Abstract
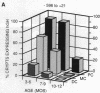
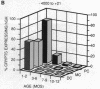
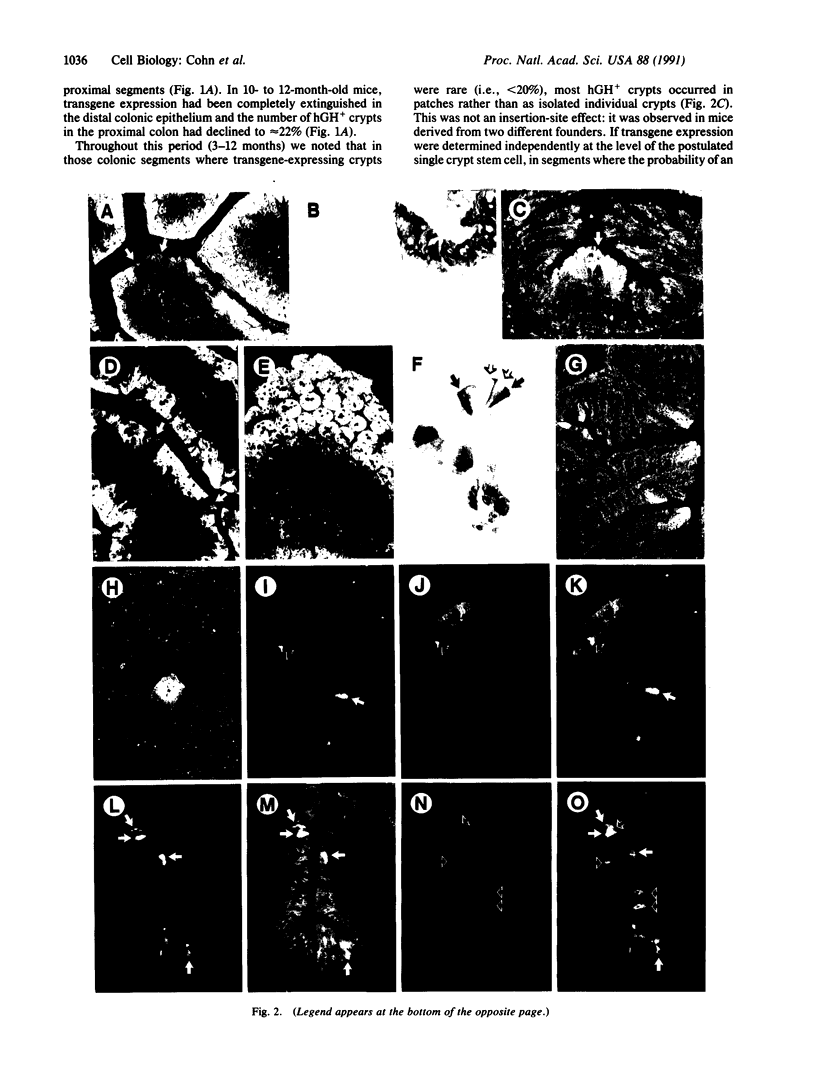
We have used liver fatty acid-binding protein/human growth hormone (L-FABP/hGH) fusion genes to explore the temporal and spatial differentiation of intestinal epithelial cells in 1- to 12-month-old transgenic mice. The intact, endogenous L-FABP gene (Fabpl) was not expressed in the colon at any time. Young adult transgenic mice containing nucleotides -596 to +21 of the rat L-FABP gene linked to the hGH gene (minus its 5' nontranscribed domain) demonstrated inappropriate expression of hGH in enterocytes and many enteroendocrine cells of most proximal and mid-colonic crypts (glands). Rare patches of hGH-negative crypts were present. With increasing age, a wave of "extinction" of L-FABP (-596 to +21)/hGH expression occurred, first in the distal colon and then in successively more proximal regions, leaving by 10 months of age only rare hGH-positive multicrypt patches. At no time during this progressive silencing of transgene expression were crypts observed that contained a mixture of hGH-positive and -negative cells at a particular cell stratum. Young (5-7 weeks) mice containing a L-FABP (-4000 to +21)/hGH transgene also demonstrated inappropriate expression of the transgene in most proximal colonic crypts. However, the additional 3.3 kilobases of upstream sequence resulted in much more rapid extinction of reporter expression, leaving by 5 months of age only scattered single crypts with detectable levels of hGH. This age-related extinction of L-FABP/hGH expression did not involve enterocytes and enteroendocrine cells in the (proximal) small intestine. These results indicate that cis-acting elements outside of nucleotides -4000 to +21 are necessary to fully modulate suppression of colonic L-FABP expression. They also define fundamental changes in colonic epithelial cell populations during adult life. Our data suggest that (i) a single stem cell gives rise to all cells that populate a given colonic crypt, (ii) stem cells represented in several adjacent crypts may be derived from a common progenitor, and (iii) such a progenitor cell may repopulate colonic crypts with stem cells during adult life. Since each colonic crypt contains the amplified descendants of its stem cell, transgenes may be powerful tools for characterizing the spatial and biological features of gut stem cells and their progenitors during life.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gordon J. I. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989 Apr;108(4):1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D. F., Davies S. J., Williams D., Williams G. T., Williams E. D. Demonstration of somatic mutation and colonic crypt clonality by X-linked enzyme histochemistry. Nature. 1988 Jun 2;333(6172):461–463. doi: 10.1038/333461a0. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Festing M. F., Wilkinson M. M. An allelic difference determines reciprocal patterns of expression of binding sites for Dolichos biflorus lectin in inbred strains of mice. J Embryol Exp Morphol. 1985 Jun;87:229–239. [PubMed] [Google Scholar]
- Ponder B. A., Schmidt G. H., Wilkinson M. M., Wood M. J., Monk M., Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985 Feb 21;313(6004):689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Gordon J. I. Spatial differentiation of the intestinal epithelium: analysis of enteroendocrine cells containing immunoreactive serotonin, secretin, and substance P in normal and transgenic mice. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6408–6412. doi: 10.1073/pnas.87.16.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth K. A., Hertz J. M., Gordon J. I. Mapping enteroendocrine cell populations in transgenic mice reveals an unexpected degree of complexity in cellular differentiation within the gastrointestinal tract. J Cell Biol. 1990 May;110(5):1791–1801. doi: 10.1083/jcb.110.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. H., O'Sullivan J. F., Paul D. Ethylnitrosourea-induced mutations in vivo involving the Dolichos biflorus agglutinin receptor in mouse intestinal epithelium. Mutat Res. 1990 Feb;228(2):149–155. doi: 10.1016/0027-5107(90)90071-b. [DOI] [PubMed] [Google Scholar]
- Schmidt G. H., Wilkinson M. M., Ponder B. A. Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell. 1985 Feb;40(2):425–429. doi: 10.1016/0092-8674(85)90156-4. [DOI] [PubMed] [Google Scholar]
- Schmidt G. H., Wilkinson M. M., Ponder B. A. Non-random spatial arrangement of clone sizes in chimaeric retinal pigment epithelium. J Embryol Exp Morphol. 1986 Feb;91:197–208. [PubMed] [Google Scholar]
- Schmidt G. H., Winton D. J., Ponder B. A. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988 Aug;103(4):785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Birkenmeier E. H., Hoppe P. C., McKeel D. W., Gordon J. I. Mechanisms underlying generation of gradients in gene expression within the intestine: an analysis using transgenic mice containing fatty acid binding protein-human growth hormone fusion genes. Genes Dev. 1988 Oct;2(10):1318–1332. doi: 10.1101/gad.2.10.1318. [DOI] [PubMed] [Google Scholar]
- Winton D. J., Blount M. A., Ponder B. A. A clonal marker induced by mutation in mouse intestinal epithelium. Nature. 1988 Jun 2;333(6172):463–466. doi: 10.1038/333463a0. [DOI] [PubMed] [Google Scholar]
- Winton D. J., Peacock J. H., Ponder B. A. Effect of gamma radiation at high- and low-dose rate on a novel in vivo mutation assay in mouse intestine. Mutagenesis. 1989 Sep;4(5):404–406. doi: 10.1093/mutage/4.5.404. [DOI] [PubMed] [Google Scholar]
- Winton D. J., Ponder B. A. Stem-cell organization in mouse small intestine. Proc Biol Sci. 1990 Jul 23;241(1300):13–18. doi: 10.1098/rspb.1990.0059. [DOI] [PubMed] [Google Scholar]