Abstract
Summary
We created a 30-item Frailty Index in the Canadian Multicentre Osteoporosis Study. A Frailty Index is a sensitive measure that can quantify fracture risk according to degree of frailty. Our results indicated that at any age, frailty was an important independent risk factor for fracture over 10 years.
Introduction
In later life, frailty has been linked to fractures. It is likely that the antecedents of fracture are seen across the life course, in ways not entirely captured by traditional osteoporosis risk factors. Using data collected from the prospective, population-based Canadian Multicentre Osteoporosis Study (CaMos), we created the 30-item CaMos Frailty Index and examined whether it was associated with incident fractures over 10 years.
Methods
All CaMos participants aged 25 years and older (n= 9,423) were included in the analysis. To examine the relationship between baseline Frailty Index scores and incident fractures, a competing risk proportional sub-distribution hazards model was used with death considered a competing risk. Analyses were adjusted for age, sex, body mass index, education level, femoral neck T-score, and antiresorptive therapy.
Results
At baseline, the mean age was 62.1 years [standard deviation (SD) 13.4], and 69.4 % were women. The mean Frailty Index score was 0.13 (SD 0.11), ranging from 0 to 0.66. For every 0.10 increase in Frailty Index scores (approximately one SD), the hazard ratio was 1.25 (p<0.001) for all fractures, 1.18 (p=0.043) for hip fractures, and 1.30 (p= 0.001) for clinical vertebral fractures.
Conclusion
The CaMos Frailty Index quantified fracture risk according to degree of frailty. Irrespective of age and bone mineral density, the Frailty Index was associated with hip, vertebral, and all-type clinical fractures. Predicting late onset illnesses may have to consider overall health status and not just traditional risk factors.
Keywords: Deficits, Fracture, Frailty, Index, Longitudinal, Osteoporosis
Introduction
Frailty can be characterized as a state of diminished reserves (energy, physical ability, cognition, health) that gives rise to vulnerability and adverse outcomes [1, 2]. While it is more common in older adults, frailty can occur across the life span and can be conceptualized as variability in health status for people of the same chronological age [3]. Despite general consensus about frailty as a construct, there is less agreement about its operational definition [4]. Three main approaches operationalize frailty. The frailty phenotype developed by Fried et al. [5] defines frailty as the presence of at least three of five components: unintentional weight loss, self-reported poor energy, weakness (poor grip strength), slow gait speed, and low physical activity. A second approach utilizes clinical judgment based on history-taking and clinical examination [1, 6]. A third approach, the focus of this paper, conceives frailty as an accumulation of health deficits (i.e., signs, symptoms, diseases, functional impairments) which are scored on a composite measure termed as cumulative deficits Frailty Index [2, 7,8].
Frailty has been linked with increased fracture risk in older adults [9–14]. However, previous studies have largely assessed frailty utilizing the frailty phenotype [12, 14] or modified versions [9–11, 15], all of which categorize individuals into discrete frail/nonfrail categories. A Frailty Index allows the quantification of risk according to the degree of frailty. More recently, it also appears to add to traditional risk factors associated with other late onset illnesses, including dementia [16], sleep disorders [17], heart disease [18], and the outcomes of intellectual disability from late middle age [19].
We created a Frailty Index in the Canadian Multicentre Osteoporosis Study (CaMos) [20] and examined (1) the age-sex distribution of frailty and (2) whether it was associated with incident fractures over 10 years in men and women age 25 years and older. We hypothesized that frailty would be an independent, nontraditional risk factor for fractures.
Methods
Study setting and design
A more detailed description of CaMos methods has been published [20] ( www.camos.org). CaMos is an ongoing, population-based, prospective cohort study that was established in 1995. The baseline cohort consisted of 9,423 noninstitutionalized individuals aged 25 years and older, including 6,539 women (70.7 %). The sampling frame consisted of randomly generated lists of noninstitutionalized residential telephone subscribers for nine study sites across Canada and represents 40 % of Canadian residents. Ethics approval was obtained at each participating center, and signed informed consent was received from all participants.
Data collection
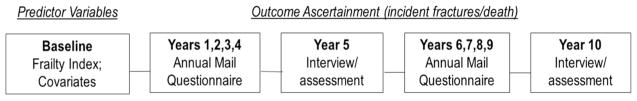
Figure 1 displays the timeline of data collection and ascertainment of predictor and outcome variables.
Fig 1.
Ascertainment of predictor and outcomes variables
Full assessments
At baseline and every 5-years thereafter, CaMos participants complete an extensive interviewer-administered questionnaire, and physical measurements are taken. Data collected include sociodemographic information, self-reported medical conditions, fracture history, family history, medication use, dietary intake, lifestyle data, quality of life (Health Utilities Index, SF-36), height and weight measurement, dual-energy X-ray absorptiometry (DXA) and, for participants 50 years and older, lateral lumbar and thoracic spine X-rays. Bone mineral density (BMD) was measured by dual-energy DXA; a European Spine Phantom was used for cross-calibration of DXA machines between sites. BMD T-scores at the femoral neck are based on published reference standards for Canadians [21].
Short assessments
Annually, in the intervening years, participants complete a mailed questionnaire regarding fractures, hospitalization, and the use of medications for bone health.
Fracture ascertainment
Self-reported, clinical fractures occurring between baseline and the tenth annual (2005–2006) follow-up were included. If consent was given, the treating physician or hospital was contacted for verification and to acquire further details. Here, we categorized fractures into three groups: (1) all fractures excluding face, fingers, toes, and skull; (2) hip fractures; and (3) vertebral fractures.
CaMos Frailty Index
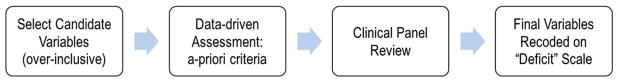
The CaMos Frailty Index was developed using a cumulative deficits framework [2, 7, 8], which specifies that variables must collectively cover a range of biological systems and not be overly representative of any particular system. Variables (termed deficits) should be added until there are at least 30–40 total deficits, with more deficits resulting in greater precision of estimates [8]. The focus is not on the exact combination of specific traits but rather on their cumulative impact, which in the aggregate characterizes physiological performance and overall function in a single variable [22]. As long as 30 or more variables are employed, various combinations of qualifying variables (randomly sampled from the same dataset) give comparable results [23]. Even so, to make serial comparisons in a given cohort, the same items need to be used.
The CaMos questionnaire and data dictionary were examined to identify variables for potential inclusion in the Frailty Index (Fig. 2). Osteoporosis, fractures, and falls were intentionally omitted. The cumulative deficits framework [2, 7, 8] specifies that variables must be present in at least 1 % of the sample, accumulate with age, not saturate too early (i.e., should not develop high prevalence at younger ages or be present in >80 % of the sample), and have fewer than 5 % missing data. A clinical panel (authors JDA, KR, AP) reviewed the final list to ensure biological plausibility and adequate representation of a range of systems.
Fig 2.
Framework for creating the CaMos Frailty Index
The final CaMos Frailty Index consisted of 30 items (Table 1). Dichotomous variables were coded as 1 when the deficit was present and 0 when absent. Variables with multilevel responses were assigned deficit values in equal cut points (e.g., not limited=0, limited a little=0.5, limited a lot=1) or were combined into logical categories (e.g., same/somewhat better/better=0, somewhat worse/worse=1).
Table 1.
The CaMos Frailty Index
| Deficit | Deficit values/description | |
|---|---|---|
| 1 | Osteoarthritis | No=0, Yes=1 |
| 2 | Rheumatoid arthritis | No=0, Yes=1 |
| 3 | Thyroid disease | No=0, Yes=1 |
| 4 | Breast cancer | No=0, Yes=1 |
| 5 | Uterine cancer/prostate cancer | No=0, Yes=1 |
| 6 | Inflammatory bowel disease | No=0, Yes=1 |
| 7 | Hypertension | No=0, Yes=1 |
| 8 | Heart attack | No=0, Yes=1 |
| 9 | Stroke | No=0, Yes=1 |
| 10 | Neuromuscular diseasea | No=0, Yes=1 |
| 11 | Diabetes | No=0, Yes=1 |
| 12 | Kidney disease | No=0, Yes=1 |
| 13 | Phlebitis/thrombophlebitis | No=0, Yes=1 |
| 14 | Vision | Six levels (0, 0.2, 0.4, 0.6, 0.8, 1); descriptive range: able to see well enough to read ordinary newsprint and recognize a friend on the other side of the street, without glasses or contact lenses=0 to unable to see at all=1 |
| 15 | Hearing | Six levels (0, 0.2, 0.4, 0.6, 0.8, 1); descriptive range: able to hear what is said in a group conversation with at least three other people, without a hearing aid=0 to unable to hear at all=1 |
| 16 | Walking | Six levels (0, 0.2, 0.4, 0.6, 0.8, 1); descriptive range: able to walk around the neighborhood without difficulty, and without walking equipment=0 to cannot walk at all=1 |
| 17 | Dexterity | Six levels (0, 0.2, 0.4, 0.6, 0.8, 1); descriptive range: full use of two hands and ten fingers=0 to limitations in use of hands or fingers to requires the help of another person for all tasks, not independent even with use of special tools=1 |
| 18 | Cognition | Six levels (0, 0.2, 0.4, 0.6, 0.8, 1); descriptive range: able to remember most things, think clearly and solve day to day problems=0 to unable to remember anything at all and unable to think or solve day to day problems=1 |
| 19 | Pain | Five levels (0, 0.25, 0.5, 0.75, 1); descriptive range: free of pain and discomfort=0 to severe pain that prevents most activities=1 |
| 20 | General health | Excellent=0; Very Good=0.25; Good=0.5; Fair=0.75; Poor=1 |
| 21 | Change in general health (past 1 year) | Same/somewhat better/better=0; Somewhat worse/worse=1 |
| 22 | Reduced daily work/other activities (last 4 weeks) | No=0, Yes=1 |
| 23 | Interference with social activities due to physical/ emotional health (last 4 weeks) | Not at all/slightly=0; Moderately=0.5; Quite a bit/extremely=1 |
| 24 | Limitation in moderate activities (e.g., moving table, vacuuming, golf, bowling) | Not limited=0; Limited a little=0.5; Limited a lot=1 |
| 25 | Limitation in lifting/carrying groceries | Not limited=0; Limited a little=0.5; Limited a lot=1 |
| 26 | Limitation in climbing a flight of stairs | Not limited=0; Limited a little=0.5; Limited a lot=1 |
| 27 | Limitation in bending, kneeling, and stooping | Not limited=0; Limited a little=0.5; Limited a lot=1 |
| 28 | Limitation in bathing/dressing | Not limited=0; Limited a little=0.5; Limited a lot=1 |
| 29 | Energy | Have a lot energy all/most/a good bit=0; Some of the time=0.5; Have a lot energy a little/none of the time=1 |
| 30 | Feel tired | Feel tired a little/none of the time=0; Some of the time=0.5; Feel tired all/most/a good bit=1 |
Parkinson’s/multiple sclerosis/other
Total Frailty Index scores represent the sum of the deficit values divided by the total number of items (n=30). Scores range from 0 to 1, with higher scores indicating greater frailty.
Statistical methods
Continuous variables were summarized as mean and standard deviation (SD) and categorical variables as the number (percent). Box plots were constructed to display the distribution of Frailty Index scores by age decade and gender. One-way analysis of variance (ANOVA) was used to examine differences in mean Frailty Index scores between groups (i.e., age decades, gender). Post hoc comparisons were performed using Tukey’s honestly significant difference (HSD) procedure. To enable comparisons with other studies, we report the prevalence of frailty using a Frailty Index cut point of ≥0.25 [24].
Simple linear regression was used to examine the relationship between the Frailty Index and age. We calculated the rate of accumulation of deficits per year (i.e., per 1-year increase in age) as the slope of the line of the logarithm of the Frailty Index versus age. A log transformation was selected to improve the symmetry of the distribution.
Unlike censoring, which merely obstructs the researcher from viewing the event (i.e., dropouts), a competing risk prevents the event of interest from occurring altogether, and the analysis should adjust accordingly to the competing factor. Thus, to examine the relationship between the Frailty Index and fractures over 10 years, we used a competing risk proportional sub-distribution hazards model where death was considered a competing risk [25]. This analysis determines probabilities where a participant is not only at risk of fracture but also from death. In the model, we adjusted for age, sex, body mass index (categorized as ≤20, 21–25, 26–30, 31–35, >35), education level, femoral neck T-score, and antiresorptive therapy. We conducted analyses for all participants combined and separately for men and women. Our analyses examined all clinical fractures combined (as above), hip and vertebral fractures. Hazard ratios (HR; p values) are expressed for each 0.10 increase in baseline Frailty Index score (approximately SD). In sensitivity analysis, we examined only participants over the age of 65 years. Statistical significance was set a priori at alpha=0.05. All statistical analyses were performed using IBM SPSS statistics v.20 and SAS v. 9.1.
Results
Baseline characteristics of CaMos participants are displayed in Table 2. The mean Frailty Index score across the entire CaMos cohort was 0.13 (SD 0.11), ranging from 0 to 0.66, with a mean increase per year of age of 3.9 %.
Table 2.
Baseline characteristics of the CaMos cohort
| All participants | Any incident fracture | Incident hip fracture | Incident vertebral fracture | |
|---|---|---|---|---|
| Sample size | 9,423 | 1,224 | 192 | 191 |
| Age (years), mean (SD) | 62.1 (13.4) | 67.1 (11.8) | 74.6 (8.4) | 69.4 (10.8) |
| Women [n (%)] | 6,539 (69.4) | 973 (79.5) | 148 (77.1) | 158 (82.7) |
| Attended university [n (%)] | 2,620 (27.8) | 334 (27.3) | 45 (24.4) | 50 (26.2) |
| Taking antiresorptive therapy [n (%)] | 1,633 (17.3) | 221 (18.1) | 38 (19.8) | 42 (22.0) |
| Body mass index [mean (SD)] | 27.0 (4.9) | 26.7 (4.7) | 25.3 (4.3) | 26.8 (4.6) |
| T-score Femoral neck [mean (SD)] | −1.08 (1.03) | −1.58 (0.98) | −2.12 (0.80) | −1.81 (0.93) |
| Fallen in past week [n (%)] | 203 (2.2) | 38 (3.1) | 2 (1.0) | 10 (5.2) |
| Fallen in past month [n (%)] | 621 (6.6) | 105 (8.6) | 4 (2.1) | 19 (10.0) |
| Frailty Index [mean (SD)] | 0.13 (0.11) | 0.17 (0.12) | 0.20 (0.12) | 0.19 (0.12) |
Frailty and incident fractures
During a mean follow-up of 7.9 years (SD=3.1), 998 participants died before a fracture occurred and 1,224 (13.0 %) had a fracture. Adjusted for education level, body mass index, femoral neck T-score, age, and antiresorptive therapy, for every 0.10 increase in Frailty Index scores (approximately one SD), HRs were 1.25 (p<0.001) for all fractures, 1.18 (p=0.043) for hip fractures, and 1.30 (p<0.001) for vertebral fractures (Table 3). For example, consider two individuals with the same characteristics (age, sex, T-score, etc.) but different Frailty Index scores. Person A has a score of 0.1 and person B a score of 0.50; over 10 years, person B had a nearly 2.5-fold risk of any fracture as person A. The HRs for men showed a similar trend as for women; however, the HR for hip fractures was not statistically significant (Table 3).
Table 3.
Adjusted hazard ratios of all hip and vertebral fractures per 0.10 increase in Frailty Index scores
| Characteristics | All participants (n=9,423)
|
Women (n=6,539)
|
Men (n=2,884)
|
|||
|---|---|---|---|---|---|---|
| Hazard ratio | p value | Hazard ratio | p value | Hazard ratio | p value | |
| All fractures | 1.25 | <0.001 | 1.22 | <0.001 | 1.29 | <0.001 |
| Hip fracture | 1.18 | 0.043 | 1.22 | 0.032 | 1.12 | 0.453 |
| Vertebral fracture | 1.30 | <0.001 | 1.27 | <0.001 | 1.38 | 0.021 |
Adjusted for education level, body mass index, femoral neck T-score, age, and antiresorptive therapy
Sensitivity analysis
When we restricted the cohort to participants 65 years and older, the magnitude of the estimates was very similar; however, the HR for hip fractures was no longer significant for women.
Frailty Index properties
Similar to other studies, the frequency distribution of the CaMos Frailty Index was right-skewed and well approximated by a gamma distribution. The maximal upper limit was 0.66. Across the entire baseline cohort (n=9423), the mean rate of deficit accumulation per year of age was 0.039. There was no significant interaction with gender when age was treated as a continuous variable.
Burden of frailty in CaMos cohort
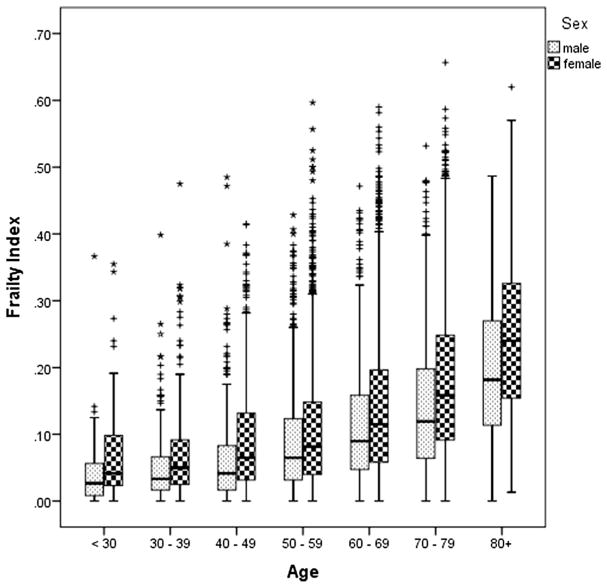
As displayed in Fig. 3, after age 30, Frailty Index scores increased significantly with each subsequent decade, and women had higher frailty scores than men. Even though the Frailty Index is a continuous measure, to facilitate comparisons with other studies, we calculated the prevalence of frailty using a threshold value for frailty of 0.25. The prevalence of frailty was 14.7 % (17.3 % women, 8.9 % men) in the overall cohort, 22.8 % (25.6 % women, 15.3 % men) in participants age 65 and older, 8.9 % (10.3 % women, 5.4 % men) in participants ages 50 to <65, and 3.8 % (4.9 % women, 2.2 % men) in those under age 50.
Fig 3.
Distribution of CaMos Frailty Index scores by age decades, men and women (ages 25–103)
Discussion
In a large cohort of community-dwelling Canadians, we examined the age-sex distribution of frailty and evaluated whether it was a nontraditional, independent risk factor for fracture. Our results demonstrate that frailty, operationalized by a cumulative deficits Frailty Index, was associated with incident fractures, irrespective of age, and traditional risk factors (i.e., low bone mass). Over the 10-year study period, the risk of a hip fracture increased by 18 % and all fractures by 25 % for each 0.10 increase in baseline Frailty Index score. Note that the magnitude of the relationship between frailty and fractures was similar both when we included all participants (25 years and older) and just those adults 65 years and older. Although frailty is strongly related to advancing age, they are not synonymous [26]. Here, frail individuals could be identified across all age decades (Fig. 3), with frailty conferring a similar risk of fracture across the life span.
Our results suggest the importance of considering overall health status in predicting late onset illnesses, in addition to traditional risk factors. To date, few studies have considered how frailty modifies outcomes for individuals identified as high risk based on traditional risk factors for osteoporosis and fractures. Although further evidence is required, the substantial prevalence of frailty observed even in middle age (i.e., 8.9 % in ages 50 to <65) may in part be explained by the fractures occurring in this age group.
Previous studies that have established a link between frailty and fractures have utilized measures which categorize individuals into discrete categories (i.e., frail/pre-frail/nonfrail) [9–12, 14]. In cohorts which utilized the frailty phenotype [5, 9, 12, 14] or a simplified version [10, 11], individuals classified as frail versus nonfrail had greater risk of incident hip and/or nonvertebral fractures. In contrast, a study which utilized a nine-item measure similar to the frailty phenotype did not find a significant association between frailty/nonfrailty status and fractures [15]. One other study [13], the Beijing Longitudinal Study of Aging, examined the relationship between a cumulative deficits Frailty Index and fractures over 8 years; although a relationship was observed, the result was not significant. All of these cohorts only included older adults with the exception of one that included women age 55 years and older [12].
Strengths
A cumulative deficits Frailty Index correlates well with the frailty phenotype [5, 24]; although unique instruments, they may serve as complementary measures [27]. A shorter measure is an advantage of the frailty phenotype; however, flexibility and generalizability may be reduced due to the highly specified items [28]. It may also be difficult to measure performance measures in patients with illness or severe physical limitations [29, 30], without special equipment (i.e., dynamometers; [27]), and these items are often not be available in population-based databases. The Frailty Index is highly generalizable, being derivable in any clinical or population-based dataset with a critical mass of relevant variables. Second, a Frailty Index does not require performance measures and can be applied to most population health databases. Third, a Frailty Index captures the multidimensional nature of the frailty construct and may offer an improvement in content validity [31]. Notably too, it can quantify the degree of frailty on a continuum rather than characterizing frailty as either an “all or none” construct. Statistically speaking, categorization assumes that the risk will not vary within categories and throws away valuable information which can reduce power [32].
Our study has some methodological strengths. The properties of the CaMos Frailty Index were comparable with other Frailty Indexes created in a wide range of datasets [2, 33], demonstrating external validity. CaMos is a randomly selected, longitudinal study across a wide age range and has detailed fracture information. Since we were interested in the relationship between frailty and fractures, our statistical modeling took death into account as a competing risk. Standard survival analyses (time-to-event) may produce biased estimates [34], as the assumption of these methods is that censored subjects (i.e., have not experienced the outcome but cannot be followed to the study endpoint) are considered “at risk” for the duration of the study, even if they died and cannot experience the event.
Limitations
Several limitations should be acknowledged. Although the CaMos cohort is population-based [20], it did not include individuals who were institutionalized or living in more remote or rural locations (i.e., >50 km from a study site), and it may have underrepresented economically marginalized groups (e.g., individuals without a telephone were not recruited). Furthermore, it is likely that the frailest individuals may have declined participation, given the effort required to participate in a study with multiple measurements. We only examined clinically recognized incident fractures, and thus, we were not able to include individuals with morphometric vertebral fractures. Missing values for Frailty Index deficits, or those coded as do not know/refused, were considered as no deficit present. This conservative approach likely attenuated any observed associations (i.e., bias toward the null); however, there was a low amount of missing data; only two variables had greater than 0.5 % missing data, and the majority were missing <0.1 %. This study may have been underpowered in relation to men, in whom, for example, we were unable to detect a relationship between frailty and hip fracture.
Conclusion
The CaMos Frailty Index was a robust measure that predicted fracture outcomes in a community-dwelling cohort of Canadian men and women ages 25–103. The ability to quantify fracture risk along a frailty continuum offers added precision, and the Frailty Index would be a useful measure in future interventional studies. In addition to traditional risk factors for fracture, including poor bone strength and low bone mass, at any age, frailty should be considered an important independent risk factor for fracture. This study joins others in suggesting that it is important to take into account overall health status, and not just traditional risk factors, in quantifying the risk of age-associated illnesses.
Acknowledgments
Courtney C. Kennedy was supported by Osteoporosis Canada–Canadian Multicentre Osteoporosis Study Fellowship Awards (2011–2013).
Footnotes
Conflicts of interest Kenneth Rockwood discloses that the Dalhousie University Industry-Liaison Office is reviewing the commercialization potential of one version of the Frailty Index (not the one used here) based on a Comprehensive Geriatric Assessment.
Dr. Alexandra Papaioannou has received grants and research support from Amgen, Eli Lilly, Merck Canada Inc., Warner Chilcott and consults for Amgen, Eli Lilly.
Dr. Jonathan Adachi consults for and has received lecture fees from Amgen, Eli Lilly, Merck, Novartis, Warner Chilcott.
Courtney C. Kennedy, George Ioannidis, Lehana Thabane, Susan Kirkland, and Laura E. Pickard declare no conflict of interest.
Contributor Information
C. C. Kennedy, Department of Medicine, Division of Geriatrics, McMaster University, St. Peter’s Hospital, GERAS Centre, 88 Maplewood Avenue, Hamilton, ON L8M 1W9, Canada
G. Ioannidis, Department of Medicine, McMaster University, Charlton Medical Centre, 25 Charlton Ave East, Hamilton, ON L8N 1Y2, Canada
K. Rockwood, Department of Medicine, Dalhousie University, 421, Veterans’ Memorial Building, 5955 Veterans’ Memorial Lane, Halifax, NS, Canada
L. Thabane, Clinical Epidemiology and Biostatistics, McMaster University, St. Joseph’s Healthcare Hamilton, 50 Charlton Avenue East, 3rd Floor Martha Wing, Room H325, Hamilton, ON L8N 4A6, Canada
J. D. Adachi, Department of Medicine, Alliance for Better Bone Health Chair in Rheumatology, McMaster University, Charlton Medical Centre, 25 Charlton Ave East, Hamilton, ON L8N 1Y2, Canada
S. Kirkland, Department of Community Health and Epidemiology, Geriatric Medicine Research Unit, Dalhousie University, 5790 University Ave, Room 423, Halifax, NS B3H 1V7, Canada
L. E. Pickard, Department of Medicine, McMaster University, St. Peter’s Hospital, GERAS Centre, 88 Maplewood Avenue, Hamilton, ON L8M 1W9, Canada
A. Papaioannou, Department of Medicine, Division of Geriatrics, Eli Lilly Canada Chair in Osteoporosis, McMaster University, St. Peter’s Hospital, GERAS Centre, 88 Maplewood Avenue, Hamilton, ON L8M 1W9, Canada
References
- 1.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crome P, Lally F. Frailty: joining the giants. CMAJ. 2011;183:889–90. doi: 10.1503/cmaj.110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–9. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–36. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, Tracy JK, Hochberg MC, Rodondi N, Cawthon PM for the Study of Osteoporotic Fractures Research Group. Frailty and risk of falls, fracture, and mortality in older women: the study of osteopo-rotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–51. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 10.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, Dam TT, Marshall LM, Orwoll ES, Cummings SR Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–8. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tom SE, Adachi JD, Anderson FA, Jr, Boonen S, Chapurlat RD, Compston JE, Cooper C, Gehlbach SH, Greenspan SL, Hooven FH, Nieves JW, Pfeilschifter J, Roux C, Silverman S, Wyman A, LaCroix AZ GLOW Investigators. Frailty and fracture, disability, and falls: a multiple country study from the global longitudinal study of osteoporosis in women. J Am Geriatr Soc. 2013;61:327–34. doi: 10.1111/jgs.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang X, Shi J, Song X, Mitnitski A, Tang Z, Wang C, Yu P, Rockwood K. Frailty in relation to the risk of falls, fractures, and mortality in older Chinese adults: results from the Beijing Longitudinal Study of Aging. J Nutr Health Aging. 2012;16:903–7. doi: 10.1007/s12603-012-0368-6. [DOI] [PubMed] [Google Scholar]
- 14.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB Initiative W’s H. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–30. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 15.de Vries OJ, Peeters GM, Lips P, Deeg DJ. Does frailty predict increased risk of falls and fractures? A prospective population-based study. Osteoporos Int. 2013;24:2397–403. doi: 10.1007/s00198-013-2303-z. [DOI] [PubMed] [Google Scholar]
- 16.Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology. 2011;77:227–34. doi: 10.1212/WNL.0b013e318225c6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10:767–75. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- 18.Wallace L, Theou O, Rockwood M, Kirkland S, Rockwood K, Shimbo D, Davidson K. Considering both non-traditional and traditional risk factors improves the prediction of coronary heart disease events. Can Ger J. 2013;16:66–103. [Google Scholar]
- 19.Schoufour JD, Mitnitski A, Rockwood K, Evenhuis HM, Echteld MA. Development of a frailty index for older people with intellectual disabilities: results from the HA-ID study. Res Dev Disabil. 2013;34:1541–55. doi: 10.1016/j.ridd.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Kreiger N, Tenenhouse A, Joseph L, Mackenzie T, Poliquin S, Brown JP, Prior JC, Rittmaster RS. The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Canadian J Aging. 1999;18:376–87. [Google Scholar]
- 21.Tenenhouse A, Joseph L, Kreiger N, Poliquin S, Murray TM, Blondeau L, Berger C, Hanley DA, Prior JC CaMos Research Group, Canadian Multicentre Osteoporosis Study. Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2000;11:897–904. doi: 10.1007/s001980070050. [DOI] [PubMed] [Google Scholar]
- 22.Kulminski A, Yashin A, Arbeev K, Akushevich I, Ukraintseva S, Land K, Manton K. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: results from analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007;128:250–8. doi: 10.1016/j.mad.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–9. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–43. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesari M, Gambassi G, Abellan van Kan G, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2013 doi: 10.1093/ageing/aft160. [DOI] [PubMed] [Google Scholar]
- 28.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbard RE, O’Mahony MS, Woodhouse KW. Characterising frailty in the clinical setting–a comparison of different approaches. Age Ageing. 2009;38:115–9. doi: 10.1093/ageing/afn252. [DOI] [PubMed] [Google Scholar]
- 30.Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, von Zglinicki T, Kirkwood TB, Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, von Zglinicki T, Kirkwood TB. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133:456–66. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hebert R, Hogan DB, Wolfson C, McDowell I. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59:1310–7. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 32.Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Methodol. 2012;12:21. doi: 10.1186/1471-2288-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Gonzalez JJ, Garcia-Pena C, Franco-Marina F, Gutierrez-Robledo LM. A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr. 2009;9:47. doi: 10.1186/1471-2318-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–7. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]