Abstract
Objective
To assess maternal and fetal outcomes associated with subclinical (pre‐systemic lupus erythematosus [SLE] and SLE presenting up to 5 years postpartum) and prevalent maternal SLE during pregnancy compared with the general population.
Methods
This prospective cohort study used population‐based Swedish registers to identify 13,598 women with first singleton pregnancies registered in the Medical Birth Register (551 prevalent SLE, 65 pre‐SLE within 0–2 years, 133 pre‐SLE within 2–5 years, and 12,847 general population). SLE was defined as ≥2 SLE‐coded discharge diagnoses in the patient register with ≥1 diagnosis from a specialist. Unadjusted risks of adverse pregnancy or birth outcomes were calculated by SLE status, and Cochran‐Armitage tests evaluated trend across exposure groups.
Results
Maternal outcomes such as preeclampsia, hypothyroidism, stroke, and infection were more common among women with SLE. Sixteen percent of prevalent‐SLE pregnancies were diagnosed with preeclampsia compared with 5% of those from the general population. Among the pre‐SLE women, preeclampsia was found in 26% of those with SLE within 2 years postpartum and 13% in those with SLE within 2–5 years postpartum. Similarly, infant outcomes, such as preterm birth, infection, and mortality, were worse among those born to mothers with prevalent SLE and pre‐SLE during pregnancy. The test for trend was significant for most outcomes.
Conclusion
Our data demonstrate that adverse maternal and fetal outcomes are more common in SLE pregnancies. Furthermore, these unfavorable outcomes are observed in pregnancies occurring prior to the diagnosis of SLE. Thus, the underlying immunologic profile of SLE and alterations preceding clinical SLE may contribute to these pregnancy complications.
INTRODUCTION
Adverse pregnancy outcomes have been reported in mothers with systemic lupus erythematosus (SLE) and their offspring 1, 2, 3. These include preeclampsia, preterm birth, stroke, and even death. Many of these adverse events impact the life expectancy and overall health of both mother and infant. The clinical phenotype and features of SLE among pregnant patients have changed. Historically, if there were previous renal involvement, even if it were quiescent, patients were counseled to avoid pregnancy. Today, although patients are counseled to conceive while the disease is inactive, complicated SLE is managed and pregnancy strategies are discussed for a myriad of presentations 3. A better understanding of the occurrence of adverse pregnancy outcomes in SLE is essential for informing patients of potential risks.
Box 1. Significance & Innovations.
This study observes a higher proportion of adverse maternal and fetal outcomes in the years preceding systemic lupus erythematosus (SLE) diagnosis, which may be due to immunologic activity in the subclinical pre‐SLE time period.
Our population‐based approach addressed whether the commonly reported adverse pregnancy events in SLE are specific to SLE or relatively common outcomes in the general population.
Despite numerous reports, we still know little about the mechanisms behind many of these outcomes. Underlying disease activity, inflammation, and other immunologic features may explain some of the pregnancy risk in SLE. Whether systemic immune alterations pre‐dating disease have an effect on pregnancy is not known. Autoantibodies associated with SLE, including antiphospholipid antibodies and SSA, both of which are known to be associated with pregnancy morbidity, are present up to 5 years before the clinical diagnosis of SLE 4, 5, 6. Furthermore, as the clinical diagnosis nears, the levels of many of these autoantibodies increase 4. These autoantibodies may directly impact the maternal immune system during pregnancy or may be manifestations of immune dysregulation, potentially even in a pre‐SLE state.
Using national population‐based Swedish registers, we examined outcomes among women first presenting with SLE up to 5 years postpartum, as well as the more conventional comparison of prevalent SLE to the general population. These women with pre‐SLE represent a group that may have immune dysregulation, autoimmunity, and increases in systemic inflammation during pregnancy. Not only will this knowledge inform and advance the understanding of clinical drivers of adverse maternal and fetal outcomes, but it may also inform clinical care, prenatal counseling, and decision‐making.
SUBJECTS AND METHODS
Study population
The study population was derived from the Swedish Lupus Linkage (SLINK), a large matched cohort using Swedish register data that has been described elsewhere in detail 7. Briefly, population‐based data yielded a large cohort of individuals with SLE and a matched random sample from the general population. In the present study we included women from the SLINK cohort also identified in the Medical Birth Register (MBR). Details on all births in Sweden (1973–2012), including maternal health, cigarette smoking, labor and delivery characteristics, and fetal outcomes, are included in the MBR from the first prenatal visit through birth/delivery. Only stillbirths of at least 28 gestational weeks were included in the register until 2008, at which time the MBR expanded to include those with a gestation of at least 22 weeks. We excluded multiple gestations and restricted to first pregnancy, because outcomes are known to be different in multiples, and later pregnancies may be influenced by outcomes of first pregnancies. We did not include previous spontaneous abortions as pregnancies, unless the losses were late enough to be captured as stillbirths. In that event, if that was the first ever birth for the mother, we included it. We restricted our data set to first pregnancies from 1987 onward in order to use International Classification of Diseases, Ninth Revision (ICD‐9) codes and ICD‐10 codes only and because national coverage in the patient register began that year.
SLE status
SLE was defined as at least 2 SLE ICD‐coded visits (inpatient, outpatient nonprimary care, or both) in the National Patient Register, with at least 1 SLE visit coded by a physician from a specialty that typically diagnoses or manages SLE (rheumatology, dermatology, internal medicine, pediatrics, and nephrology). The first observed SLE interaction with the health care system was used as a proxy for diagnosis. We categorized SLE as prevalent at delivery, incident within 0–2 years after delivery, and incident 2–5 years after delivery, assuming that this would serve as a proxy for immunologic burden during pregnancy. The general population comparator in the original matched cohort provided the women and pregnancies assumed to be unaffected by SLE. The study population for the primary analysis included a total of 13,598 women with their first singleton pregnancies registered in the MBR.
Outcomes
Maternal and fetal outcomes were identified in the MBR, the patient register, and the causes of death register. Pregnancy comorbidities included preeclampsia, gestational diabetes, hypothyroidism, and hypertension. Severe infection, also considered during pregnancy, was defined as hospitalization with an infection as the primary or as a contributory diagnosis. One‐year postpartum incidence of pulmonary embolism, deep vein thrombosis (DVT), and severe infections were identified, as well as any postpartum hemorrhage and stroke. (For a list of ICD codes, see Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22791/abstract). One‐year and 5‐year postpartum maternal mortality was defined using the causes of death register for the general population and prevalent‐SLE groups.
Delivery characteristics from the MBR included cesarean section (C‐section) and gestational duration in weeks, using the new American College of Obstetrics and Gynecology classification (very preterm <32 weeks, preterm 32 to <37 weeks, early term 37 to <39 weeks, term 39 to <41 weeks, full term 41 to <42 weeks, and post‐term ≥42 weeks) 8. Fetal outcomes included traditional perinatal characteristics, such as size for gestational age, birth weight, and 1‐minute Apgar scores. Congenital malformations were identified using the MBR and patient register, considered cumulatively and separately by chromosomal abnormalities versus other, power permitting.
Offspring infections were identified in the MBR and patient register (inpatient and outpatient visits) during the first year of life and for all available followup time until age 5 years. Infant death was identified using MBR and the causes of death register; power was not sufficient to consider different neonatal mortality periods or fetal death.
Additional covariates
Body mass index (BMI), maternal age, maternal country of birth, and self‐reported cigarette smoking at the first prenatal clinic visit were obtained from the MBR. BMI was calculated using self‐reported maternal height and measured weight, usually in the first trimester. Maternal prepregnancy comorbid conditions were identified using both the MBR and the patient register. Antiphospholipid syndrome (APS) was broadly defined as the presence of at least 1 of the following APS‐related diagnoses or features: ICD‐10 code D68.8 for APS, ICD‐9 289W or 298X, heparin prescription during pregnancy (ATC B01AB from June 2005 onward), and a history of any of the following conditions prior to pregnancy: DVT, pulmonary embolism, or stroke. If no mention of any comorbidity or complication was made in the patient register or MBR, we assumed that it was not present.
Statistical analysis
Study population characteristics were summarized by SLE status during pregnancy using frequencies and means with SDs for noncontinuous and continuous covariates, respectively. We compared the frequency of maternal and fetal outcomes by SLE exposure at pregnancy/birth (prevalent, in 0–2 years, in 2–5 years, and none). The Cochran‐Armitage test for trend was used to determine whether there was a significant trend across SLE status/exposure groups. For maternal mortality outcomes, we only compared prevalent SLE to the general population, because to be included in the 0–2 years pre‐SLE or 2–5 years pre‐SLE groups, the mothers had to survive in order to receive an SLE diagnosis within 5 years of delivery.
To investigate the degree of possible selection bias due to our study design (i.e., requiring survival 2–5 years after pregnancy to enter the pre‐SLE group), we required, in a sensitivity analysis, that all women be living 5 years after their pregnancy. This additional restriction reduced the sample slightly (548 women prevalent SLE, 65 women 0–2 years to SLE, 133 women 2–5 years to SLE, and 12,846 women general population/no SLE). This work was approved by the Institutional Review Board at Karolinska Institute and received a Notice of Determination of Non‐Human Subjects Research from Stanford University.
RESULTS
In this large population‐based cohort of women with SLE in Sweden, we observed 551 first singleton births between 1987 and 2012 to women receiving care for SLE and evaluated their maternal and fetal outcomes in relation to the general population (12,847 pregnancies). One hundred ninety‐eight pre‐SLE women, first identified with SLE 0–2 years (n = 65) and 2–5 years (n = 133) later, had first singleton pregnancies during this same time period. Maternal age at first birth was similar among the 4 groups of women, with prevalent‐SLE mothers being slightly older (29 versus 27 years) during pregnancy. Women who had SLE during pregnancy or were close to receiving the diagnosis (0–2 years postpartum register identification) were less likely to report first‐trimester cigarette smoking (Table 1). Using a constellation of features as a proxy for APS, 70 women from the general population (0.5%) fit the description, 77 (14%) with prevalent SLE, 3 (4.6%) with SLE within 2 years, and 1 (0.8%) with SLE within 2 to 5 years.
Table 1.
Maternal characteristics during pregnancy by SLE exposurea
| SLE exposure during pregnancy | |||||
|---|---|---|---|---|---|
| Variable | Prevalent (n = 551) | Within 2 years (n = 65) | Within 2–5 years (n = 133) | No SLE (n = 12,847) | Pb |
| Maternal age at delivery, mean ± SD years | 29.3 ± 4.6 | 27.7 ± 4.6 | 27.3 ± 4.6 | 27.7 ± 5.0 | < 0.0001 |
| Maternal smoking history | |||||
| First trimester smoking | 8.7 | 1.5 | 14.3 | 15.1 | 0.0001 |
| No smoking first trimester | 84.4 | 83.1 | 79.7 | 79.2 | |
| Smoking info missing | 6.9 | 15.4 | 5.7 | 5.7 | |
| Maternal country of birth | |||||
| Sweden | 89.7 | 72.3 | 79.0 | 87.8 | 0.1 |
| Other, Nordic | 1.1 | 1.5 | 1.5 | 2.0 | |
| Other, non‐Nordic | 9.3 | 26.2 | 18.1 | 9.6 | |
| Unknown/missing | 0 | 0 | 1.5 | 0.7 | |
| Time period | |||||
| 1987–1993 | 16.7 | 0 | 3.0 | 37.0 | < 0.0001 |
| 1994–2000 | 18.2 | 24.6 | 41.4 | 25.4 | |
| 2001–2006 | 26.0 | 40.0 | 29.3 | 21.0 | |
| 2007–2012 | 39.2 | 35.4 | 26.3 | 16.7 | |
| Maternal BMI, mean ± SD kg/m2 c | 23.7 ± 3.8 | 25.4 ± 5.9 | 24.0 ± 4.4 | 23.8 ± 4.1 | 0.55 |
| Maternal BMI missing | 13.9 | 15.4 | 13.5 | 14.2 | 0.89 |
| Family situation | |||||
| Living with child's father | 86.9 | 89.2 | 88.0 | 86.8 | 0.37 |
| Not living with child's father | 5.6 | 0 | 5.3 | 6.8 | |
| Missing family situation | 7.4 | 10.8 | 6.8 | 6.4 | |
Values are percentages unless otherwise specified. SLE = systemic lupus erythematosus; BMI = body mass index.
Comparing prevalent SLE at delivery to no SLE. P from chi‐square test for categorical variables and t‐test for continuous variables.
BMI data available from 1992 onward.
SLE diagnosis or underlying autoimmunity as represented by pre‐SLE was associated with a higher risk of C‐section, preterm birth, preeclampsia, serious infection during pregnancy, hypothyroidism, postpartum stroke, and infection in the mother. For many maternal delivery and clinical outcomes, those with prevalent SLE and those women soon diagnosed with SLE after their first pregnancy had the highest likelihood of poor outcomes. Women with SLE first identified 2–5 years postpartum tended to be less at risk than these groups, but more at risk than the general population (Figure 1). For example, approximately 5% of women without SLE had preeclampsia, whereas the risk was 26% for women diagnosed with SLE at 0–2 years postpartum, 13% for women diagnosed at 2–5 years, and 16% for women with prevalent SLE. The pattern was similar for serious infections both during pregnancy and in the first year postpartum, although the risks were smaller. Data were too sparse to perform comparisons for a number of other outcomes, including DVT, pulmonary embolism, postpartum hemorrhage, pregestational diabetes, gestational hypertension, and hyperthyroidism.
Figure 1.
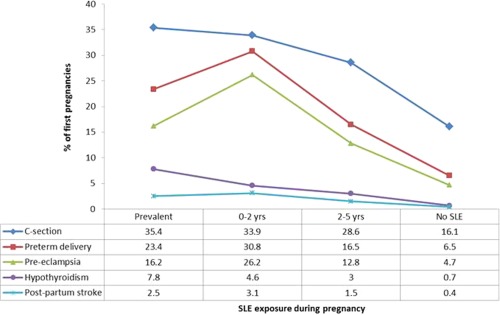
Maternal outcomes by systemic lupus erythematosus (SLE) exposure during pregnancy, presented as percentages. C‐section = cesarean section.
Fetal outcomes followed a similar pattern both for birth characteristics (nonaverage size for gestational age, low birth weight, low 1‐minute Apgar score) and for clinical outcomes such as infant mortality. Twenty‐one percent of infants born to mothers with SLE during pregnancy had an infection during their first year of life, compared with nearly 29% and 25% of infants with pre‐SLE mothers (0–2 and 2–5 years, respectively) and 14% of the general population of infants (Figure 2). This pattern was similar for infections in the first 5 years of life as well. Data were too sparse to compare groups for congenital malformations and infant mortality. Excluding women who died within 5 years of delivery (3 SLE and 1 general population), our results remained the same.
Figure 2.
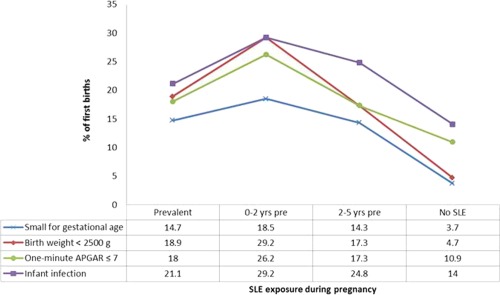
Birth and infant outcomes by maternal systemic lupus erythematosus (SLE) exposure during pregnancy, presented as percentages.
DISCUSSION
The present descriptive study compares the pregnancy and postpartum experiences of pre‐SLE and prevalent‐SLE mothers to mothers from the general population. Not only did we observe the previously reported higher risks of stroke, death, preeclampsia, and serious infection in SLE pregnancy, but we also observed significantly higher risks of adverse maternal, fetal, and infant outcomes in pregnancies prior to SLE diagnosis. These risks were elevated in women with prevalent SLE, who may have a substantial autoimmune and inflammatory load, and were also higher in women who present with SLE within 5 years postpartum. For most outcomes the association appeared to be roughly “dose‐dependent,” with increased proximity to SLE diagnosis (as a proxy for immunologic burden) correlating with higher risk. Because the present study is descriptive, we did not account for possible mediators or confounders.
Prepregnancy counseling encourages patients with prevalent SLE to conceive when their SLE is quiescent or under good control. Patients identified as having SLE within 2 years postpartum may have the highest average disease activity. We observed a higher risk of numerous adverse outcomes in pre‐SLE women, which is unlikely to be due to heightened surveillance. In Sweden the subclinical pre‐SLE pregnancies may lack the label of high‐risk pregnancy and are most likely cared for by midwives in ordinary maternal care, which is different from the special maternal care that patients known to have SLE usually receive. As a result, the outcomes in this pre‐SLE population may point to underlying mechanisms related to autoimmunity and inflammation prior to diagnosis. This group of women may represent unrecognized and untreated SLE, which may lead to more disease‐related inflammation and autoimmunity during pregnancy.
As with all observational research, this study is not without potential limitations. The purpose of this work was to describe outcomes related to pregnancy in women with SLE or pre‐SLE, and therefore our risks are unadjusted. Generalizability may be limited to populations similar to the Swedish population, which is predominantly white. Administrative and register data are imperfect data. Our recent classification work showed that the present register‐based definition of SLE has 98.3% sensitivity, 97.6% positive predictive value, and nearly 100% sensitivity and negative predictive value in women 9. An extensive followup validation study is currently under way. We may have misclassified people with pre‐SLE because of the protracted diagnostic period. These data do not identify the time of diagnosis but instead provide the first interaction (defined by SLE ICD code) with the health care system, represented by the patient register, and no validation work in these data has examined onset date and date of diagnosis. Furthermore, these data do not define subtypes of SLE or SLE phenotypes. The validity of other outcomes has been examined in numerous studies in the patient register and in the MBR 10, 11.
Because the patient register was inpatient only until 2001, we may have underestimated prevalent SLE in this early period and missed pre‐SLE within our prespecified postpartum time windows. However, because SLE status was defined based on the date of the first mention of SLE, including coding in the MBR, we assume that those with prevalent SLE during this time likely had SLE noted in the MBR. Furthermore, because this study covers a long time span, in which the management of pregnancy in SLE has changed, we wanted to examine a more recent time period. For these reasons we conducted an ad hoc analysis restricted to births from 2003 onward at a time when the outpatient register was available and found similar results.
We also cannot exclude the possibility that women with an adverse event during pregnancy or in the postpartum period might get diagnosed with SLE sooner than they would have otherwise, or that undiagnosed lupus nephritis might be misclassified as preeclampsia. Nor can we exclude the possibility that a woman with prevalent SLE managed in primary care exclusively before and during her pregnancy would present to her rheumatologist afterward due to a postpartum flare and be misclassified as pre‐SLE in our study. However the latter is unlikely, because a woman with SLE would likely receive some specialty maternal care and be seen at the rheumatology clinic, at least once during her pregnancy, even if her disease were largely inactive for many years.
Pregnancy has been investigated as a potential risk factor for SLE, although generally with an emphasis on parity, age at first birth, and pregnancy losses 12, 13, 14. Scandinavian registers have been used in other studies of SLE pregnancy. In the MBR of Norway, first and subsequent births to women with prevalent SLE requiring 1 ICD code were compared with outcomes in all other deliveries in the register among women with no inflammatory rheumatic disease during the study period 15. Using Swedish data, Soh et al focused on cardiovascular mortality as their outcome among women, also with a minimum of 1 ICD‐coded visit (in the patient register), and did not distinguish between prevalent and postpartum SLE 16. In 220 Gullah African American women with at least 1 pregnancy, there were 577 pregnancies (the majority of which were either pre‐SLE or the temporality was unknown). Although the authors had some details about pre‐SLE outcomes, they did not report these results stratified by time to SLE diagnosis, although they expressed an interest in determining whether there is a “predisease state that negatively affects pregnancy outcomes” 17. A retrospective case–control study in the Johns Hopkins Lupus Cohort assessed pregnancy loss and preterm births, comparing outcomes in women before their SLE diagnosis to outcomes among women after their SLE diagnosis and found more loss and preterm delivery among the prevalent‐SLE group. Data on time between pregnancy and SLE diagnosis were not reported, and comparisons with non‐SLE populations (friends and relatives) pooled all SLE pregnancies 18. Our study supports the idea that the underlying immunologic profile of subclinical SLE may be associated with a higher likelihood of adverse maternal and fetal outcomes. For example, autoantibodies (anti‐SSA/Ro) in patients with insufficient clinical symptoms to be classified as SLE have increased levels of interferon‐α, and interferon‐α increases maternal vascular susceptibility to preeclampsia 19, 20.
Unfavorable pregnancy outcomes were observed prior to the diagnosis of SLE and more likely to occur when compared to a large sample from the general population. The present study also confirms previous findings of maternal and fetal risks in SLE pregnancy, such as preterm birth and preeclampsia, while overcoming the limitations of autocorrelated data and limited statistical power, and we were able to also look at infant outcomes, as well as pre‐SLE pregnancies. We did not examine adjusted measures of association but instead our goal was to describe the pregnancy and postpartum experience in SLE and pre‐SLE in context with background risks from the general population. Future work will examine these outcomes in more detail, accounting not only for traditional confounders but for mediators that cannot be included in traditional regression models as covariates.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Simard had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Arkema, Simard.
Acquisition of data
Arkema, Simard.
Analysis and interpretation of data
Arkema, Palmsten, Sjöwall, Svenungsson, Salmon, Simard.
Supporting information
Supplementary Table 1. International classification of disease (ICD) codes used to define comorbidities
Dr. Arkema's work was supported by the Strategic Research Program in Epidemiology at Karolinska Institute. Dr. Sjöwall's work was supported by the County Council of Östergotland, the Swedish Society for Medical Research, the Swedish Rheumatism Association, the Swedish Society of Medicine, the Professor Nanna Svartz Foundation, and the King Gustaf V 80‐Year Foundation. Dr. Svenungsson's work was supported by the Swedish Heart‐Lung Foundation, the Swedish Research Council, the Swedish Rheumatism Association, and the King Gustaf V 80‐Year Foundation, and she has received funding from the Stockholm City Council and Karolinska Institute. Dr. Simard's work was supported by the Strategic Research Program in Epidemiology at Karolinska Institute and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant K01‐AR‐06687801).
REFERENCES
- 1. Clowse ME, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol 2008;199:127e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal N, Sawhney H, Vasishta K, Chopra S, Bambery P. Pregnancy in patients with systemic lupus erythematosus. Aust N Z J Obstet Gynecol 1999;39:28–30. [DOI] [PubMed] [Google Scholar]
- 3. Clowse ME. Managing contraception and pregnancy in the rheumatologic diseases. Best Pract Res Clin Rheumatol 2010;24:373–85. [DOI] [PubMed] [Google Scholar]
- 4. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]
- 5. Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapaa‐Dahlqvist S. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 2011;13:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borella E, Lojacono A, Gatto M, Andreoli L, Taglietti M, Iaccarino L, et al. Predictors of maternal and fetal complications in SLE patients: a prospective study. Immunol Res 2014;60:170–6. [DOI] [PubMed] [Google Scholar]
- 7. Arkema EV, Simard JF. Cohort profile: systemic lupus erythematosus in Sweden. The Swedish Lupus Linkage (SLINK) cohort. BMJ Open 2015;5:E008259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA 2013;309:2445–6. [DOI] [PubMed] [Google Scholar]
- 9. Arkema EV, Jonsen A, Ronnblom L, Svenungsson E, Sjowall C, Simard JF. Case definitions in Swedish register data to identify systemic lupus erythematosus. BMJ Open 2016;6:E007769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centre for Epidemiology . The Swedish Medical Birth Register: a summary of content and quality. 2003. URL: https://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/10655/2003-112-3_20031123.pdf.
- 12. Cooper GS, Dooley MA, Treadwell EL, StClair EW, Gilkeson GS. Hormonal and reproductive risk factors for development of systemic lupus erythematosus: results of a population‐based, case–control study. Arthritis Rheum 2002;46:1830–9. [DOI] [PubMed] [Google Scholar]
- 13. Ulff‐Moller CJ, Jorgensen KT, Pedersen BV, Nielsen NM, Frisch M. Reproductive factors and risk of systemic lupus erythematosus: nationwide cohort study in Denmark. J Rheumatol 2009;36:1903–9. [DOI] [PubMed] [Google Scholar]
- 14. Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum 2007;56:1251–62. [DOI] [PubMed] [Google Scholar]
- 15. Wallenius M, Salvesen KA, Daltveit AK, Skomsvoll JF. Systemic lupus erythematosus and outcomes in first and subsequent births based on data from a national birth registry. Arthritis Care Res (Hoboken) 2014;66:1718–24. [DOI] [PubMed] [Google Scholar]
- 16. Soh MC, Nelson‐Piercy C, Dib F, Westgren M, McCowan L, Pasupathy D. Association between pregnancy outcomes and death from cardiovascular causes in parous women with systemic lupus erythematosus: a study using Swedish population registries. Arthritis Rheumatol 2015;67:2376–82. [DOI] [PubMed] [Google Scholar]
- 17. Barnado A, Wheless L, Meyer AK, Gilkeson GS, Kamen DL. Pregnancy outcomes among African‐American patients with systemic lupus erythematosus compared with controls. Lupus Sci Med 2014;1:E000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petri M, Allbritton J. Fetal outcome of lupus pregnancy: a retrospective case‐control study of the Hopkins Lupus Cohort. J Rheumatol 1993;20:650–6. [PubMed] [Google Scholar]
- 19. Niewold TB, Rivera TL, Buyon JP, Crow MK. Serum type I interferon activity is dependent on maternal diagnosis in anti‐SSA/Ro–positive mothers of children with neonatal lupus. Arthritis Rheum 2008;58:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andrade D, Kim M, Blanco LP, Karumanchi SA, Koo GC, Redecha P, et al. Interferon‐α and angiogenic dysregulation in pregnant lupus patients who develop preeclampsia. Arthritis Rheumatol 2015;67:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. International classification of disease (ICD) codes used to define comorbidities


