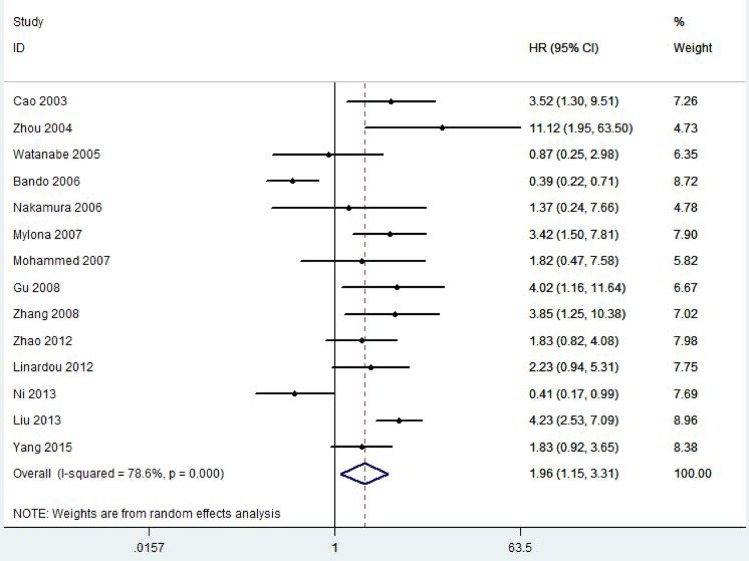
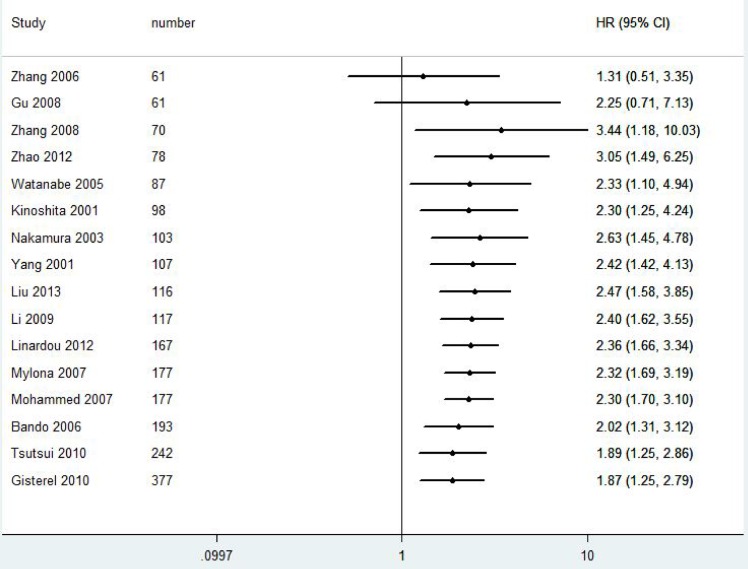
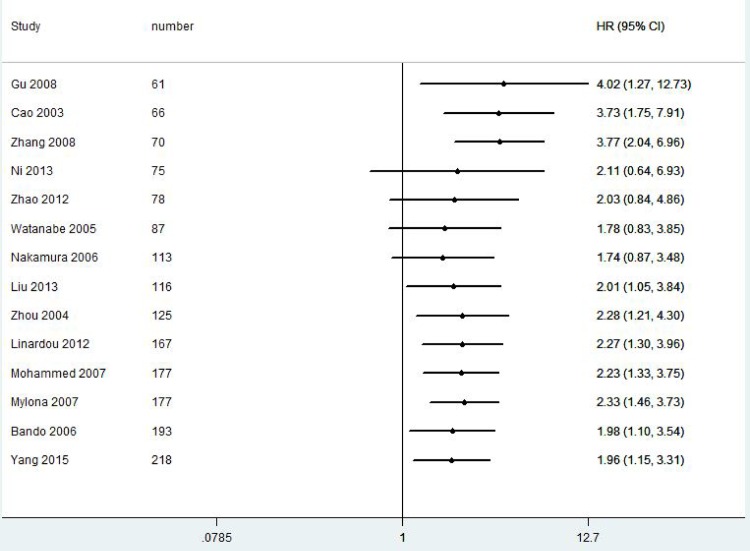
A total of 2828 patients with BC from 21 eligible studies were included and analyzed for prognostic significance of VEGF-C expression (Fig 2 and Fig 3). The combined HRs were 1.87(95% CI 1.25–2.79, P = 0.001) for DFS and 1.96(95% CI 1.15–3.31, P = 0.001) for OS in BC patients.
Fig 2. Forest plot diagrams of hazard ratios for correlations between VEGF-C expression and DFS.
Fig 3. Forest plot diagrams of hazard ratios for correlations between VEGF-C expression and OS.
Publication bias
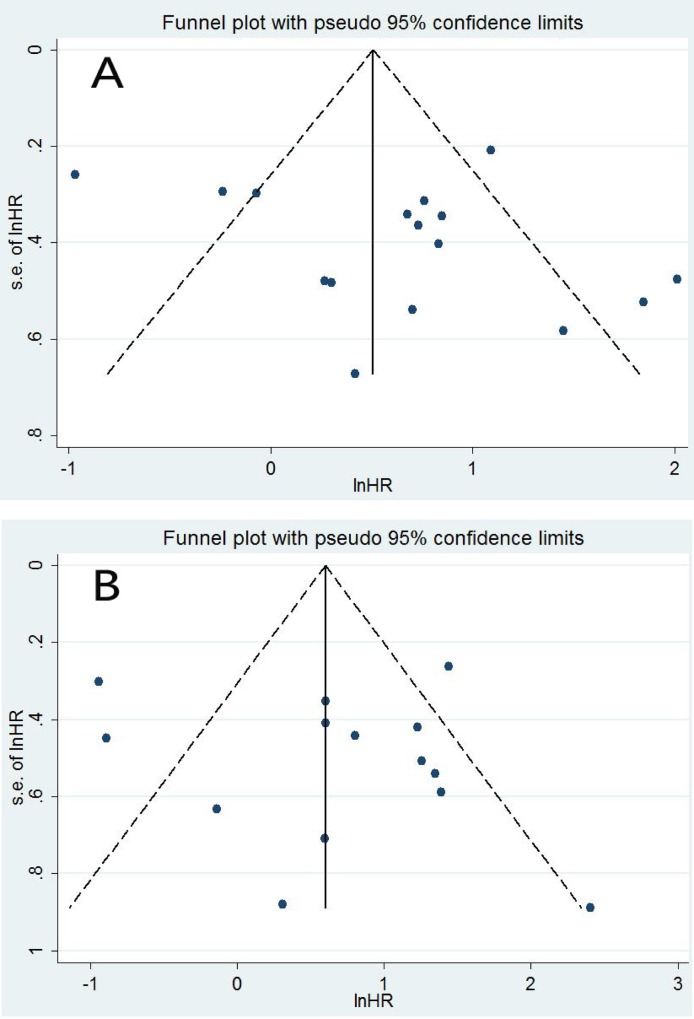
The funnel plot, Begg's test, and Egger's test were further performed to assess the publication bias. The funnel plot for publication bias was symmetrical for both DFS and OS (Fig 4). There was not significant publication bias according to Egger's test (P = 0.275) and Begg's test (P = 0.392) in the present study for DFS. Similarly, the publication bias was not significant for OS according to Egger's test (P = 0.646) and Begg's test (P = 0.913).
Fig 4.
Funnel plot for all eligible studies which provided HRs of high VEGF-C expression for DFS (A) and OS (B).
Subgroup analyses and meta-regression analyses
Subgroup analyses and meta-regression analyses were further performed to explore the sources of heterogeneity. Subgroup analyses (Table 2) demonstrated that regions and treatments might contribute to the clinical heterogeneity. Further meta-regression analysis suggested that treatments might be a potential source of heterogeneity for DFS (P = 0.045) and for OS (P = 0.017).
Table 2. Subgroup analyses for association between VEGF-C expression and survival in BC patients.
| Group factors | Subgroup | Study | HR | LCI | HCI | P value | I2 | P value |
|---|---|---|---|---|---|---|---|---|
| DFS | ||||||||
| Patients number≥100 | Yes | 10 | 1.65 | 0.97 | 2.83 | 0.067 | 83.3 | 0.001 |
| No | 6 | 2.3 | 1.25 | 4.24 | 0.007 | 70.5 | 0.005 | |
| Regions | Asian | 12 | 1.86 | 1.12 | 3.1 | 0.017 | 84.6 | 0.001 |
| Non- Asian | 4 | 2.04 | 1.36 | 3.05 | 0.001 | 0 | 0.975 | |
| IHC method | Yes | 12 | 2.2 | 1.51 | 3.21 | 0.001 | 68.7 | 0.001 |
| No | 4 | 1.09 | 0.41 | 2.9 | 0.869 | 84.8 | 0.001 | |
| Treatments | S | 6 | 2.97 | 1.87 | 4.71 | 0.001 | 51.1 | 0.069 |
| S+C | 10 | 1.41 | 0.88 | 2.27 | 0.151 | 76.9 | 0.001 | |
| Positive rate≥50% | Yes | 9 | 1.64 | 0.87 | 3.1 | 0.126 | 85.4 | 0.001 |
| No | 7 | 2.18 | 1.41 | 3.37 | 0.001 | 58.8 | 0.024 | |
| OS | ||||||||
| Patients number≥100 | Yes | 8 | 2.11 | 0.99 | 4.5 | 0.053 | 83.7 | 0.001 |
| No | 6 | 1.78 | 0.83 | 3.85 | 0.140 | 71.7 | 0.003 | |
| Regions | Asian | 11 | 1.85 | 0.96 | 3.55 | 0.064 | 82.7 | 0.001 |
| Non- Asian | 3 | 2.61 | 1.51 | 4.52 | 0.001 | 0 | 0.670 | |
| IHC method | Yes | 12 | 2.28 | 1.43 | 3.62 | 0.001 | 63.4 | 0.002 |
| No | 2 | 0.91 | 0.16 | 4.99 | 0.910 | 90.6 | 0.001 | |
| Treatments | S | 7 | 3.3 | 2.37 | 4.6 | 0.001 | 6 | 0.382 |
| S+C | 7 | 1.12 | 0.54 | 2.32 | 0.756 | 75.6 | 0.001 | |
| Positive rate≥50% | Yes | 7 | 1.55 | 0.64 | 3.75 | 0.327 | 87.9 | 0.001 |
| No | 7 | 2.46 | 1.58 | 3.83 | 0.001 | 21.4 | 0.266 |
HR, hazard ratio; CI, confidence interval; LCI, lower value of confidence interval; HCI, higher value of confidence interval; S, surgery; C, chemotherapy; OS, overall survival; DFS, disease free survival.
For DFS, the pooled HR of non-Asian subgroup was 2.04(95%CI 1.36–3.05, P = 0.001), which was higher than that of Asian subgroup. Similarly, the pooled HR for non-Asian subgroup for OS was 2.61(95%CI 1.51–4.52, P = 0.001), which was significantly higher than 1.85(95%CI 0.96–3.55, P = 0.064) for Asian subgroup, suggesting that high VEGF-C expression might be more closely associated with poor survival for BC patients in non-Asian countries.
The pooled HRs of IHC subgroup were 2.20(95%CI 1.51–3.21, P< 0.001) and 2.28(95%CI 1.43–3.62, P< 0.001) for DFS and OS respectively, which were significantly higher than that of non-IHC subgroup.
Sensitivity analyses
All studies were sequentially removed to assess that whether or not any individual study had a significant influence to the pooled HRs. The pooled HRs of sensitivity analyses varied from 1.71(95%CI: 1.15–2.53) to 2.08 (95%CI: 1.52–2.85) for DFS, demonstrating that the pooled HRs were not significantly influenced by any individual study for DFS(Table 3). Similarly, the pooled HRs for OS ranged from 1.86(95%CI: 1.07–3.23) to 2.27 (95%CI: 1.45–2.51), suggesting that the results of the present meta analysis was stable and reliable.
Table 3. Effect of individual studies on the pooled HRs of VEGF-C expression for DFS and OS.
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| Study omitted | HR | LCI | HCI | HR | LCI | HCI |
| 1 | 1.9083867 | 1.2520173 | 2.90886 | 1.9550176 | 1.1543682 | 3.3109832 |
| 2 | 1.8456045 | 1.2074366 | 2.82106 | 1.9550176 | 1.1543682 | 3.3109832 |
| 3 | 1.7387738 | 1.164133 | 2.59707 | 1.8697225 | 1.0708288 | 3.2646322 |
| 4 | 1.9721094 | 1.2912425 | 3.01199 | 1.9550176 | 1.1543682 | 3.3109832 |
| 5 | 2.0827684 | 1.5197888 | 2.85429 | 1.7930649 | 1.0554458 | 3.0461838 |
| 6 | 1.9120828 | 1.2544384 | 2.9145 | 2.0678508 | 1.1913106 | 3.58933 |
| 7 | 1.8624746 | 1.2243328 | 2.83323 | 1.9550176 | 1.1543682 | 3.3109832 |
| 8 | 1.8585508 | 1.2116612 | 2.85081 | 2.272918 | 1.489395 | 3.4686273 |
| 9 | 1.7095664 | 1.1548227 | 2.53079 | 1.9931914 | 1.151803 | 3.4492115 |
| 10 | 1.7887729 | 1.1859893 | 2.69792 | 1.8671988 | 1.0640525 | 3.2765595 |
| 11 | 1.8663094 | 1.213935 | 2.86927 | 1.9668693 | 1.1298384 | 3.4240075 |
| 12 | 1.8880523 | 1.2454731 | 2.86216 | 1.8583227 | 1.0703709 | 3.226324 |
| 13 | 1.9948787 | 1.317013 | 3.02164 | 1.859373 | 1.0684728 | 3.2357099 |
| 14 | 1.8423521 | 1.2013937 | 2.82527 | 1.9550176 | 1.1543682 | 3.3109832 |
| 15 | 1.8550151 | 1.2047843 | 2.85618 | |||
| 16 | 1.7989184 | 1.1788964 | 2.74503 | |||
| Combined | 1.8677404 | 1.2509044 | 2.78875 | 1.9550176 | 1.1543682 | 3.3109832 |
HR, hazard ratio; CI, confidence interval; LCI, lower value of 95% CI; HCI, high value of 95% CI; OS, overall survival; DFS, disease free survival.
Stability assessment of the pooled HRs of VEGF-C expression for survival by cumulative meta-analyses
We further performed cumulative meta-analyses to determine the stability of VEGF-C expression for survival in BC patients (Fig 5 and Fig 6).With inclusions of studies that patient number more than 70, the pooled HRs for DFS ranged from 1.89 to 3.44. The pooled HRs for OS varied from 1.98 to 2.33 with inclusions of studies that patient number more than 116, indicating that the prognostic significance of VEGF-C expression for survival in BC patients was stable.
Fig 5. Cumulative meta-analyses for stability of the pooled HRs of VEGF-C expression for DFS in BC patients.
Fig 6. Cumulative meta-analyses for stability of the pooled HRs of VEGF-C expression for OS in BC patients.
Discussion
The present meta analysis demonstrated that high VEGF-C expression is significantly associated with poor survival in BC patients. The combined HRs were 1.87 for DFS and 1.96 for OS in BC patients. The pooled HRs of non-Asian subgroup were 2.04 for DFS and 2.61 for OS, which were significantly higher that of Asian subgroup. Further sensitivity analyses demonstrated that the relation between high VEGF-C expression and poor prognosis of BC patients did not change after removing any individual study. Furthermore, cumulative meta-analyses also demonstrated that the predictive value of VEGF-C expression for prognosis of BC patients was stable and reliable.
There were two opposite opinions in four previous meta analyses [26–29]. In 2012, Wang J et al. reported that VEGF-C expression could predict poor prognosis in BC patients, with a pooled HR of 2.164 (95% CI 1.256–3.729) for DFS and a pooled HR of 2.613 (95% CI 1.256–3.729) for OS [26]. In 2014, Liang B et al. reported that VEGF-C expression was significantly associated with poor OS (OR = 2.46, 95% CI 1.46–4.14) and DFS (OR = 2.10, 95% CI 1.32–3.35) in BC patients [27]. By contrast, Gao S et al. reported that high VEGF-C expression was not associated with poor DFS (HR = 0.80, 95% CI 0.51–1.51) or OS (HR = 1.08, 95% CI 0.37–1.78) in BC patients in 2014[28]. In 2016, Wang F et al. also reported that there was no significant association between high VEGF-C expression and OS (HR = 0.76, 95% CI 0.43–1.33) in BC patients [29]. The contradictory conclusions in different meta analyses leaded to great confusion on whether or not high VEGF-C expression was associated with prognosis in BC patients. Remarkably, according to Cochrane handbook for meta analysis, odds ratio is not suitable for survival analysis with time-to-event data in consideration of censored data and time to study endpoint. Meanwhile, one original study [35] included in the meta analysis performed by Wang J et al. did not extract the right survival data.
The conclusions of the current meta analysis was generally similar to that of two previous meta analyses performed by Wang J et al. and Liang B et al.[26–27]. Compared with four previous meta analyses above, the present meta analysis had six strengths which provided powerful support to the conclusions in the present meta analysis. Firstly, the present meta analysis totally included 21 eligible studies and 2828 BC patients. The numbers of studies and patients were significantly more than that of the previous meta analyses and could significantly increase persuasiveness of the conclusions. Secondly, sensitivity analyses demonstrated that the pooled HRs were not significantly affected by any individual study. Thirdly, cumulative meta-analyses provided reliable evidences to support the final conclusions. Fourthly, subgroup analysis further demonstrated that the pooled HRs of non-Asian subgroup were significantly higher than that of Asian subgroup for both DFS and OS, suggesting that high VEGF-C expression might be more closely associated with poor survival of BC patients in non-Asian countries. Fifthly, considering that detection method might be a source of clinical heterogeneity and affect the pooled HR, we conducted a comparison between IHC subgroup and non-IHC subgroup. The comparison results demonstrated that the pooled HRs of IHC subgroup were significantly higher than that of non-IHC subgroup for both DFS and OS, suggesting that prognostic significance of VEGF-C expression for prognosis of BC patients was stable and reliable by using IHC method. Sixthly, studies published in Chinese were also included in the present meta analysis as English studies, increasing the representation of the included studies. These six strengths significantly enhanced persuasive power of the conclusions in the present study. In addition, some meta analyses have explored the clinical value of VEGF-C expression as a predictive tool for prognosis in different tumors, such as colorectal cancer and non-small cell lung cancer [36–37].
The heterogeneity was significant in the present meta analysis. The heterogeneity in the present meta analysis might be caused by the following reasons. First, both subgroup analyses and meta regression analyses showed that treatments might be a potential source of clinical heterogeneity. Second, there was no significant heterogeneity in non-Asian subgroup by subgroup analyses, indicating that regions might be a potential source of heterogeneity. Third, the true heterogeneity might be caused by differences in the intensity of interventions or differences in underlying risk between studies with different sizes.
Publication bias is important for interpreting the conclusions. The funnel plot for publication bias was symmetrical in the present meta analysis. The further Egger's test and Begg's test did not detect significant publication bias (all P>0.05), suggesting that the results might not be influenced by the publication bias.
The conclusions of the present meta analysis should be interpreted cautiously for the following reasons: First, the positive status of VEGF-C expression was defined according to different cut-off values in various studies, which might result in clinical heterogeneity. Second, different baseline characteristics, such as tumor stages and races, might result in clinical heterogeneity and reduce the persuasiveness of the conclusions in the current meta analysis.
In conclusion, the present meta analysis strongly supported the prognostic role of VEGF-C expression for DFS and OS in BC patients. Furthermore, stratification by VEGF-C expression may help to optimize the treatments and the integrated managements for BC patients.
Supporting Information
(DTA)
(DTA)
(DOC)
(DOCX)
Acknowledgments
This study was funded in part by Guangdong Provincial Health Department. The Grant Numbers were No: A2013695(Grant Recipient: peng wang) and No: A2016450(Grant Recipient: zhiqiao zhang).The total funding account was RMB 15000.The funding was given by Finance Department of Guangdong Province. The received authors of the funding was Peng Wang (No: A2013695) and Zhiqiao Zhang(A2016450). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded in part by Guangdong Provincial Health Department. The Grant Numbers were No: A2013695 (Grant Recipient: Peng Wang) and No: A2016450 (Grant Recipient: Zhiqiao Zhang). The total funding account was RMB 15000. The funding was given by Finance Department of Guangdong Province. The received authors of the funding was Peng Wang (No: A2013695) and Zhiqiao Zhang (A2016450). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D,et al. Global cancer statistics.CA Cancer J Clin 2011V61N2:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Hayes D.F. Prognostic and predictive factors revisited. Breast 2005V14N6: 493–499. [DOI] [PubMed] [Google Scholar]
- 3.Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer 2008V8N: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 2010V17N4: 229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang GH, Zeng Y, Yang WT, Shi DR. Study of mRNA expression of vascular endothelial growth factor-(A, C, D) genes and its effect on prognosis of breast cancer. Zhonghua Bing Li Xue Za Zhi 2006V35N8: 473–477(Chinese). [PubMed] [Google Scholar]
- 6.Linardou H, Kalogeras KT, Kronenwett R, Kouvatseas G, Wirtz RM, Zagouri F, et al. The prognostic and predictive value of mRNA expression of vascular endothelial growth factor family members in breast cancer: a study in primary tumors of high-risk early breast cancer patients participating in a randomized Hellenic Cooperative Oncology Group trial. Breast Cancer Res 2012V14N6: R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe O, Kinoshita J, Shimizu T, Imamura H, Hirano A, Okabe T,et al. Expression of a CD44 variant and VEGF-C and the implications for lymphatic metastasis and long-term prognosis of human breast cancer.J Exp Clin Cancer Res 2005V24N1:75–82. [PubMed] [Google Scholar]
- 8.Yang W, Klos K, Yang Y, Smith TL, Shi D, Yu D, et al. ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C, and D in human breast carcinoma. Cancer 2002V94N11:2855–2861. [DOI] [PubMed] [Google Scholar]
- 9.Zhao YC, Ni XJ, Li Y, Dai M, Yuan ZX, Zhu YY,et al. Peritumoral lymphangiogenesis induced by vascular endothelial growth factor C and D promotes lymph node metastasis in breast cancer patients. World J Surg Oncol 2012V10N:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y D, Li YY, Qi RH. The expression of VEGF-C and Fhit in breast cancer and its association with lymph node metastasis. Journal of Practical Oncology 2003,18;206–208(Chinese). [Google Scholar]
- 11.Li P, Teng YE, PU X, Liu YP, Qu XJ, Hou KZ. Expression of COX-2 and VEGF-C in breast cancer and its significance for prognosis. Chin J Clin Oncol 2009,4;207–210(Chinese). [Google Scholar]
- 12.Liu XL, Huang Y, Chen P. Expression of hMAN, VEGF-C and VEGFR-3 in breast cancer tissues and its relation with prognosis. Acad J Sec Mil Med Uni 2013,10; 1078–1082 (Chinese). [Google Scholar]
- 13.Bando H, Weich HA, Horiguchi S, Funata N, Ogawa T, Toi M. The association between vascular endothelial growth factor-C, its corresponding receptor, VEGFR-3, and prognosis in primary breast cancer: a study with 193 cases.Oncol Rep 2006V15N3: 653–659. [PubMed] [Google Scholar]
- 14.Gisterek I, Matkowski R, Lacko A, Sedlaczek P, Szewczyk K, Biecek P,et al. Serum vascular endothelial growth factors a, C and d in human breast tumors.Pathol Oncol Res 2010V16N3:337–344. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG,et a.Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis.Br J Cancer 2007V96N7:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsutsui S, Matsuyama A, Yamamoto M, Takeuchi H, Oshiro Y, Ishida T,et al. The Akt expression correlates with the VEGF-A and -C expression as well as the microvessel and lymphatic vessel density in breast cancer. Oncol Rep 2010V23N3: 621–630. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Qi X, Guo S.Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: a retrospective study of 61 cases. Clin Exp Metastasis 2008V25N7:717–725. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita J, Kitamura K, Kabashima A, Saeki H, Tanaka S, Sugimachi K,et al. Clinical significance of vascular endothelial growth factor-C (VEGF-C) in breast cancer. Breast Cancer Res Treat 2001V66N2: 159–164. [DOI] [PubMed] [Google Scholar]
- 19.Mylona E, Alexandrou P, Mpakali A, Giannopoulou I, Liapis G, Markaki S, et al. Clinicopathological and prognostic significance of vascular endothelial growth factors (VEGF)- C and -D and VEGF receptor 3 in invasive breast carcinoma.Eur J Surg Oncol 2007V33N3: 294–300. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura Y, Yasuoka H, Tsujimoto M, Yoshidome K, Nakahara M, Nakao K,et al. itric oxide in breast cancer: induction of vascular endothelial growth factor-C and correlation with metastasis and poor prognosis.Clin Cancer Res 2006V12N4: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Tsukiyama A, Imabun S,et al. Clinicopathological significance of vascular endothelial growth factor-C in breast carcinoma with long-term follow-up.Mod Pathol 2003V16N4: 309–314. [DOI] [PubMed] [Google Scholar]
- 22.Ni X, Zhao Y, Ma J, Xia T, Liu X, Ding Q, et al. Hypoxia-induced factor-1 alpha upregulates vascular endothelial growth factor C to promote lymphangiogenesis and angiogenesis in breast cancer patients. J Biomed Res 2013V27N6: 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Liu F, Fan Y, Qian X, Lang R, Gu F, et al. Both high expression of pyruvate kinase M2 and vascular endothelial growth factor-C predicts poorer prognosis in human breast cancer.nt J Clin Exp Pathol 2015V8N7: 8028–8037. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XH, Huang DP, Guo GL, Chen GR, Zhang HX, Wan L,et al. Coexpression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC Cancer 2008V8N:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L,Wang XL,Wang YJ. Expression of CX43,CX26 in breast carcinoma and their prognosis significance. Carcinogenesis, Teratogenesis & Mutagenesis 2004,17;291–294. (Chinese). [Google Scholar]
- 26.Wang J, Guo Y, Wang B, Bi J, Li K, Liang X,et al. Lymphatic microvessel density and vascular endothelial growth factor-C and -D as prognostic factors in breast cancer: a systematic review and meta-analysis of the literature. ol Biol Rep 2012V39N12:11153–11165. [DOI] [PubMed] [Google Scholar]
- 27.Liang B, Li Y. Prognostic Significance of VEGF-C Expression in Patients with Breast Cancer: A Meta-Analysis.Iran J Public Health 2014V43N2:128–135. [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S, Ma JJ, Lu C. Prognostic significance of VEGF-C immunohistochemical expression in breast cancer: a meta-analysis. Tumour Biol 2014V35N2: 1523–1529. [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Li S, Zhao Y, Yang K, Chen M, Niu H,et al. Predictive role of the overexpression for CXCR4, C- Met, and VEGF-C among breast cancer patients: A meta-analysis.Breast 2016V28N:45–53. [DOI] [PubMed] [Google Scholar]
- 30.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D,et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000V283N15: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions.Version 5.1.0. The Cochrane Collaboration. 2011. Retrieved from: http://handbook.cochrane.org/.
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986V 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994V50:1088–1101. [PubMed] [Google Scholar]
- 34.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997V315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tezuka K. Onoda N, Takashima T, Ishikawa T, Wakasa T, Wakasa K,et al. Clinical significance of intra-tumoral sinusoidal structures showing lympho- endothelial immunoreactivity in breast cancer.Oncol Rep 2008V20N1:25–32. [PubMed] [Google Scholar]
- 36.Zong S, Li H, Shi Q, Liu S, Li W, Hou F,et al. Prognostic significance of VEGF-C immunohistochemical expression in colorectal cancer: A meta-analysis.Clin Chim Acta 2016V458N: 106–114. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Shao W, Zhao W.VEGF-C in non-small cell lung cancer: meta-analysis.Clin Chim Acta 2014V427N: 94–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DTA)
(DTA)
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.