Abstract
In vivo peripheral quantitative computed tomography (pQCT) and peripheral magnetic resonance imaging (pMRI) modalities can measure apparent bone microstructure at resolutions 200 μm or higher. However, validity and in vivo test-retest reproducibility of apparent bone microstructure have yet to be determined on 1.0 T pMRI (196 μm) and pQCT (200 μm). This study examined 67 women with a mean age of 74 ± 9 yr and body mass index of 27.65 ± 5.74 kg/m2, demonstrating validity for trabecular separation from pMRI, cortical thickness, and bone volume fraction from pQCT images compared with high-resolution pQCT (hr-pQCT), with slopes close to unity. However, because of partial volume effects, cortical and trabecular thickness of bone derived from pMRI and pQCT images matched hr-pQCT more only when values were small. Short-term reproducibility of bone outcomes was highest for bone volume fraction (BV/TV) and densitometric variables and lowest for trabecular outcomes measuring microstructure. Measurements at the tibia for pQCT images were more precise than at the radius. In part I of this 3-part series focused on trimodality comparisons of precision and validity, it is shown that pQCT images can yield valid and reproducible apparent bone structural outcomes, but because of longer scan time and potential for more motion, the pMRI protocol examined here remains limited in achieving reliable values.
Keywords: MRI, pQCT, segmentation, short-term precision, validity
Introduction
Bone volumetric structure has proven useful in monitoring treatment success of bone formation agents beyond changes in areal bone mineral density (BMD) (1,2). Over the last decade, several noninvasive techniques for quantifying volumetric bone outcomes have been developed including the peripheral quantitative computed tomography (pQCT) (3), high-resolution pQCT (hr-pQCT) (4), and magnetic resonance imaging (MRI) (5). Although the first 2 modalities had been constructed for the investigation of bone, MRI has mostly been used for soft tissue investigations. The 2 computed tomography (CT)–based modalities hinge on the linear attenuation of X-ray photons to determine density and structure by calibration. With MRI, however, bone structure can be measured by virtue of the lack of proton signals in solid bone. Application of full body MRI to bone structure quantification is limited by poor accessibility, but dedicated high-field (>1.0 T) peripheral MRI (pMRI) units have been developed. These 3 peripheral modalities (pQCT, hr-pQCT, and pMRI) enable the examination of trabecular bone at the ultradistal radius and tibia.
In vivo short-term precision error for volumetric bone outcomes has been quantified from test-retest images obtained by hr-pQCT but not by either pQCT or pMRI. Boutroy et al (4) demonstrated that all hr-pQCT image–derived bone outcomes yielded root mean square coefficients of variation (RMSCV) below 5% (2.5%–4.4%). Short-term precision error for bone volume fraction (BV/TV), trabecular separation (Tb.Sp), and trabecular thickness (Tb.Th) derived from 1.5 T MRI of the calcaneus was between 1% and 2% in vivo (6). One study quantified the test-retest precision for trabecular, cortical, and integral bone density (0.8%–1.6%) using pQCT imaging at the radius (7). The manufacturer software of pQCT does not yield bone microstructural outcomes, although at an in-plane resolution of 200 μm, apparent structural measurements can be obtained. Such outcome measures have formerly been reported in the Osteoporotic Fractures in Men Study by Sheu et al (8), but the test-retest precision of these measurements remains unknown in this or any of the Osteoporotic Fractures in Men Study studies using pQCT. The validity of these pQCT apparent bone structure measurements has been demonstrated ex vivo using specimens by Lala et al (9) (R2 = 0.61–0.98), but in vivo validity is important to ensure that soft tissue attenuation and participant motion do not significantly interfere with the accuracy of measurements. Similar to pQCT, 1.0 T pMRI is capable of imaging at 195 μm in-plane resolution, but measurements derived from this modality have not been validated. Apparent bone microstructure from other MRI modalities has been validated using cadavers, and externally validated using BMD derived from dual-energy X-ray absorptiometry and vertebral fractures. However, there is a lack of construct validity data. To date, hr-pQCT–derived bone outcomes have been validated against μCT (R2 = 0.59–0.96) at 19 μm resolution on ex vivo specimens (10).
An appreciation of the differences among these modalities by characterizing their short-term precision and validity will assist in selecting one vs another for specific applications. The present study aimed to compare short-term precision error of apparent volumetric bone measures computed from images obtained by pQCT and 1.0 T pMRI vs hr-pQCT. In addition, outcomes quantified from pQCT and pMRI were validated against hr-pQCT equivalents.
This tri-modality comparison is presented as the first component of a three-part series discussing intermodality differences in technological limitations versus advantages for in vivo volumetric bone imaging.
Methods
This study was designed as a cross-sectional observational analysis comparing the short-term technical precision of 3 in vivo technologies for imaging bone volumetrically. All study procedures were performed within 1.5 yr in a cohort of women ≥50 yr of age enrolled in the Canadian Multicentre Osteoporosis Study (CaMOS) and living within a 50 km radius of the Hamilton (Ontario, Canada) CaMOS site (sampling pool N = 340). CaMOS is an ongoing, prospective cohort study of community-dwelling, randomly selected women and men ≥25 yr of age at 9 major Canadian cities. The main CaMOS objectives, methodology, and sampling framework are described in detail elsewhere (11). Those having valid contraindications to MRI (pacemaker and insulin pumps) were excluded from 1.0 T pMRI procedures. Participants weighing more than 250 lbs were excluded from hr-pQCT and 1.0 T pMRI procedures because of the chair weight limit. Women with self-reported tremors were also excluded to avoid significant motion artifact.
Participants volunteered in the completion of a pQCT, hr-pQCT, and 1.0 T pMRI ultradistal radius scan. For pQCT and pMRI, scans were repeated once within the same day. A second scan was not completed for hr-pQCT within this cohort because this procedure has already been completed on the same scanner by the same technician previously. Repeated imaging was also performed at the ultradistal tibia for pQCT. Because of the limitations of the gantry diameter and depth, ultradistal tibia scans were not completed using the pMRI. All study procedures were approved by the St. Joseph’s Healthcare Research Ethics Board in Hamilton and the University Health Network in Toronto (Ontario, Canada).
hr-pQCT
The standard hr-pQCT imaging protocol was followed (4). Briefly, a region of interest located 9.5 and 22.5 mm proximal to the end plate of the radius and tibia, respectively, was scanned on the hr-pQCT (XtremeCT v1; Scanco Medical AG, Bassersdorf, Switzerland) machine acquiring 110 transaxial CT slices in the proximal direction at an isotropic voxel resolution of 82 μm. Only images with motion grade 3 and below (12) were accepted for analyses. Each slice was semiautomatically segmented using Scanco software as guided by a dual-threshold edge-detection algorithm. Segmented images were reconstructed in three dimension (3D) and were used to automatically compute apparent microstructural outcomes (BV/TV, Tb.Sp, Tb.Th, Tb.N, Ct.Th, integral, cortical, and trabecular vBMD, as denoted by subscripts i, c, and tr). Hydroxyapatite rod phantoms were scanned daily for quality control.
pQCT
Limb positioning followed previous pQCT studies (13). Scans of the radius were completed using a model XCT2000 pQCT (Stratec Medizintechnik, Pforzheim, Germany) at sites 11.5 and 16.5 mm proximal to a reference line identified at the midpoint of the radial tilt. The base and tip of the radial tilt was identified as the most medial and most lateral articulating aspects of the distal radius, respectively. Tibial scans were completed at sites that were 24.5 and 29.5 mm proximal to a reference line located along the distal tibia end plate, which was defined as the outer cortical border of the plateau portion of the ultradistal tibia. At each region of interest for both radius and tibia scans, 2.5 ± 0.3 mm thick slices were acquired with an in-plane resolution of 200 μm, a CT scan speed of 10 mm/s, 38 kVp X-ray beam energy, a tube current of 0.3 mA, and reconstructed by filtered back projection on a matrix size of 256 × 256. Both slices obtained on each limb were acquired in sequence without interruption. Hydroxyapatite phantoms were assessed on days in which scans were obtained. Only images with no discontinuities in the cortical bone were accepted for image analyses. Densitometric (vBMDi, vBMDc, and vBMDtr) measures were obtained from Stratec v5.2.1 software after threshold- and edge-detection-based segmentation. Primary measures of apparent trabecular microstructure (Ct.Th, Tb.Sp, BV/TV, Tb.N, and Tb.Th) and Ct.Th were computed with custom software package pQCT OsteoQ (Inglis Software Solutions Inc., Hamilton, Ontario, Canada), which applied a combination of threshold-based and region-growing algorithms.
1.0 T pMRI
Distal radius scans were performed on a 1.0 T pMRI OrthOne scanner (GE Healthcare, Pittsburgh, PA) using a 100 mm diameter transmit/receive coil. The wrist was positioned in the isocentre of the bore of the magnet. On the coronal scout view, a reference line was placed at the midpoint of the radial tilt as prescribed for pQCT reference line positioning. Scans began 9.5 mm proximal to this reference location. All slices were obliquely positioned so that slices were aligned perpendicular to the long axis of the radius.
A T1-weighted spoiled 3D gradient recalled echo (SPGR) sequence was acquired transaxially with number of excitations = 3, echoes = 1, flip angle = 40°, bandwidth = 15 kHz, repetition/echo time = 47 ms/23.8 ms, field of view = 100 × 100 mm, and a matrix size of 512 × 256 pixels (interpolated to 512 × 512), resulting in an in-plane resolution of 195 μm. A series of 20 slices each 1.0 mm thick were obtained in tandem with 0.0 mm gap. The total scan time was 12:09 min. A geometric phantom was assessed on days in which scans were obtained.
A single reader evaluated image quality on all participant scans. Only images that were judged by the reader to have preserved sufficient sharpness and textural pattern in the trabecular region were accepted for image analyses. Bone structural measures were obtained from the central 18 slices because signal loss at end slices in SPGR scans precludes sufficient image contrast between bone and marrow. Using a custom-designed software package, MRI OsteoQ (Inglis Software Solutions, Inc.), trabecular bone was separated from marrow space using an image-foresting transform algorithm adapted from Falcao et al (14,15), which relied on pixel greyscale value, gradient transition between pixels and relative position from a seeded point. Trabecular apparent microstructural (Tb.Sp, Tb.Sp SD, BV/TV, Tb.N, and Tb.Th) outcomes were computed on a per-slice basis and averaged to yield a final structural outcome.
Volumetric Bone Outcome Computation
All volumetric bone outcomes were derived from equations previously reported for hr-pQCT (16) and for histomorphometry. The latter was based on the model of parallel plates and derived from single slices as described by Parfitt et al (17)—hereon forward termed “model-dependent” outcomes. The former was computed from 3D volumes and was not based on the model by Parfitt.
Image Coregistration
Manual coregistration for test-retest acquisitions of pMRI was achieved on 3D Slicer (v4.2.1) (18). The first image was fixed and the repeated image transformed. Rigid orthogonal transformations were performed without oblique rotation to prevent multiplanar reformatting. Successfully coregistered slices were decided by visually inspecting the degree of alignment in the endocortical perimeter for each slice. For pMRI-hr-pQCT coregistrations, pMRI slices were thicker and often oblique compared with thinner hr-pQCT slices. Hence, a single pMRI slice may match with features on multiple hr-pQCT slices. To address this multilayer challenge, the range of matching hr-pQCT slices was identified by scanning across the surface of an MRI slice. A summary of matching slice numbers was compiled for all participants, and only the commonly matched slices across all participants were used. Coregistration between pQCT and hr-pQCT images was not performed because wide variability in slice matching is possible for single slice coregistration.
Image Set Misregistration Angle
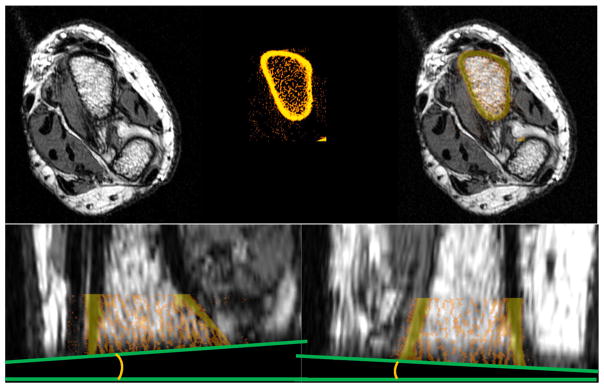
Interimage set angulation error between the actual imaged axis on hr-pQCT vs the axial plane on pMRI was quantified using the end slices of the already coregistered image sets. At this slice location, a contrasting edge formed an angle (Fig. 1), which was quantified from both coronal and sagittal views using PixelStick (Plum Amazing, LLC).
Fig. 1.
Coregistration of 1.0 T pMRI with hr-pQCT scans. The base image was the pMRI scan, remaining in greyscale (top left). The hr-pQCT image set was colored in an “iron” scheme and transparency set at 40% (top middle). Successful coregistrations showed endocortical boundary of the radius from both modalities aligning (top right). Cortical bone from sagittal and coronal views (bottom) was also used as guides for correct matching. hr-pQCT, high-resolution peripheral quantitative computed tomography; pMRI, peripheral magnetic resonance imaging.
Data Analyses
RMSCV and root mean square standard deviation (SD) estimated short-term precision error for test-retest measurements as previously described (19), using 5% error as the benchmark for acceptable reproducibility. A linear regression model determined the relationship between hr-pQCT (dependent) and each of 1.0 T pMRI and pQCT (independent) image-derived measures. Slopes and intercepts were reported along with 95% confidence intervals. A generalized additive model was explored for analyzing the most skewed and kurtotic variable, Tb.Sp, and showed no difference from a linear regression model for quantifying slope and intercept estimates. A Bland-Altman analysis depicted the relationship between mean value for each volumetric bone variable and the degree of intermodality deviation. Test-retest analyses for pMRI and linear regression analyses between pMRI and hr-pQCT were performed on original and coregistered image sets. The effect of angular deviation between image sets and the degree of intermodality deviation, as measured by SD and coefficients of variation, was assessed using a linear regression model. All statistical analyses were performed on SAS/STAT v9.3 (SAS Institute Inc., Cary, NC). Because results at the more proximal slice for pQCT were similar to those at the more distal slice, only the distal slice data were reported here.
Results
Of the 67 study participants who completed one or more study procedures, 49 completed all 3 imaging techniques, 51 completed both pQCT and hr-pQCT scans, and 65 completed both 1.0 T pMRI and hr-pQCT scans. Anthropometrics for all study participants were similar for all subgroups involved in different analyses (Table 1). The majority of volumetric bone outcomes acquired from either pQCT or pMRI were significantly different from those obtained using hr-pQCT (Table 2). Only model-dependent Tb.Sp did not exhibit significant differences across modalities after adjusting for the 20 pairwise comparisons. Both BV/TV and Tb.Th from hr-pQCT were less than 30% of what was observed on either pQCT or pMRI.
Table 1.
Participant Characteristics for All Study Procedures
| Variable | Meana/medianb | Standard deviationa/Q1–Q3b | Minimum | Maximum |
|---|---|---|---|---|
| Age (yr)a | 74 | 9 | 56 | 94 |
| BMI (kg/m2)a | 27.65 | 5.74 | 18.73 | 48.28 |
| Height (m)a | 1.60 | 0.07 | 1.48 | 1.79 |
| Weight (kg)a | 70.46 | 13.86 | 45.00 | 119.00 |
| Antiresorptive therapy (yr)b | 0.0 | 0–4.5 | 0.0 | 22.0 |
| Vitamin D3 (yr)b | 5.0 | 0.6–11.0 | 0.0 | 42.0 |
| Calcium (yr)b | 5.5 | 0.0–12.0 | 0.0 | 42.0 |
Note: Anthropometrics and medication use descriptive statistics for study participants completing any of the local Hamilton study procedures.
Abbr: BMI, body mass index.
Parametric variable described using mean and standard deviations.
Nonparametric variable characterized by median and first (Q1) and third (Q3) quartiles.
Table 2.
Summary Statistics and Comparison of Volumetric Bone Outcomes Across the 3 Modalities
| Variable | hr-pQCT, mean ± SD | hr-pQCT, median (Q1–Q3) | pQCT, mean ± SD | pQCT, median (Q1–Q3) | MRI, mean ± SD | MRI, median (Q1–Q3) |
|---|---|---|---|---|---|---|
| BV/TVP | 0.113 ± 0.034 | 0.116 (0.089–0.139) | 0.422 ± 0.113 | 0.434 (0.340–0.500) | 0.471 ± 0.050 | 0.486 (0.465–0.501) |
| Tb.Sp MI (mm)NP | 0.527 ± 0.193 | 0.468 (0.420–0.558) | 0.593 ± 0.275 | 0.479 (0.392–0.740) | 0.622 ± 0.169 | 0.573 (0.527–0.643) |
| Tb.Sp (mm)NP | 0.595 ± 0.276 | 0.479 (0.394–0.740) | 0.641 ± 0.196 | 0.579 (0.532–0.657) | ||
| Tb.Th MI (mm)P | 0.062 ± 0.012 | 0.062 (0.054–0.068) | 0.391 ± 0.056 | 0.383 (0.347–0.430) | 0.535 ± 0.034 | 0.529 (0.512–0.560) |
| Tb.Th (mm)P | 0.391 ± 0.056 | 0.383 (0.347–0.430) | 0.539 ± 0.036a | 0.533 (0.513–0.563) | ||
| Tb.N (number/mm2)NP | 1.8 ± 0.4 | 1.9 (1.6–2.1) | 1.1 ± 0.2 | 1.1 (0.9–1.2) | 0.9 ± 0.1 | 0.9 (0.8–1.0) |
| Ct.Th (mm)P | 0.669 ± 0.178 | 0.670 (0.540–0.790) | 0.960 ± 0.126 | 0.940 (0.864–1.050) | ||
| vBMDi (mg/cm3)P | 277.43 ± 62.61 | 275.70 (242.50–325.40) | 337.54 ± 68.45 | 332.80 (307.50–377.10) | ||
| vBMDc (mg/cm3)P | 804.52 ± 63.16 | 804.00 (764.10–844.00) | 980.29 ± 64.31 | 979.80 (931.70–1032.90) | ||
| vBMDtr (mg/cm3)P | 136.14 ± 40.10 | 139.70 (106.60–167.20) | 162.43 ± 33.98 | 173.00 (135.40–189.40) |
Note: Measures of central tendency (mean for parametric [P] and median for nonparametric [NP] variables) and error (SD for parametric and interquartile range (Q1–Q3) for nonparametric variables) were reported. MI is the variables not based on the model of parallel plates by Parfitt et al. If at least 2 of the 3 modalities’ bone outcome is nonparametric, a nonparametric Friedman comparison of medians was applied. Otherwise parametric repeated measures analysis of variances compared means of volumetric bone outcomes. Post-hoc Tukey’s HSD was applied to parametric data and a Wilcoxon signed ranks test applied to nonparametric pairwise comparisons. An adjustment for multiple comparison was performed using Bonferroni’s correction. Bold indicates significantly different compared with the corresponding variable from hr-pQCT.
Abbr: BV/TV, bone volume fraction; Ct.Th, cortical thickness; hr-pQCT, high-resolution peripheral quantitative computed tomography; MI, model-independent measures; MRI, magnetic resonance imaging; pQCT, peripheral quantitative computed tomography; vBMDc, cortical volumetric bone mineral density; vBMDi, integral volumetric bone mineral density; vBMDtr, trabecular volumetric bone mineral density; SD, standard deviation; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
Bold indicates significantly different compared with pQCT.
Motion Artifacts Across Modalities
A larger number of images obtained by pMRI failed quality assurance (5.3%), followed by pQCT (2.6%), and then hr-pQCT (1.5%), which had only 1 quality failure at the distal tibia. The majority of MRI exhibited at least some mild to moderate (grades 1–3) level of motion but were still acceptable for analyses. The same was true for pQCT of the radius. However, at the same site for hr-pQCT, most images showed no or minor motion. At the tibia, motion grades appeared to be lower than at the radius for both pQCT and hr-pQCT.
Validation
pMRI
Only Tb.Th did not display a significant relationship with its counterpart on hr-pQCT even after coregistration. Model-independent Tb.Sp showed the highest correlation that was also closest to unity and had near zero intercept (Table 3). However, values of both model-dependent and model-independent Tb.Sp were underestimated by pMRI after coregistration. Bone volume fraction was overestimated compared with hr-pQCT, but Tb.N was underestimated. Coregistration strengthened the pMRI-hr-pQCT relationship for BV/TV without changing the regression coefficient.
Table 3.
Linear Relationships Between pMRI and hr-pQCT Bone Structure Values With and Without Coregistration
| pMRI bone outcome | Slope, 95% CI (lower, upper) | Intercept, 95% CI (lower, upper) | R2 | p Value |
|---|---|---|---|---|
| Un-coregistered | ||||
| BV/TV | 0.484 (0.361, 0.607) | −0.119 (−0.179, −0.059) | 0.526 | <0.001 |
| Tb.Sp | 0.744 (0.576, 0.911) | 0.048 (−0.060, 0.157) | 0.586 | <0.001 |
| Tb.Sp MI | 0.970 (0.781, 1.159) | −0.075 (−0.192, 0.043) | 0.654 | <0.001 |
| Tb.Th | 0.016 (−0.039, 0.071) | 0.053 (0.022, 0.084) | 0.006 | 0.554 |
| Tb.Th MI | 0.016 (−0.04, 0.071) | 0.054 (0.023, 0.085) | 0.006 | 0.578 |
| Tb.N | 2.1 (1.4, 2.9) | 0.0 (−0.7, 0.6) | 0.376 | <0.001 |
| Coregistered | ||||
| BV/TV | 0.767 (0.568, 0.967) | −0.263 (−0.362, −0.165) | 0.514 | <0.001 |
| Tb.Sp | 1.640 (1.357, 1.924) | −0.414 (−0.575, −0.252) | 0.706 | <0.001 |
| Tb.Sp MI | 1.712 (1.419, 2.004) | −0.447 (−0.613, −0.282) | 0.710 | <0.001 |
| Tb.Th | −0.010 (−0.064, 0.044) | 0.068 (0.039, 0.097) | 0.003 | 0.700 |
| Tb.Th MI | −0.011 (−0.065, 0.044) | 0.068 (0.038, 0.098) | 0.003 | 0.700 |
| Tb.N | 2.2 (1.3, 3.0) | −0.1 (−0.9, 0.7) | 0.296 | <0.001 |
Note: Hr-pQCT bone structural variables were used as the dependent variable, and pMRI bone structure was treated as an independent variable.
Abbr: BV/TV, bone volume fraction; CI, confidence interval; hr-pQCT, high-resolution peripheral quantitative computed tomography; MI, model-independent measures; pMRI, peripheral magnetic resonance imaging; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
pQCT
pQCT-hr-pQCT slopes were similar in directionality to pMRI-hr-pQCT comparisons, except the magnitude of parameter estimates were smaller. Like pMRI, there was a lack of validity for Tb.Th (Table 4). Both model-dependent and model-independent measures yielded near identical validation slopes to one another. Ct.Th and vBMDtr showed linear relationships near unity, but both integral and cortical vBMD were overestimated from pQCT images.
Table 4.
Linear Relationships Between pQCT and hr-pQCT Bone Structure Values at the Ultradistal Radius (11.5 mm From End Plate) and Ultradistal Tibia (24.5 mm From End Plate)
| pQCT bone outcome | Slope, 95% CI (lower, upper) | Intercept, 95% CI (lower, upper) | R2 | p Value |
|---|---|---|---|---|
| Ultradistal radius | ||||
| BV/TV | 0.293 (0.257, 0.33) | −0.011 (−0.026, 0.005) | 0.849 | <0.001 |
| Tb.Sp | 0.696 (0.497, 0.895) | 0.160 (0.047, 0.272) | 0.514 | <0.001 |
| Tb.Sp MI | 0.695 (0.471, 0.919) | 0.167 (0.041, 0.294) | 0.497 | <0.001 |
| Tb.Th | 0.158 (0.114, 0.201) | 0.005 (−0.011, 0.021) | 0.531 | <0.001 |
| Tb.Th MI | 0.159 (0.116, 0.202) | 0.000 (−0.017, 0.017) | 0.542 | <0.001 |
| Tb.N | 1.7 (1.3, 2.2) | −0.2 (−0.7, 0.3) | 0.583 | <0.001 |
| Ct.Th | 0.768 (0.255, 1.281) | −0.036 (−0.506, 0.435) | 0.162 | 0.004 |
| vBMDi | 0.90 (0.72, 1.08) | 45.26 (−1.70, 92.22) | 0.685 | <0.001 |
| vBMDc | 0.59 (0.38, 0.80) | 297.93 (115.48, 480.38) | 0.400 | <0.001 |
| vBMDtr | 1.15 (1.00, 1.30) | −67.01 (−93.61, −40.40) | 0.837 | <0.001 |
| Ultradistal tibia | ||||
| BV/TV | 0.334 (0.267, 0.401) | −0.015 (−0.043, 0.013) | 0.682 | <0.001 |
| Tb.Sp | 0.724 (0.386, 1.062) | 0.124 (−0.078, 0.325) | 0.283 | <0.001 |
| Tb.Sp MI | 0.724 (0.386, 1.062) | 0.124 (−0.078, 0.326) | 0.283 | <0.001 |
| Tb.Th | 0.217 (0.128, 0.307) | −0.014 (−0.048, 0.020) | 0.337 | <0.001 |
| Tb.Th MI | 0.217 (0.128, 0.307) | −0.014 (−0.048, 0.020) | 0.337 | <0.001 |
| Tb.N | 1.8 (1.3, 2.3) | −0.1 (−0.7, 0.4) | 0.526 | <0.001 |
| Ct.Th | 1.249 (1.017, 1.481) | −0.726 (−1.029, −0.422) | 0.714 | <0.001 |
| vBMDi | 0.89 (0.73, 1.04) | −49.86 (−102.27, 2.56) | 0.737 | <0.001 |
| vBMDc | 1.33 (0.81, 1.86) | −545.70 (−1059.23, −32.16) | 0.356 | <0.001 |
| vBMDtr | 1.10 (0.83, 1.37) | −35.10 (−80.18, 9.98) | 0.592 | <0.001 |
Note: hr-pQCT bone structural variables were used as the dependent variable, and pQCT bone structure was treated as an independent variable. Where unspecified, model-dependent equations were used in the computation of the variable.
Abbr: BV/TV, bone volume fraction; CI, confidence interval; Ct.Th, cortical thickness; hr-pQCT, high-resolution peripheral quantitative computed tomography; MI, model-independent measures; pQCT, peripheral quantitative computed tomography; vBMDc, cortical volumetric bone mineral density; vBMDi, integral volumetric bone mineral density; vBMDtr, trabecular volumetric bone mineral density; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
Effect of Angular Deviation on Linear Relations Between pMRI and hr-pQCT
The angular deviation measured from coregistered pMRI-hr-pQCT images ranged from 0.14° to 10.50° with a mean centered at 4.46 ± 2.28°. None of the regression models examining the contribution of angular deviation to pMRI-hr-pQCT bone structure deviations were found to be significant, explaining less than 5% variance. Between-modality SD for both model-dependent and model-independent Tb.Th was explained up to only 3% by intermodality angular deviation.
Intermodality Limits of Agreement From Bland-Altman Plots
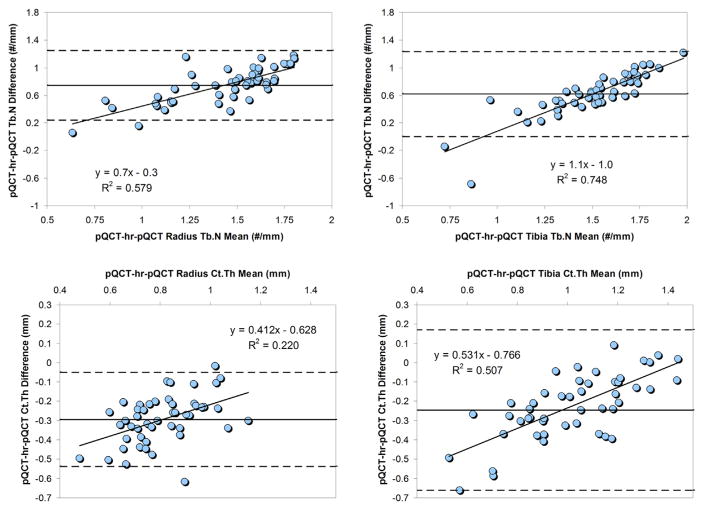
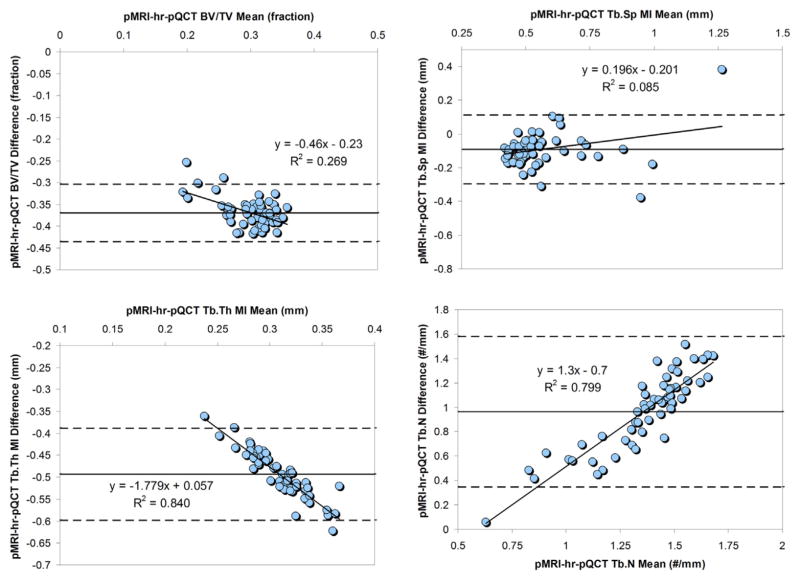
The pMRI-hr-pQCT limits of agreement for BV/TV and Tb.Sp were, on average, tighter than that for pQCT-hr-pQCT comparisons. The opposite was true for Tb.Th and Tb.N. pQCT showed significant correlations between mean bone values and degree of pQCT-hr-pQCT discrepancy at both the radius and the tibia (Figs. 2 and 3) particularly for BV/TV and Tb.Th. For pMRI, the same was true for Tb.Th and Tb.N (Fig. 4). A larger amount of bone in general seemed to be responsible for a larger intermodality deviation. In contrast, larger Tb.Sp was modestly associated with a larger intermodality difference for both pMRI and pQCT at the distal radius.
Fig. 2.
Bland-Altman plots for pQCT radius and tibia volumetric bone measure agreement with hr-pQCT. Bland-Altman plots for pQCT radius (left column) and tibia (right column) image-derived volumetric bone outcomes compared with hr-pQCT at the corresponding anatomical location. Dashed lines indicate upper and lower limits. Linear regression was performed on all means vs intermodality differences. Linear equations and regression coefficients are displayed in graphs where relevant. BV/TV, bone volume fraction; hr-pQCT, high-resolution peripheral quantitative computed tomography; MI, model-independent measures; pQCT, peripheral quantitative computed tomography; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation.
Fig. 3.
Bland-Altman plots for pQCT radius and tibia bone measure agreement with hr-pQCT. Bland-Altman plots for pQCT radius (left column) and tibia (right column) image-derived bone structural variables compared with hr-pQCT at the corresponding anatomical location. Dashed lines indicate upper and lower limits. Linear regression was performed on all means vs intermodality differences. Linear equations and regression coefficients are displayed in graphs where relevant. Ct.Th, cortical thickness; hr-pQCT, high-resolution peripheral quantitative computed tomography; MI, model-independent measures; pQCT, peripheral quantitative computed tomography; Tb.N, trabecular number.
Fig. 4.
Bland-Altman plots for pMRI radius bone structure agreement with hr-pQCT. Bland-Altman plots for pMRI bone structural variables compared with hr-pQCT were plotted with dashed lines indicating upper and lower limits. Linear regression was performed on all means vs intermodality differences. Linear equations and regression coefficients are displayed in graphs where relevant. BV/TV, bone volume fraction; hr-pQCT, high-resolution peripheral quantitative computed tomography; MI, model-independent measures; pMRI, peripheral magnetic resonance imaging; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
Short-term Precision Error
Of all volumetric bone measures, BV/TV was the most reproducible across all modalities followed by Tb.N, Tb.Th, and then Tb.Sp (Tables 5 and 6). Cortical vBMD appeared to be more reproducible than integral vBMD for pQCT at the radius. At the tibia, the opposite was true. Precision of model-dependent and model-independent measures were nearly identical to one another. Test-retest reproducibility for all volumetric bone measures appeared similar between pMRI and pQCT scans of the radius. Tibial bone variables for pQCT were, in general, more reproducible than radial bone variables. Coregistration and selection of anatomically analogous pMRI slices across participants resulted in only a slight improvement in reproducibility for all but Tb.Th and Tb.N (Table 5). Test-retest limits of agreement for BV/TV, Tb.Sp, and Tb.N were similar between pMRI and pQCT. Both pQCT and pMRI modalities also yielded trabecular connectivity and hole geometry measures, but because of its unavailability from hr-pQCT image analyses and poor test-retest reproducibility (>5%), the data were not reported.
Table 5.
pMRI Short-term Test-Retest Statistics for All Participants’ Full Image Sets With and Without Coregistration
| MRI bone variable | Un-coregistered
|
Coregistered
|
||
|---|---|---|---|---|
| RMSCV | RMSSD | RMSCV | RMSSD | |
| BV/TV (fraction) | 0.043 | 0.019 | 0.037 | 0.017 |
| Tb.Sp (mm) | 0.079 | 0.066 | 0.059 | 0.043 |
| Tb.Sp MI (mm) | 0.067 | 0.047 | 0.058 | 0.042 |
| Tb.Th (mm) | 0.059 | 0.033 | 0.070 | 0.043 |
| Tb.Th MI (mm) | 0.062 | 0.034 | 0.070 | 0.043 |
| Tb.N (number/mm) | 0.049 | 0.0 | 0.055 | 0.0 |
Note: Distal radius scans obtained twice at the same study visit were analyzed with and without coregistration. Root mean square coefficients of variation (RMSCV) and root mean square standard deviations (RMSSD) were reported.
Abbr: BV/TV, bone volume fraction; MI, model-independent measures; MRI, magnetic resonance imaging; pMRI, peripheral magnetic resonance imaging; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
Table 6.
pQCT and hr-pQCT Short-term Test-Retest Statistics
| pQCT distal slice | Distal radius
|
Distal tibia
|
||
|---|---|---|---|---|
| RMSCV | RMSSD | RMSCV | RMSSD | |
| BV/TV (fraction) | 0.035 | 0.010 | 0.016 | 0.010 |
| Tb.Sp (mm) | 0.041 | 0.030 | 0.025 | 0.010 |
| Tb.Sp MI (mm) | 0.041 | 0.030 | 0.025 | 0.010 |
| Tb.Th (mm) | 0.040 | 0.010 | 0.017 | 0.010 |
| Tb.Th MI (mm) | 0.040 | 0.010 | 0.017 | 0.010 |
| Tb.N (number/mm) | 0.029 | 0.0 | 0.016 | 0.0 |
| Ct.Th (mm) | 0.050 | 0.040 | 0.050 | 0.060 |
| vBMDi (mg/cm3) | 0.050 | 0.06 | 0.052 | 0.07 |
| vBMDc (mg/cm3) | 0.070 | 18.57 | 0.026 | 7.98 |
| vBMDtr (mg/cm3) | 0.030 | 26.12 | 0.011 | 10.32 |
| hr-pQCT volume | Radius
|
Tibia
|
||
|---|---|---|---|---|
| RMSCV | RMSSD | RMSCV | RMSSD | |
| BV/TV (fraction) | 0.007 | 0.001 | 0.004 | <0.000 |
| Tb.Sp (mm) | 0.048 | 0.023 | 0.041 | 0.022 |
| Tb.Th (mm) | 0.046 | 0.003 | 0.037 | 0.003 |
| Tb.N (mm−1) | 0.048 | 0.09 | 0.041 | 0.07 |
| Ct.Th (mm) | 0.013 | 0.010 | 0.005 | 0.006 |
| vBMDi (mg/cm3) | 0.005 | 1.77 | 0.002 | 0.76 |
| vBMDc (mg/cm3) | 0.005 | 3.93 | 0.002 | 1.73 |
| vBMDtr (mg/cm3) | 0.007 | 1.01 | 0.004 | 0.69 |
Note: Distal radius and tibia scans obtained twice at the same study visit were analyzed for root mean square coefficients of variation (RMSCV) and root mean square standard deviations (RMSSD) in the present study for pQCT (above). A separate study of participants with a mean age of 44.2 yr (range, 20–69 yr) with 25 women and 6 men were scanned twice at the same study visit on the same hr-pQCT scanner used in the present study, also reporting RMSCV and RMSSD values (below). Reproduced with permission from Cheung et al (27).
Abbr: BV/TV, bone volume fraction; Ct.Th, cortical thickness; hr-pQCT, high-resolution peripheral quantitative computed tomography; MI, model-independent measures; pQCT, peripheral quantitative computed tomography; vBMDc, cortical volumetric bone mineral density; vBMDi, integral volumetric bone mineral density; vBMDtr, trabecular volumetric bone mineral density; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
Discussion
Summary of Results
In the local cohort of women with a mean age of 74 ± 9 yr and body mass index of 27.65 ± 5.74 kg/m2, model-independent Tb.Sp from pMRI, Ct.Th, and vBMD from pQCT images all showed strong correlations with the corresponding values derived from hr-pQCT images. However, Bland-Altman analyses suggested a relationship between mean value of the outcome and degree of intermodality agreement for multiple pQCT- and pMRI-derived volumetric bone outcomes. The angular difference in anatomical orientation between MRI and hr-pQCT images made little impact on these linear relationships. Coregistration and selection of only anatomically comparable slices between participants resulted in an improvement in BV/TV correlation but worsened Tb.Sp correlation between pMRI and hr-pQCT. Short-term reproducibility of bone outcomes was highest for BV/TV and densitometric variables, and lowest for microstructural outcomes. Measurements at the tibia were more precise than those at the radius for pQCT images. Coregistration of repeated pMRI contributed to mild improvement in precision error.
Validity of Volumetric Bone Measurements From pQCT and 1.0 T pMRI
Tb.Sp
pMRI
Examining the same 9.5 mm site of the ultradistal radius in 52 women with a mean age of 55 yr, Kazakia et al (20) observed an R2 of 0.54 (B = 1.69) between hr-pQCT and 3T MRI (156 × 156 μm × 2 mm)–derived Tb.Sp that was slightly smaller than the present study (R2 = 0.65, B = 0.97). Folkesson et al (21) saw Tb.Sp correlations at the radius with hr-pQCT (R2 = 0.67, NS) similar to what was reported here using dual-threshold segmentation. However, using fuzzy logic cluster segmentation, an algorithm with the basis of pixel belongingness being similar to the image-foresting segmentation technique used on MRI here, the correlation became larger (R2 = 0.88, p < 0.001) (22). Like Krug et al (23), the present study demonstrated that model-independent measures derived from MRI better correlated with hr-pQCT equivalents than parallel plate model–derived measures (R2 = 0.92 vs 0.83, respectively).
pQCT
The stronger validity of Tb.Sp derived from pQCT images at the radius vs the tibia can be explained by the greater heterogeneity of bone at the radius vs the tibia, as shown by Calder et al (24). Thus, a single pQCT slice centered about the tibia may actually be reasonably representative of the full volume from hr-pQCT images. Unlike the high in vivo validity for Tb.Sp shown here, Lala et al (9) did not observe a significant correlation (R2 = 0.11, p = 0.22) ex vivo at the tibia.
BV/TV
pMRI
The bone-soft tissue segmentation threshold values used for pMRI were based on the surrounding soft tissue and were fixed for hr-pQCT. In both cases, what was defined as bone remained very similar. In contrast to threshold-based techniques, the image-foresting algorithm for pMRI segmented bone based on greyscale contrast, cost-minimizing functions, and Euclidean distances. Bone volume fraction was calculated as the trabecular vBMD divided by a mean of 1200 mg HA/cm3 for hr-pQCT and as the area of total bone divided by total mask area for pQCT and pMRI. Kazakia et al (20) observed a smaller correlation between 3T MRI and hr-pQCT–derived BV/TV (R2 = 0.25) than reported here (R2 = 0.53) at the ultradistal radius but underestimated BV/TV (B = 1.43). Using the fuzzy logic segmentation, Folkesson et al (22) found an R2 of 0.75 for BV/TV compared with an R2 of 0.39 when dual-threshold segmentation was used. In both cases, BV/TV was still underestimated vs hr-pQCT. Krug et al (23) showed that a spin echo sequence generated smaller BV/TV values (by more than 9%) compared with gradient echo sequences, as was used in the present study.
pQCT
A single slice from pQCT yielding a higher BV/TV may over represent the full volume examined on hr-pQCT because of the lack of bone in the more proximal region of the hr-pQCT volume of interest. However, the strong correlation for BV/TV between pQCT and hr-pQCT could enable pQCT to estimate BV/TV representing a full volume by using the derived slope and intercept. The same magnitude of correlation was demonstrated by the ex vivo validation study by Lala et al (9).
Tb.Th
Both pMRI and pQCT images have approximately 200 μm resolution. With only 1 or 2 pixels spanning most trabeculae, the interpolation of any Tb.Th value would be limited to a small range of values.
pMRI
For this reason, pMRI correlations were not significant, and slopes were near zero. The difference between fuzzy logic (MRI-hr-pQCT Tb.Th R2 = 0.15, p < 0.05) and dual-threshold segmentation (MRI-hr-pQCT Tb.Th R2 = 0.32, NS) techniques described by Folkesson et al (22) accounted for a 17% difference in Tb.Th validity. In either case, the correlations were larger than what was observed here using the image-foresting technique.
pQCT
No reports of Tb.Th validity were identified for pQCT.
Ct.Th and vBMD
pMRI
Kazakia et al (20) was also unable to measure Ct.Th in the distal half of the corresponding hr-pQCT radius volume on MRI. Although significant chemical shift artifact was noted at the radial cortex in MRI described here (Fig. 1, top), MRI observed from the articles by Kazakia et al (20) or Folkesson et al (22) did not display as dramatic a chemical shift (20,22,23) by virtue of the 10 times larger bandwidth compared with that used here.
pQCT
The poorer validity observed for Ct.Th and cortical vBMD for pQCT radius images could also be explained by poor spatial resolution. By averaging bone mass over an overestimated Ct.Th extrapolated by partial volume effects, the resultant cortical vBMD would be underestimated. However, in ex vivo tibia specimens, Lala et al (9) also demonstrated a higher validity for Ct.Th measured at the distal tibia (R2 = 0.83), which could be explained by the thicker cortex at this region of interest.
Overall
In addition to in-plane partial volume effects, the larger slice thickness of pQCT and pMRI was a major culprit for the inaccurate quantification of trabecular geometry. Within a thicker slice, bone and marrow signals are integrated during image reconstruction. Depending on the threshold selected for segmentation, these voxels may or may not be considered bone in the final analyses. The relationship between mean bone variable and intermodality deviation observed for Ct.Th, Tb.Th, and Tb.N could also be explained by the low variability of measurements under subvoxel conditions, thus minimizing deviation between modalities. The reason why this pattern was observed for BV/TV for pQCT, and not for pMRI, is because both pMRI and hr-pQCT average values over a larger volume including the more proximal locations where BV/TV is low. Thus, when pQCT yields lower BV/TV for certain individuals, it is likely that these values better represent the full volume from which BV/TV is computed for hr-pQCT.
Short-term Test-Retest Precision Error
The high reproducibility of BV/TV could be reconciled by the fact that this measurement was computed as a ratio, thus reducing the variance contribution of both bone and total volumes. Moreover, the fraction of bone may not differ as dramatically with repositioning as more minute features of bone such as trabecular geometries. Although a similarly reproducible BV/TV measure was observed using hr-pQCT images of the radius and tibia by Kazakia et al (20) and Boutroy et al (4), 3T MRI yielded a precision error of 6.7% for BV/TV at the radius, which was higher than all other bone outcomes (20). The fact that short-term precision errors for all measures were similar between pMRI and pQCT could in part be explained by the similar in-plane resolution. The increased error in computing bone perimeter after coregistration may be derived from the reduction in the number of slices averaged for analyses.
Short-term Reproducibility of hr-pQCT Bone Outcomes
Study participants were not scanned twice at baseline to quantify short-term precision error for hr-pQCT as this had already been done on the same hr-pQCT scanner by the same technician used for baseline hr-pQCT scans. These data were reported previously by Cheung et al and are shown in Table 6. In addition to having the smallest precision error out of the 3 modalities, hr-pQCT–derived bone outcomes’ RMSCV at the tibia was smaller than that at the radius. However, the mean age (44.2 yr) of this cohort was younger than the present study. Comparing data from the study by Cheung in which Ct.Th displayed a precision error of 1.3% at the radius and 0.5% at the tibia, Burghardt et al (25) saw a larger 3.9% error at the radius, and 1.5% error at the tibia in older women (mean age, 61 ± 10 yr).
Study Limitations
Short-term reproducibility could be poorer in those who are less mobile and unable to travel to complete hr-pQCT scans and who may confer additional error due to motion. The longer duration of pMRI scans (12 min acquisition + 5 min scan setup) led to a higher degree of motion and a consequential loss in image quality, translating to greater precision error and poorer validity of bone measures. Coregistration of pMRI was manually guided by anatomical features and matched slice-by-slice while only orthogonal transformations were applied. However, Blumenfeld et al (26) demonstrated that trabecular bone structural measures calculated from automatic vs manual registration methods did not differ significantly, and test-retest precision error for both methods remained well within 5%.
Recommendations
As it was noted that the longer scan time for a 2-slice pQCT acquisition may have resulted in more motion artifact, it is recommended that a single, more distal, slice be acquired to minimize the potential for more motion. pQCT presents as a feasible alternative to hr-pQCT for estimating apparent bone microstructure and volumetric density. The short-term results demonstrated here suggest that the derived measurements are valid and reasonably reproducible. Although the comparability of BV/TV, Tb.Th, Tb.N, and Ct.Th measures to hr-pQCT depended on mean value, this corollary is mostly related to differences in volumes of interest selection and resolutions. In comparison, pMRI-derived bone measures were unable to yield any densitometric information, generated less reproducible outcomes, but exhibited a similar degree of validity for the same measurements.
Acknowledgments
This three-part series is dedicated to Dr. Colin E. Webber. Andy Kin On Wong was funded by a Vanier CGS Doctoral Award at the time of this project (CGV-104858). All CaMOS participants are thanked for their dedication and over a decade of volunteerism. The CaMOS study staff are thanked for overseeing the operation of the parent CaMOS study.
References
- 1.Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int. 2011;22:357–362. doi: 10.1007/s00198-010-1226-1. [DOI] [PubMed] [Google Scholar]
- 2.Zanchetta JR, Bogado CE, Ferretti JL, et al. Effects of teriparatide [recombinant human parathyroid hormone (1–34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18(3):539–543. doi: 10.1359/jbmr.2003.18.3.539. [DOI] [PubMed] [Google Scholar]
- 3.Schneider P, Butz S, Allolio B, et al. Multicenter German reference data base for peripheral quantitative computer tomography. Technol Health Care. 1995;3(2):69–73. [PubMed] [Google Scholar]
- 4.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–6515. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 5.Wehrli FW, Song HK, Saha PK, Wright AC. Quantitative MRI for the assessment of bone structure and function. NMR Biomed. 2006;19(7):731–764. doi: 10.1002/nbm.1066. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang X, Selby K, Lang P, et al. High resolution magnetic resonance imaging of the calcaneus: age-related changes in trabecular structure and comparison with dual X-ray absorptiometry measurements. Calcif Tissue Int. 1997;60(2):139–147. doi: 10.1007/s002239900204. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmi G, De Serio A, Fusilli S, et al. Age-related changes assessed by peripheral QCT in healthy Italian women. Eur Radiol. 2000;10(4):609–614. doi: 10.1007/s003300050972. [DOI] [PubMed] [Google Scholar]
- 8.Sheu Y, Zmuda JM, Boudreau RM, et al. Bone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2011;26(1):63–71. doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lala D, Cheung AM, Lynch CL, et al. Measuring apparent trabecular structure with pQCT: a comparison with HR-pQCT. J Clin Densitom. 2014;17(1):47–53. doi: 10.1016/j.jocd.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 10.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29(10):1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Kreiger N, Tenenhouse A, Joseph L, et al. Research notes: the Canadian Multicentre Osteoporosis Study (CaMos)—background, rationale, methods. Can J Aging. 1999;18(3):12. [Google Scholar]
- 12.Pauchard Y, Liphardt AM, Macdonald HM, et al. Quality control for bone quality parameters affected by subject motion in high-resolution peripheral quantitative computed tomography. Bone. 2012;50(6):1304–1310. doi: 10.1016/j.bone.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 13.MacIntyre NJ, Adachi JD, Webber CE. In vivo measurement of apparent trabecular bone structure of the radius in women with low bone density discriminates patients with recent wrist fracture from those without fracture. J Clin Densitom. 2003;6(1):35–43. doi: 10.1385/jcd:6:1:35. [DOI] [PubMed] [Google Scholar]
- 14.Falcao AX, Bergo FP. Interactive volume segmentation with differential image foresting transforms. IEEE Trans Med Imaging. 2004;23(9):1100–1108. doi: 10.1109/TMI.2004.829335. [DOI] [PubMed] [Google Scholar]
- 15.Falcao AX, Stolfi J, de Alencar Lotufo R. The image foresting transform: theory, algorithms, and applications. IEEE Trans Pattern Anal Mach Intell. 2004;26(1):19–29. doi: 10.1109/tpami.2004.1261076. [DOI] [PubMed] [Google Scholar]
- 16.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24(1):35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 17.Parfitt AM, Mathews CH, Villanueva AR, et al. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983;72(4):1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gluer CC, Blake G, Lu Y, et al. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5(4):262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 20.Kazakia GJ, Hyun B, Burghardt AJ, et al. In vivo determination of bone structure in postmenopausal women: a comparison of HR-pQCT and high-field MR imaging. J Bone Miner Res. 2008;23(4):463–474. doi: 10.1359/jbmr.071116. [DOI] [PubMed] [Google Scholar]
- 21.Folkesson J, Carballido-Gamio J, Eckstein F, et al. Local bone enhancement fuzzy clustering for segmentation of MR trabecular bone images. Med Phys. 2010;37(1):295–302. doi: 10.1118/1.3264615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folkesson J, Goldenstein J, Carballido-Gamio J, et al. Longitudinal evaluation of the effects of alendronate on MRI bone microarchitecture in postmenopausal osteopenic women. Bone. 2011;48(3):611–621. doi: 10.1016/j.bone.2010.10.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krug R, Carballido-Gamio J, Burghardt AJ, et al. Assessment of trabecular bone structure comparing magnetic resonance imaging at 3 Tesla with high-resolution peripheral quantitative computed tomography ex vivo and in vivo. Osteoporos Int. 2008;19(5):653–661. doi: 10.1007/s00198-007-0495-9. [DOI] [PubMed] [Google Scholar]
- 24.Calder K, Inglis D, MacIntyre NJ. Comparison of pQCT-based measures of radial bone geometry and apparent trabecular bone structure using manufacturer and in-house developed algorithms. J Clin Densitom. 2010;13:433–440. doi: 10.1016/j.jocd.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Burghardt AJ, Buie HR, Laib A, et al. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–528. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenfeld J, Carballido-Gamio J, Krug R, et al. Automatic prospective registration of high-resolution trabecular bone images of the tibia. Ann Biomed Eng. 2007;35(11):1924–1931. doi: 10.1007/s10439-007-9365-z. [DOI] [PubMed] [Google Scholar]
- 27.Cheung AM, Chan C, Ahmed F, et al. ISCD, editor. Intra-operator precision for in vivo high resolution pQCT scans. International Society for Clinical Densitometry 14th Annual Meeting; San Francisco, CA. 2008. pp. 12–15. [Google Scholar]