Abstract
The amygdalar nuclear complex and hippocampal/parahippocampal region are key components of the limbic system that play a critical role in emotional learning and memory. This review discusses what is currently known about the neuroanatomy and neurotransmitters involved in amygdalohippocampal interconnections, their functional roles in learning and memory, and their involvement in mnemonic dysfunctions associated with neuropsychiatric and neurological diseases. Tract tracing studies have shown that the interconnections between discrete amygdalar nuclei and distinct layers of individual hippocampal/parahippocampal regions are robust and complex. Although it is well-established that glutamatergic pyramidal cells in the amygdala and hippocampal region are the major players mediating interconnections between these regions, recent studies suggest that long-range GABAergic projection neurons are also involved. Whereas neuroanatomical studies indicate that the amygdala only has direct interconnections with the ventral hippocampal region, electrophysiological studies and behavioral studies investigating fear conditioning and extinction, as well as amygdalar modulation of hippocampal-dependent mnemonic functions, suggest that the amygdala interacts with dorsal hippocampal regions via relays in the parahippocampal cortices. Possible pathways for these indirect interconnections, based on evidence from previous tract tracing studies, are discussed in this review. Finally, memory disorders associated with dysfunction or damage to the amygdala, hippocampal region, and/or their interconnections are discussed in relation to Alzheimer's disease, posttraumatic stress disorder (PTSD), and temporal lobe epilepsy.
Keywords: amygdala, hippocampus, parahippocampal region, tract tracing, fear conditioning, fear extinction
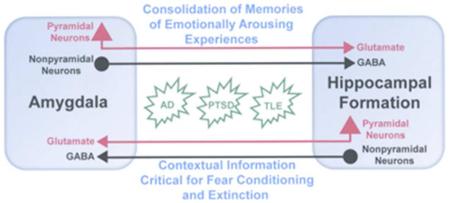
Graphical Abstract
This review discusses the neuroanatomy of amygdalohippocampal interconnections, including the participation of glutamatergic pyramidal neurons (red) and GABAergic nonpyramidal neurons (black), their functional roles in learning and memory (blue), and their involvement in the memory impairments seen in Alzheimer's disease (AD), posttraumatic stress disorder (PTSD), and temporal lobe epilepsy (TLE).
Introduction
The amygdalar nuclear complex and hippocampal/parahippocampal region are key components of the limbic system that play a critical role in emotion, learning and memory, and complex behavior. The interconnections between discrete amygdalar nuclei and distinct hippocampal/parahippocampal regions are robust and complex. There has been a resurgence of interest in the finer details of the anatomy of these interconnections with the advent of optogenetic techniques to selectively activate different components of amygdalohippocampal circuits in brain slices and conscious behaving animals (Goshen et al., 2011; Sparta et al., 2014; Hübner et al., 2014; Felix-Ortiz and Tye, 2013, 2014; Bazelot et al., 2015). This review discusses what is currently known about the neuroanatomy and neurotransmitters involved in amygdalohippocampal interconnections, their functional roles in learning and memory, and their involvement in mnemonic dysfunction in Alzheimer's disease, posttraumatic stress disorder (PTSD), and temporal lobe epilepsy.
Anatomy of the amygdalar nuclear complex and hippocampal region
In humans and non-human primates the amygdalar nuclear complex and hippocampal region are juxtaposed in the anterior part of the medial temporal lobe, with the amygdala located just in front of the hippocampus. In rodents and other mammals with less developed neocortices they exhibit a similar juxtaposition in the caudal halves of the cerebral hemispheres. In all mammals the amygdalar nuclear complex consists of over a dozen nuclei, each with distinct extrinsic and intra-amygdalar connections (Price et al., 1987; Pitkänen, 2000; Fig. 1A). Several different nomenclatures are used to denote the nuclei of the rat amygdala, the main focus of this review (Table 1). While the text of this review will mainly use the nomenclature of the Paxinos and Watson atlas (1997), some of the figures use other nomenclatures. Both the cortical amygdalar nuclei (anterior, posterolateral, and posteromedial cortical nuclei, and nucleus of the lateral olfactory tract) and the basolateral amygdalar nuclear complex (BLC; lateral, basolateral, and basomedial nuclei, and the amygdalohippocampal area), which are located deep to the cortical nuclei, have cortex-like cell types. As in the cortex, the projection neurons of the cortical and basolateral nuclei, which constitute about 85% of neurons, are glutamatergic pyramidal or pyramidal-like neurons with spiny dendrites (McDonald, 1992a, b). The remaining neurons in the cortical and basolateral nuclei are mainly GABAergic nonpyramidal neurons with spine-sparse dendrites. Most of the nonpyramidal neurons appear to be interneurons and, as in the cerebral cortex, several distinct subpopulations can be distinguished on the basis of their content of calcium-binding proteins and peptides, including: (1) parvalbumin (PV), (2) somatostatin (SOM), (3) vasoactive intestinal peptide (VIP), and (4) cholecystokinin (CCK) (Kemppainen and Pitkänen, 2000; McDonald and Betette, 2001; McDonald and Mascagni, 2001, 2002; Mascagni and McDonald, 2003; Mascagni et al., 2009; Spampanato et al., 2010). In addition, it has been demonstrated that the expression of neuropeptide Y (NPY) defines a distinct subpopulation of SOM+ nonpyramidal neurons (McDonald, 1989; McDonald et al., 1995). In contrast, the so called “extended amygdalar nuclei” consisting of the central and medial amygdalar nuclei, as well as areas in the bed nucleus of the stria terminalis (BNST) with phenotypically-similar neurons, contain predominantly GABAergic spiny projection neurons, like the striatum (McDonald, 1992a, 2003).
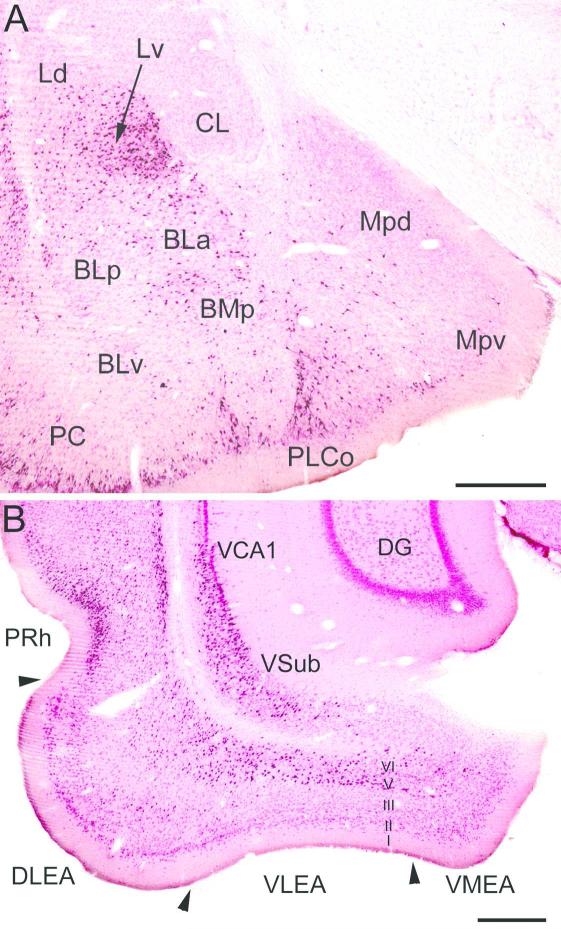
Fig. 1.
(A) Photomicrograph of retrogradely-labeled neurons in the amygdala (black) at the bregma −3.1 level in a rat that received an injection of Fluorogold (FG) into the dorsolateral entorhinal area (DLEA) (immunoperoxidase technique with pink pyronin Y Nissl counterstain). The nomenclature used to denote the amygdalar nuclei is that used in the atlas by Paxinos and Watson (1997) (see Table 1). Abbreviations: BLa, anterior basolateral nucleus; BLp, posterior basolateral nucleus; BLv, ventral basolateral nucleus; BMp, posterior basomedial nucleus; CL, lateral central nucleus; Ld, dorsolateral lateral nucleus; Lv, ventromedial lateral nucleus; Mpd, posterodorsal medial nucleus; Mpv, posteroventral medial nucleus; PC, piriform cortex; PLCo, posterolateral cortical nucleus. (B) Photomicrograph showing the locations of FG+ retrogradely-labeled neurons (black) in the ventral hippocampus and parahippocampal area at the bregma −6.3 level in a rat that received an injection of FG into the basolateral amygdalar complex (immunoperoxidase technique with pink pyronin Y Nissl counterstain). Note dense retrograde labeling in the perirhinal cortex (PRh; mainly layers II and VI), the deep layers (layers V and VI) of the dorsolateral and ventrolateral entorhinal cortex (DLEA and VLEA, respectively), and the ventral subiculum (VSub) and adjacent ventral part of CA1 (VCA1). Fewer FG+ neurons are seen in the ventromedial entorhinal cortex (VMEA) and layer III of the DLEA and PRh. Scale bars = 500 μm. Modified from McDonald and Zaric, 2015a, b.
Table 1.
The main nuclei of the rat amygdala in the atlas by Paxinos and Watson (1997), and the alternative nomenclatures used by Price and co-workers (1987), and in the atlas by Swanson (2004).
| Nuclei of Paxinos and Watson (1997) | Nuclei of Price et al., 19871 | Nuclei of Swanson (2004) |
|---|---|---|
| Amygdalohippocampal area (AHA) | Amygdalohippocampal area (AHA) | Posterior (PA) |
| Anterior amygdaloid area (AA) | Anterior amygdaloid area (AAA) | Anterior amygdaloid area (AAA) |
| Anterior basolateral (BLa) | Basal magnocellular (Bmg) | Anterior basolateral (BLAa) |
| Posterior basolateral (BLp) | Basal parvicellular (Bpc) | Posterior basolateral (BLAp) |
| Anterior basomedial (BMa) | Anterior cortical (Coa; deep part) | Anterior basomedial (BMAa) |
| Posterior basomedial (BMp) | Accessory basal (AB) | Posterior basomedial (BMAp) |
| Central (Ce) | Central (Ce) | Central (CEA) |
| Anterior cortical (ACo) | Anterior cortical (Coa; superficial part) | Anterior cortical (COAa) |
| Posterolateral cortical (PLCo) | Periamygdaloid cortex (PAC) | Posterolateral cortical (COApl) |
| Posteromedial cortical (PMCo) | Posterior cortical (Cop) | Posteromedial cortical (COApm) |
| Intercalated (IN) | Intercalated (IN) | Intercalated (IA) |
| Lateral (L) | Lateral (L) | Lateral (LA) |
| Nucleus of the lateral olfactory tract (LOT) | Nucleus of the lateral olfactory tract (NLOT) | Nucleus of the lateral olfactory tract (NLOT) |
| Anterodorsal medial (Mad) | Dorsocentral medial (Mcd) | Anterodorsal medial (MEAad) |
| Anteroventral medial (Mav) | Rostral medial (Mr) | Anteroventral medial (MEAav) |
| Posterodorsal medial (Mpd) | Caudal medial (Mc) | Posterodorsal medial (MEApd) |
| Posteroventral medial (Mpv) | Caudoventral medial (Mcv) | Posteroventral medial (MEApv) |
Medial nuclei subdivided according to Pitkanen, 2000
Each nucleus of the amygdala has a characteristic set of interconnections with extrinsic brain regions, as well as with other amygdalar nuclei (Pitkänen, 2000). The amygdala receives sensory information from all sensory modalities via inputs from the cortex and thalamus, including complex polymodal contextual inputs from the hippocampal region (McDonald, 1998). The cortical and medial nuclei mainly have interconnections with olfactory areas, including the olfactory bulb, whereas the BLC has additional interconnections with cortical association areas that process non-olfactory sensory information. The BLC has one-way projections to the central nucleus and striatum, which serve as output pathways for motor/autonomic responses and emotional behavior (LeDoux, 2000; McDonald, 1992a, 2003).
The term hippocampal region, as used in this review, consists of the hippocampal formation (dentate gyrus, cornu ammonis fields CA1-CA3, and subiculum) as well as the adjacent parahippocampal cortices (including the entorhinal cortex, perirhinal cortex, postrhinal cortex, parasubiculum and presubiculum) (Fig. 1B). In all mammals information is transmitted through the hippocampal region by a series of connections that includes the so-called “trisynaptic circuit” of the hippocampus (Witter et al., 1989, 2000). Olfactory inputs and highly-processed information from sensory association cortices target the perirhinal and postrhinal cortices. The latter areas in turn project to the entorhinal cortex (ERC), which has been called the gateway to the hippocampal formation. The efferents of the ERC to the hippocampal formation, which constitute the perforant pathway, target the hippocampus, including the dentate gyrus. Following the processing of information by the classic trisynaptic circuit of the hippocampus, the subiculum then projects back to the ERC. The ERC then sends information, including mnemonic information, processed by the hippocampus to the neocortex via a relay in the perirhinal and postrhinal cortices. These structures and circuits of the hippocampal region are critical for memory formation and constitute the medial temporal lobe memory system (MTLMS; Squire and Zola-Morgan, 1991). As discussed in this review the amygdala has extensive, complex interconnections with the MTLMS that are critical for emotional memory.
Anatomy of hippocampal/parahippocampal projections to the amygdala
The connectivity of the amygdala with the hippocampal region is very complex, involving interconnections of discrete amygdalar nuclei with distinct hippocampal/parahippocampal laminae in all species studied. Interconnections between the amygdala and hippocampal/parahippocampal areas in the rat and other species have been discussed in previous reviews (e.g., Price et al., 1987; Witter, et al., 1989; McDonald, 1998; McDonald et al. 1999; Pitkänen, 2000; Pitkänen et al., 2000; Petrovich et al., 2001). The following account is based on these reviews and the references therein, as well as on more recent studies, and focuses on the connections seen in the rat (see these reviews for additional details and references). Virtually all of the information on the anatomy of these interconnections in the rat was obtained from anterograde tracing studies. There have been few studies in which sensitive retrograde tracers such as Fluorogold or cholera toxin B have been injected into discrete amygdalar nuclei or hippocampal/parahippocampal areas.
The hippocampal region consists of polymodal association areas that integrate highly-processed sensory information from all sensory modalities into complex configurational representations such as context. Projections from the hippocampal formation proper to the amygdala arise solely from CA1 and the subiculum (Fig. 2; Pitkänen, 2000; Pitkänen et al., 2000; McDonald, 1998), both of which receive outputs from the trisynaptic circuit. There are no projections to the amygdala from the dentate gyrus, CA3, or CA2. Moreover, the inputs from CA1 and the subiculum arise only from the temporal part of the septotemporal axis of the hippocampal formation (i.e., the ventral subiculum and adjacent portions of CA1). This ventral portion of the rodent hippocampus, which corresponds to the anterior part of the primate hippocampus, is mainly involved in stress, emotion, and affect, whereas the dorsal hippocampus, which corresponds to the posterior part of the primate hippocampus, performs primarily cognitive functions (Fanselow and Dong, 2010; Strange et al., 2014). The ventral subiculum (VSub) has widespread projections to the amygdala that target the BLC, cortical nuclei, and anterior portions of the medial nucleus (Figs. 2 and 3; Canteras and Swanson, 1992; McDonald, 1998; Kishi et al., 2006). Most portions of the central nucleus receive only light projections. The ventral part of CA1 (VCA1), located adjacent to the VSub, has projections to the amygdala that are lighter than those of VSub. The rostral portion of VCA1 mainly targets the amygdalohippocampal area and medial part of the anterior basolateral nucleus (BLa), whereas the caudal part of VCA1 has more widespread projections that target most amygdalar nuclei with the exception of the caudal medial nucleus, rostral part of the BLC, and most portions of the central nucleus (Kishi et al., 2006). In general, the rostral-to-caudal axis of origin of VSub/VCA1 projections corresponds to the medial-to-lateral axis of termination in the amygdala (Kishi et al., 2006). Electron microscopic studies have shown that the projections from the VSub/VCA1 to the BLC mainly form asymmetrical (excitatory) synapses with distal dendrites and spines of pyramidal cells, but dendrites of GABAergic interneurons are also targeted (Müller et al., 2011; Bazelot et al., 2015). A recent in vivo study that combined optogenetic stimulation of VCA1 pyramidal neurons with recording in the BLa found that some of the interneurons that received excitatory inputs from VCA1 mediated feedforward inhibition onto neighboring BLa pyramidal neurons (Bazelot et al., 2015).
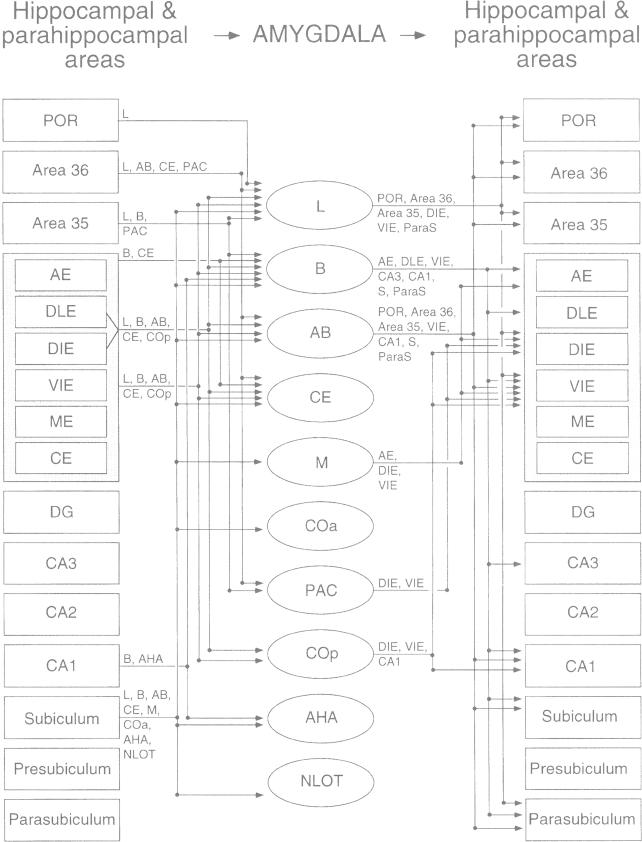
Fig. 2.
Summary of inputs and outputs of the amygdala with the hippocampal region in the rat. The nomenclature used to denote the amygdalar nuclei is that used by Price and coworkers (1987) (see Table 1), and the nomenclature of entorhinal areas (AE, DLE, DIE, VIE, ME, and CE) is that used by Insausti and coworkers (1997) (see text). Only projections that are moderate to heavy in density are illustrated. Abbreviations (with nomenclature of Paxinos and Watson [1997] in parentheses): AB, accessory basal nucleus (posterior basomedial nucleus); AE, amygdalo-entorhinal transitional area (amygdalopiriform transition area); AHA, amygdalohippocampal area; Areas 36 and 35, dorsal and ventral perirhinal areas; B, basal nucleus (basolateral nucleus); CE entorhinal area, caudal entorhinal area; CE amygdalar nucleus, central amygdalar nucleus; COa, anterior cortical nucleus; Cop, posterior cortical nucleus (posteromedial cortical nucleus); DIE, dorsal intermediate entorhinal area (DLEA of Krettek and Price, 1977); DG, dentate gyrus; DLE, dorsal lateral entorhinal area; L, lateral nucleus; M, medial nucleus; ME, medial entorhinal area; NLOT, nucleus of the lateral olfactory tract; PAC, periamygdalar cortex (posterolateral cortical nucleus); POR, postrhinal cortex; VIE, ventral intermediate entorhinal area (VLEA of Krettek and Price, 1977). Reproduced from Pitkänen et al. (2000) with permission.
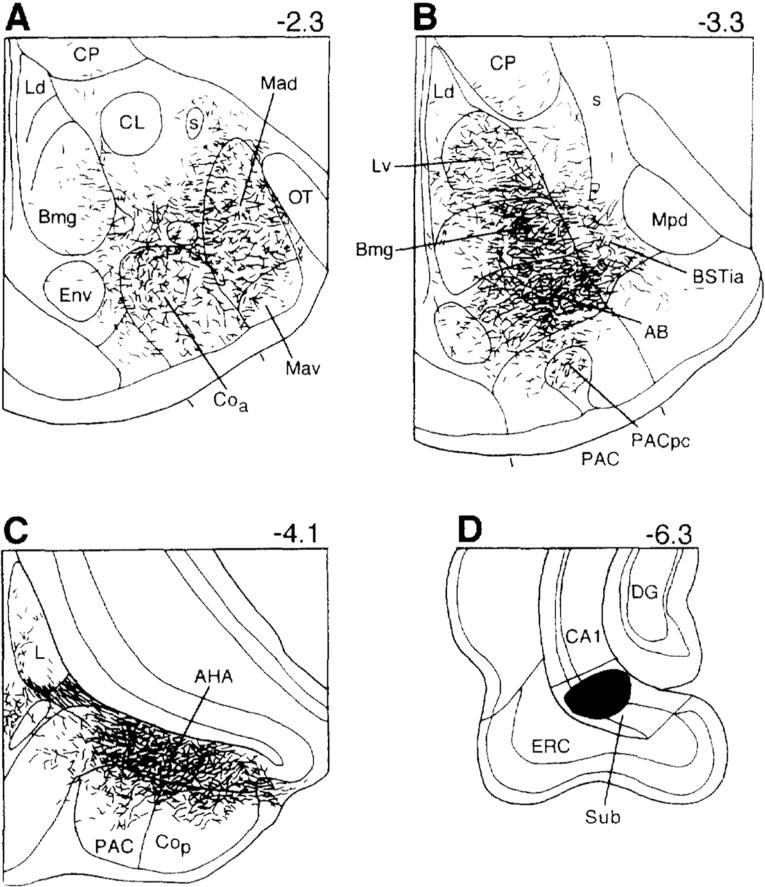
Fig. 3.
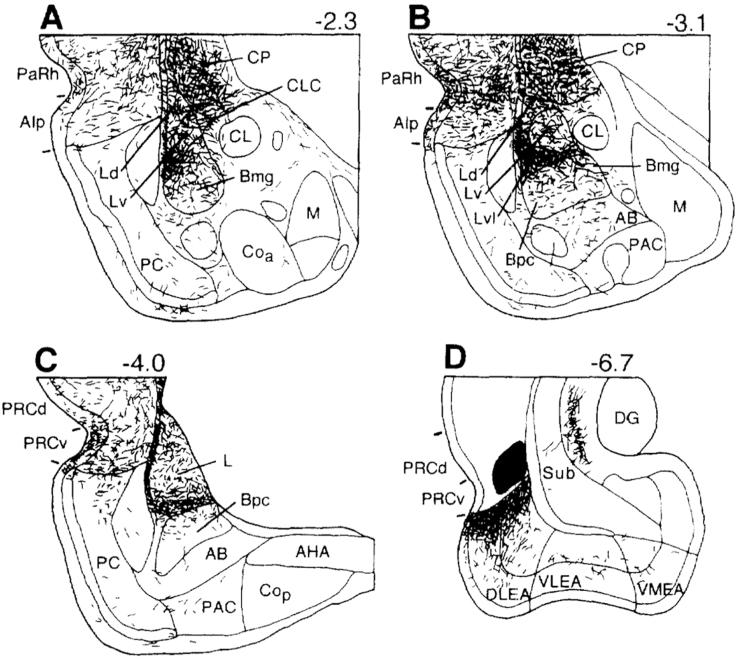
Drawing of coronal sections arranged from rostral (A) to caudal (C) showing terminations of labeled axons in the rat amygdala seen with an injection of the anterograde tracer PHA-L (D) into the ventral subiculum in the rat. Section D was drawn at half the magnification of sections A-C. Bregma levels are indicated in upper right corner for each level. The nomenclature used to denote the amygdalar nuclei is mainly that used by Price and coworkers (1987) (see Table 1). Reproduced from McDonald (1998) with permission.
The entorhinal, perirhinal, and postrhinal cortices of the parahippocampal region have widespread and complex projections to the amygdala. Entorhinal projections to the amygdala arise mainly from the lateral entorhinal cortex (LERC). Krettek and Price (1977) divided the rat LERC into three fields arranged from lateral to medial, termed the dorsolateral (DLEA), ventrolateral (VLEA), and ventromedial (VMEA) entorhinal areas (Fig. 1B). The DLEA and VLEA correspond, respectively, to the dorsal intermediate (DIE) and ventral intermediate (VIE) entorhinal fields of Insausti and coworkers (1997). The rostral portion of the VLEA of Krettek and Price (1977), located just caudolateral to the amygdala, corresponds to the “amygdalo-entorhinal transitional field” (AE) of Insausti and coworkers (1997), the “postpiriform transition area” of Petrovich and coworkers (2001), and the “amygdalopiriform transitional area” (APIR) of Paxinos and Watson (1997); it is arguably not a portion of the entorhinal cortex since it does not have projections to the dentate gyrus, although it does project to temporal portions of CA3, CA1, and the subiculum (Jolkonen et al., 2001).
Most of the projections of the LERC to the amygdala arise from layers 5 and 6 of the DLEA and VLEA (Fig. 1B). These deep layers of the LERC do not have significant projections to the hippocampal formation, but instead receive outputs from the hippocampus (Amaral and Witter, 1995). The main amygdalar targets of the DLEA and VLEA are the BLC, lateral capsular portion of the central nucleus (CLC), and all three subdivisions of the cortical nuclear complex (Figs. 2 and 4). These projections are topographically organized such that the lateral-to-medial gradient in the LERC origins of these projections coincides with the lateral-to-medial gradient in their terminations in the BLC and cortical amygdalar complex (McDonald and Mascagni, 1997; Fig. 4). APIR is distinct from more caudal portions of the entorhinal region by having very widespread projections to the amygdala and robust projections to the lateral subdivision of the central nucleus and its “homolog” in the bed nucleus of the stria terminalis (BNST), the dorsolateral subdivision of the BNST (McDonald et al., 1999; Jolkonen et al., 2001). Both the dorsal (area 36) and ventral (area 35) portions of the perirhinal cortex project to the BLC (especially the lateral nucleus), lateral capsular portion of the central nucleus (CLC), and the posterolateral cortical nucleus (Copl) (McDonald, 1998; Shi and Cassell, 1999) (Figs. 2 and 5). The postrhinal cortex has a projection to the lateral nucleus (Fig. 2). Electron microscopic studies have shown that the projections from the entorhinal and perirhinal cortices to the lateral and basolateral amygdalar nuclei form asymmetrical (excitatory) synapses with spines, and to a lesser extent distal dendrites, of pyramidal cells (Smith et al., 2000).
Fig. 4.
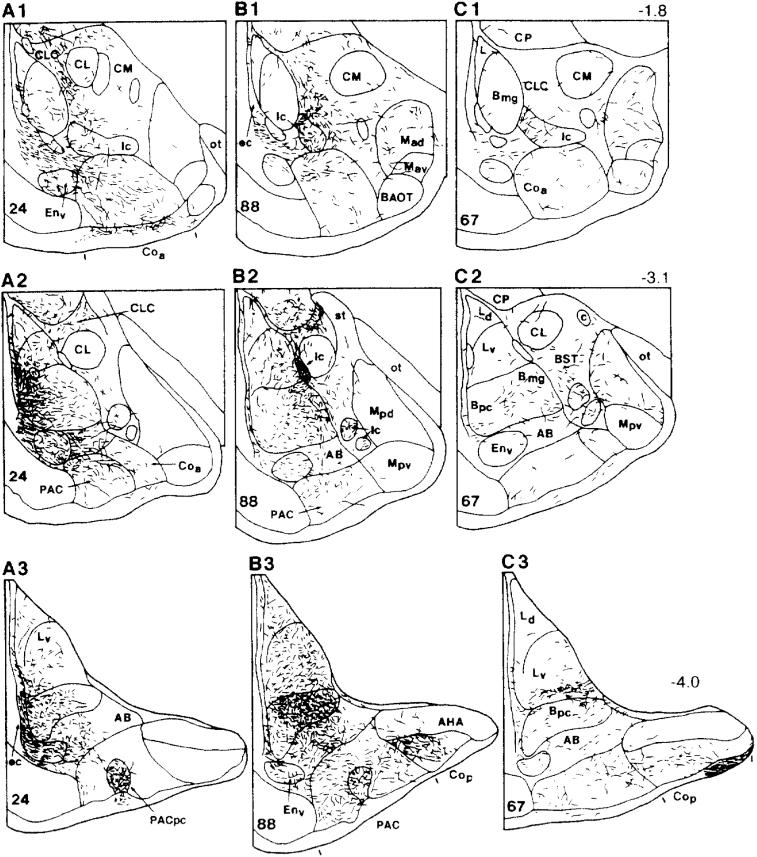
Drawing illustrating the terminations of labeled axons in the rat amgydala in cases with injections of the anterograde tracer PHA-L into the DLEA (A1-A3), VLEA (B1-B3), and VMEA (C1-C3). Bregma levels are indicated in upper right corner for each level. The nomenclature used to denote the amygdalar nuclei is mainly that used by Price and coworkers (1987) (see Table 1). Reproduced with modification from McDonald and Mascagni (1997) with permission.
Fig. 5.
Drawing of coronal sections arranged from rostral (A) to caudal (C) showing terminations of labeled axons in the rat amygdala seen with an injection of the anterograde tracer PHA-L (D) into the perirhinal cortex in the rat. In section D the fibers in the perirhinal cortex and dorsally adjacent neocortex were not drawn. Bregma levels are indicated in upper right corner for each level. The nomenclature used to denote the amygdalar nuclei is mainly that used by Price and coworkers (1987) (see Table 1). Reproduced from McDonald (1998) with permission.
Anatomy of amygdalar projections to hippocampal/parahippocampal areas
The projections from the nuclei of the amygdala to hippocampal/parahippocampal areas largely reciprocate the projections of hippocampal/parahippocampal areas to the amygdala with the exception of the central nucleus and the dorsolateral BNST. The central nucleus and the dorsolateral BNST have spiny GABAergic neurons that closely resemble the medium spiny neurons of the striatum (McDonald, 1982, 1992a; 2003). Like the striatum these amygdalar nuclei receive cortical inputs but do not project back to the cortex (McDonald, 2003). In addition, the intercalated nuclei and the rostral pole of BLa do not project to hippocampal/parahippocampal areas. The remaining amygdalar nuclei project to various hippocampal/parahippocampal areas and target distinct layers in these regions (Fig. 6), thus interacting with different sets of extra-amygdalar inputs to these layers (see Pitkänen et al. [2000] and Petrovich et al. [2001] for a detailed description of these laminar terminations). Since each amygdalar nucleus receives convergent inputs from a characteristic set of sensory association areas and thalamic nuclei, and also has a characteristic set of intra-amygdalar connections, each amygdalar nucleus would appear to transmit distinct information to its targets in the hippocampal region. In regards to emotional learning and memory, this information would pertain to the emotional salience of sensory stimuli, based on the association of these stimuli with aversive stimuli or reward in the amygdala.
Fig. 6.
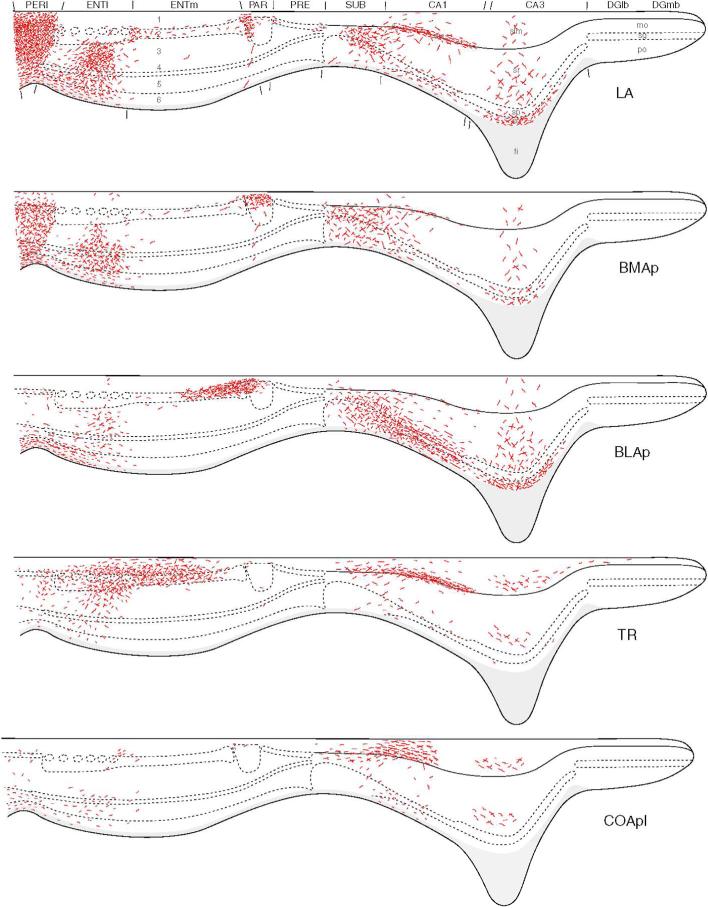
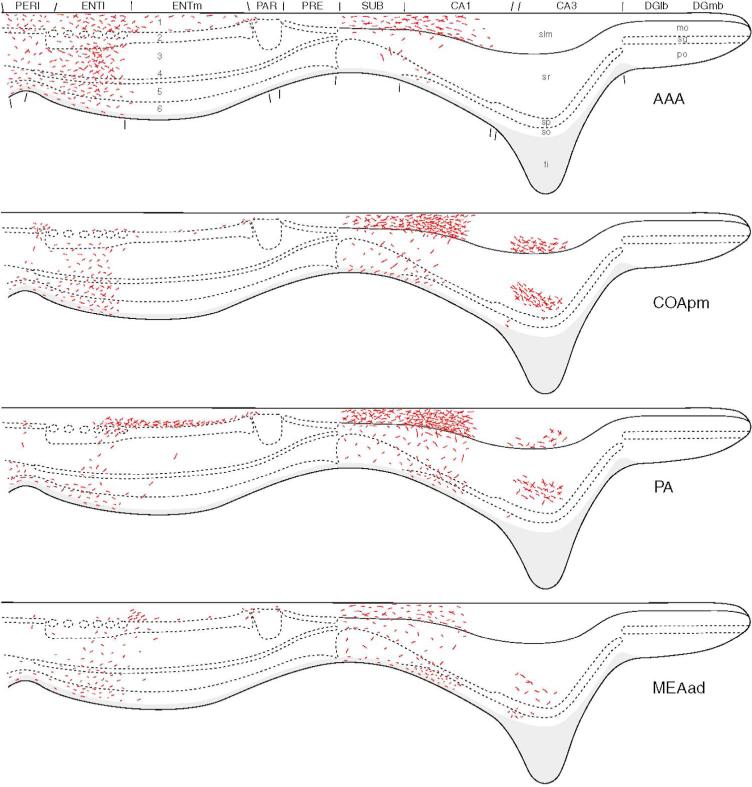
Summary of the laminar distributions of amygdalar inputs (red) to the hippocampal region based on the results of PHA-L anterograde tracing studies. The findings are illustrated on an idealized section through an unfolded “flatmap” of the hippocampal region (see Petrovich et al., 2001, for details). The nomenclature used to denote the amygdalar nuclei is that used by Swanson (2004) (see Table 1). The postpiriform transition area, TR, corresponds to APIR of Paxinos and Watson (1997). Reproduced from Petrovich et al, 2001.
The dentate gyrus receives no direct inputs from the amygdala (Figs. 2, 6), and has no direct projections to the amygdala (see above). Therefore, all information transfer between these two structures is indirect. However the fact that injections of the excitatory amino acids into the amygdala, or electrical stimulation of the basolateral amygdala, induce c-fos expression in the DG clearly indicates that indirect pathways do exist (Cahill and McGaugh, 1998; Nakao et al., 2004). Although CA3 and CA2 do not have projections to the amygdala, the temporal parts of CA3 and CA2 receive projections from the amygdala. Pikkarainen and coworkers (1999) using PHA-L as an anterograde tracer found that the only amygdalar nucleus with significant projections to the temporal part of CA3 was the caudomedial part of the posterior basolateral nucleus (BLp). However, in a later PHA-L study, Petrovich and coworkers (2001) reported significant projections from the lateral, posterior basolateral, amygdalohippocampal, and posterior cortical nuclei, and lighter projections from the posterolateral cortical and posterior basomedial nuclei (Fig. 6).
The VSub and VCA1 receive more robust amygdalar inputs. The amygdalar projections to these regions arise from the lateral, anterior and posterior basolateral, posterior basomedial, posteromedial cortical and posterolateral cortical nuclei, as well as from the amygdalohippocampal and anterior amygdaloid areas (Figs. 2, 6; Pikkarainen et al., 1999; Pitkänen et al., 2000; Petrovich et al., 2001; Kempainnen and Pitkänen, 2004). Electron microscopic studies have shown that the projections from the basolateral nucleus to VSub form asymmetrical (excitatory) synapses with dendritic spines and distal dendritic shafts of subicular pyramidal cells (French et al., 2003). The parasubiculum receives projections mainly from the lateral, posterior basolateral, and basomedial nuclei (Figs. 2, 6; Pikkarainen et al., 1999; Pitkänen et al., 2000; Petrovich et al., 2001).
The two main entorhinal targets of amygdalar projections are the DLEA and VLEA of the LERC. Anterograde tracing studies have shown that the predominant inputs to DLEA and VLEA are from the lateral, posterior basolateral, basomedial, medial, posterolateral cortical, and posteromedial cortical nuclei (Figs. 2, 6; Pikkarainen et al., 1999; Pitkänen et al., 2000; Petrovich et al., 2001). The densest projections originate from BLC and mainly target layers 3 and 5. In contrast to layer 2, which sends information mainly to the dentate gyrus and CA3 (i.e., early stages of the trisynaptic circuit), layer 3 projects to CA1 and the subiculum, which receive information already processed by the trisynaptic circuit of the hippocampus (Amaral and Witter, 1995). The main LERC target of CA1 and subiculum is layer 5, which is the other main layer targeted by the amygdala. Thus, hippocampal areas (VCA1/VSub) and LERC layers (layers 3 and 5) that receive the most robust amygdalar projections are areas that receive, directly or indirectly, information already processed by the trisynaptic circuit. Hippocampal areas VCA1/VSub and the deep layers of the LERC layers (layers 5 and 6) are also the main origin of projections from the hippocampal region to the amygdala. It is also of interest that the deeper layers of the LERC are the main origins of projections to the perirhinal cortex (Burwell and Amaral, 1998), which in turn has widespread connections to the neocortex that are important for memory storage. In regards to the perirhinal and postrhinal cortices, most of the amygdalar inputs to these areas arise from the lateral and posterior basomedial nuclei (Figs. 2, 6).
However, there are some direct amygdalar inputs to layer 2 of the entorhinal cortex, the origin of the perforant pathway to the dentate gyrus (and other hippocampal areas). Thus, the posterolateral cortical nucleus has stronger projections to layer 2 than to the deeper layers of the LERC (Krettek and Price, 1977; Luskin and Price, 1983). In addition, there is evidence that the posterior basolateral nucleus has a strong projection to a portion of layer 2 of the medial entorhinal area (MERC) as well as to the adjacent part of the parasubiculum (Fig. 6; Petrovich et al., 2001). Finally, the amygdalohippocampal area appears to have a projection layer 1 of the MERC which contains dendrites of layer 2 neurons (Fig. 6; Petrovich et al., 2001).
In a recent study performed in our laboratory the amygdalar projections to the DLEA and VLEA were investigated using retrograde tracing with Fluorogold (McDonald and Zaric, 2015a). The pattern of retrograde labeling in the amygdala produced by injections of FG into each area was distinct (Fig. 7). Injections into the DLEA produced retrograde labeling in all amygdalar nuclei with the exception of the central nucleus, intercalated nuclei, posterodorsal medial nucleus, and the rostral pole of the anterior basolateral nucleus. The density of labeled neurons was much greater with injections into the rostral DLEA compared to the caudal DLEA. In contrast to DLEA injections, injections into the VLEA produced retrograde labeling that was mainly confined to the caudal lateral nucleus and the cortical nuclei (Fig. 7). In general these findings are consistent with anterograde tract tracing studies in the rat (Krettek and Price, 1977; Luskin and Price, 1983; Canteras et al., 1992, 1995; Petrovich et al., 1996, 2001; Pikkarainen et al., 1999; Kemppainen et al., 2002; Majak and Pitkänen, 2003a). It is not clear, however, why we did not see evidence for projections of the VLEA to the medial and basolateral nuclei as seen in anterograde tracing studies of these nuclei (Canteras et al., 1995; Pikkarainen et al., 1999; Petrovich et al., 2001).
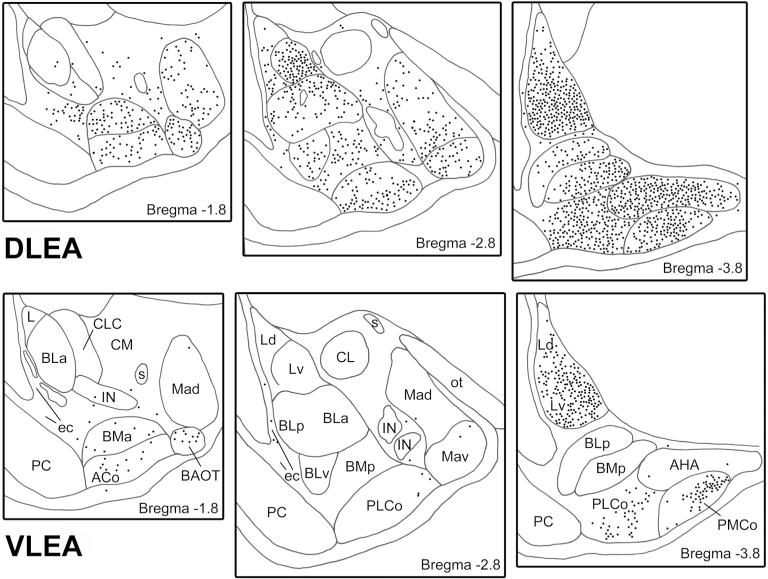
Fig. 7.
Plots of FG+ retrogradely-labeled neurons at three rostrocaudal levels (bregma −1.8, −2.8, and −3.8) after FG injections into the DLEA (top) or VLEA (bottom). Each section is 50μm thick; each dot represents one cell. The nomenclature used to denote the amygdalar nuclei is that used in the atlas by Paxinos and Watson (see Table 1). See Fig. 1A for most abbreviations. Additional abbreviations: ACo, anterior cortical nucleus; AHA, amygdalohippocampal area; BAOT, bed nucleus of the accessory olfactory tract; BMa, anterior basomedial nucleus; CLC, lateral capsular subdivision of the central nucleus; CM, medial central nucleus; ec, external capsule; IN, intercalated nucleus; L, lateral nucleus; Mad, anterodorsal medial nucleus; Mav, anteroventral medial nucleus; ot, optic tract; PMCo, posteromedial cortical nucleus; S, commissural portion of the stria terminalis. Modified from McDonald and Zaric, 2015a.
Glutamatergic pyramidal neurons and a small subpopulation of GABAergic nonpyramidal neurons participate in amygdalohippocampal interconnections
It is well established that the interconnections of the hippocampal and parahippocampal regions involved in the medial temporal lobe memory system, including the trisynaptic circuit, are mediated primarily by glutamatergic projection neurons (Johnson and Amaral, 2004; Amaral and Witter, 1995). Neuroanatomical and electrophysiological studies have also shown that glutamatergic pyramidal neurons are the main neuronal type associated with projections from the hippocampal/parahippocampal region to the amygdala (Brothers and Finch, 1985; Mello et al., 1992; Maren and Fanselow, 1995; Lang and Paré, 1998; McDonald and Zaric, 2015b) as well as projections from the cortical and basolateral amygdalar nuclei back to the hippocampal/parahippocampal region (Finch et al., 1986; Colino and Fernandez de Molina, 1986; Paré et al., 1995; Sparta et al., 2014; McDonald and Zaric, 2015a).
However, as early as the 1980s evidence began to accumulate that amygdalohippocampal interconnections also involve long-range nonpyramidal projection neurons (LRNP neurons), which are known to be inhibitory GABAergic neurons. Köhler and coworkers (1986) found that a small number of nonpyramidal NPY+ neurons in the lateral amygdalar nucleus of the rat were retrogradely-labeled by injections of Fast Blue into the entorhinal cortex. These investigators mainly focused their attention on interconnections of the parahippocampal region in horizontal sections that did not include most portions of the amygdala, and they did not report on projections from amygdalar regions ventral to the lateral nucleus. NPY+ neurons in the basolateral amygdala are now known to present a subpopulation of SOM+ nonpyramidal neurons in both rat and monkey (McDonald 1989; McDonald et al., 1995). In the other direction, Ino and coworkers (1990) found that large injections of horseradish peroxidase into the cat basolateral amygdala resulted in the retrograde labeling of nonpyramidal neurons in Ammons horn and the entorhinal cortex, and Müller and coworkers (2011) reported that large injections of Fluorogold (FG) into the mouse amygdala resulted in retrograde labeling of nonpyramidal GABAergic neurons in the stratum oriens and pyramidal layer of the VSub and VCA1.
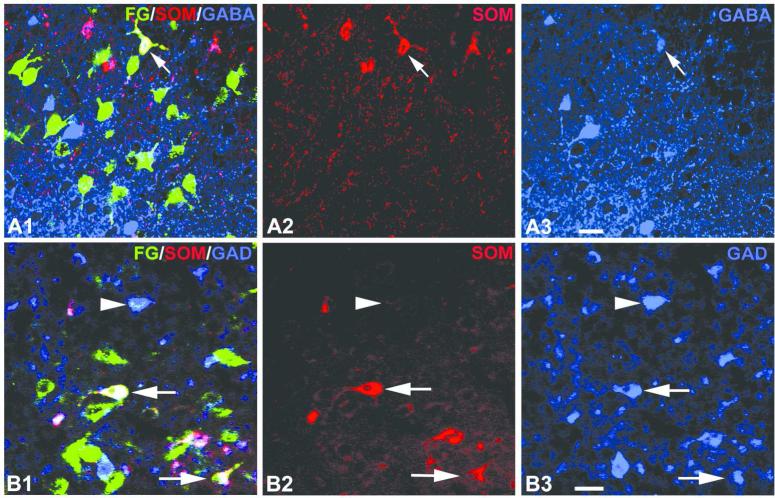
Recent studies in our laboratory combining FG retrograde tract tracing with markers for distinct subpopulations of amygdalar and hippocampal/parahippocampal nonpyramidal neurons have provided a more comprehensive, quantitative analysis of these inhibitory amygdalohippocampal interconnections in the rat (McDonald and Zaric, 2015a, b). Injections of FG into the BLC produced widespread retrograde labeling in the cerebral hemispheres and diencephalon (McDonald and Zaric, 2015b). Triple-labeling for FG, somatostatin (SOM), and neuropeptide Y (NPY) revealed a small number of nonpyramidal FG+ neurons that were SOM+/NPY+ or SOM+/NPY− in the LERC, perirhinal cortex, APIR, and piriform cortex, but not in the subiculum, cornu ammonis, prefrontal cortex, or diencephalon (Fig. 8). In most cases these LRNP neurons were interspersed with SOM−/NPY− pyramidal cells in layers 5 and 6. In the LERC these LRNP neurons constituted only 2% of the total population of FG+ projection neurons; the remaining FG+ projection neurons all appeared to be pyramidal neurons. Half of the SOM+ neurons in the LERC labeled by FG expressed GABA immunoreactivity (Fig. 9A), and these GABAergic projection neurons constituted 2% of the total population of GABAergic neurons (McDonald and Zaric 2015b). No neurons expressing parvalbumin (PV) or vasoactive intestinal peptide (VIP) (i.e., two other important nonpyramidal neuronal subpopulations) were observed in the parahippocampal region, or in any other cortical area.
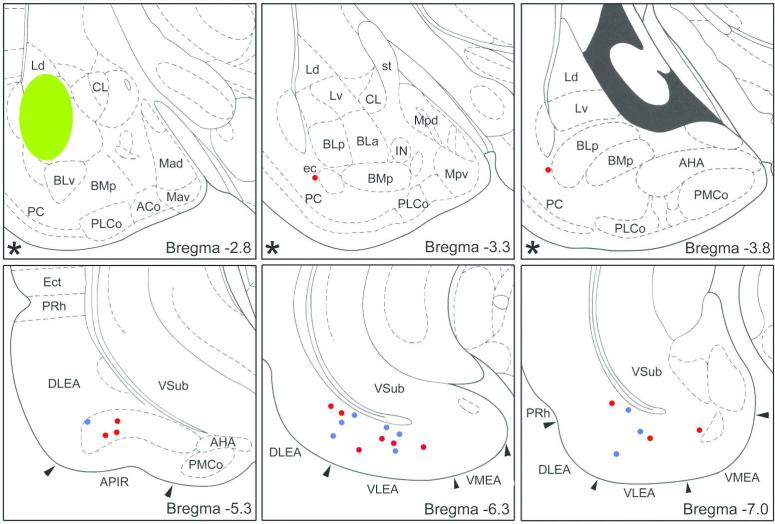
Fig. 8.
Sections arranged from rostral (upper left, Bregma −2.8 level) to caudal (lower right, Bregma −7.0 level) depicting the locations of retrogradely labeled FG+ neurons expressing both SOM and NPY (red dots) or just SOM (blue dots) in a rat that received an injection of FG into the BLC (green). Each dot represents one neuron. Neurons are plotted from two non-adjacent 50μm-thick sections at most levels; asterisks in the lower left corner of a pane indicate levels at which only one section was available for plotting. Templates are modified from the atlas by Paxinos and Watson (1997). The nomenclature used to denote the amygdalar nuclei is that used in the atlas by Paxinos and Watson (see Table 1). Modified from McDonald and Zaric, 2015b.
Fig. 9.
Confocal immunofluorescence photomicrographs of FG+ retrogradely-labeled neurons (green), SOM immunoreactive neurons (red), and GABA or GAD immunoreactive neurons (blue) in the DLEA (A) or lateral amygdalar nucleus (B) seen with injections of FG into the BLC or lateral entorhinal cortex, respectively. (A1) A single FG+/SOM+/GABA+ neuron (arrow) in the DLEA in a rat that received a FG injection into the BLC. (A2) The same field as A1, but only showing SOM+ structures. (A3) The same field as A1, but only showing GABA+ structures. B1) FG/SOM/GAD triple labeling in a case with a FG injection that involved VLEA and VMEA. Two FG+/SOM+/GAD+ triple-labeled neurons in the lateral nucleus (arrows) are white. This field also contains a GAD+ neuron with very low levels of FG (arrowhead). B2) The same field as B1 but only showing SOM+ structures. B3) The same field as B1 but only showing GAD+ structures. Scale bars = 20 μm for both A and B. Modified from McDonald and Zaric, 2015a, b.
Although the great majority of nonpyramidal neurons in cortical areas are known to be GABAergic, only about half of the LRNP neurons in the entorhinal cortex projecting to the basolateral amygdala were GABA+ (McDonald and Zaric 2015b). However, GABAergic neurons with long projections typically have low levels of somatic GABA and glutamic acid decarboxylase (GAD, the synthetic enzyme for GABA), since GABA and GAD are rapidly transported from the soma to the distant axon terminals of these neurons (Onteniente et al., 1986; Tóth and Freund, 1992). Thus, the GABA-negative SOM+ nonpyramidal projection neurons in the entorhinal cortex seen in our study may be GABAergic but have levels of somatic GABA that are below the threshold for immunohistochemical detection using immunofluorescence. This interpretation is consistent with previous studies performed in GAD67-GFP transgenic mice which have shown that all SOM+ neurons in both neocortical and paleocortical areas are GABAergic (Tamamaki et al., 2003; Suzuki and Bekkers, 2010).
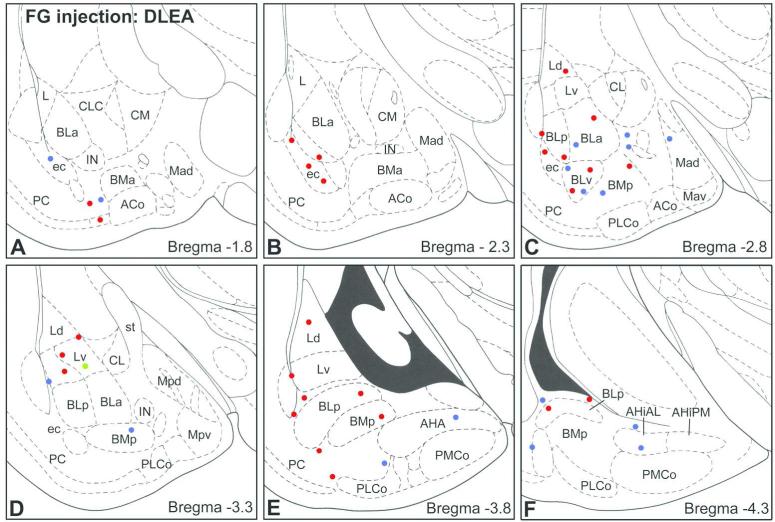
In a second recent study performed in our laboratory injections of FG were made into different areas of the rat LERC to determine the extent to which amygdalar LRNP neurons might contribute to the amygalohippocampal network (McDonald and Zaric, 2015a). Neurons retrogradely labeled with FG were mainly located in the cortical nuclei and BLC. Although most of the FG+ neurons in the cortical nuclei and BLC labeled by entorhinal injections were putative pyramidal cells, about 2%-6% were nonpyramidal neurons that expressed SOM, or both SOM and NPY (Fig. 10). These LRNP neurons were interspersed among SOM−/NPY− pyramidal cells in these nuclei. No FG+ neurons in the amygdala expressed PV or VIP. Cell counts revealed that LRNP neurons labeled by injections into the LERC constituted about 10%-20% of the total SOM+ population, and 20%-40% of the total NPY population in the lateral amygdalar nucleus. Approximately half of the SOM+ neurons in the LERC labeled by FG expressed GAD (Fig. 9B), but it is most likely that all are GABAergic (see above; McDonald and Zaric 2015a). It is of interest that while injections of FG into the prefrontal cortex resulted in many FG+ neurons in the amygdala, none were SOM+, NPY+, or GAD+.
Fig. 10.
Sections arranged from rostral (A) to caudal (F) depicting the locations of FG+ neurons in the amygdala co-expressing SOM and NPY (red dots), expressing SOM but not NPY (blue dots), or expressing NPY but not SOM (green dot) in with a FG injection into DLEA. Each bregma level shows the locations of neurons plotted from two non-adjacent 50 μm-thick sections at this level; each dot represents one neuron. Templates are modified from the atlas by Paxinos and Watson (1997). Modified from McDonald and Zaric, 2015a.
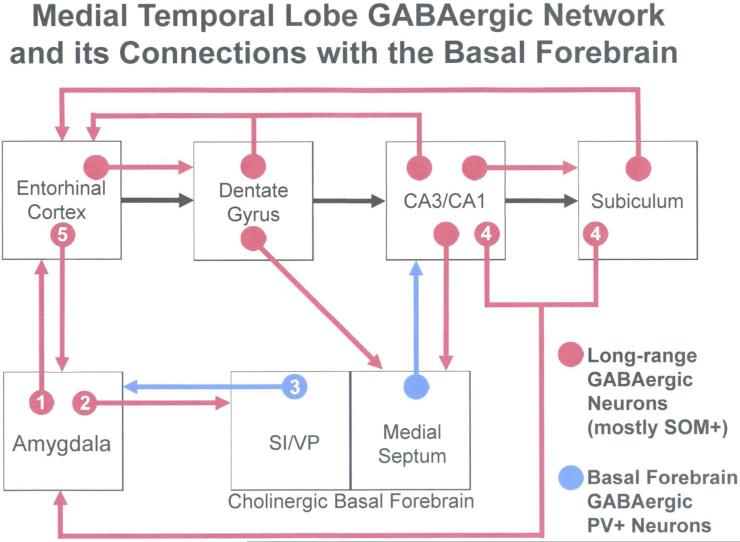
Thus, several studies in a variety of species indicate that interconnections between the amygdala and hippocampal region are mediated in part by inhibitory GABAergic LRNP neurons. It is also of interest that all major portions of the medial temporal lobe memory system are interconnected by various populations of LRNP neurons, including many that are SOM+ (see Jinno, 2009 for a review). Thus, LRNP neurons have been shown to contribute to the following projections: (1) neocortex to perirhinal cortex (Pinto et al., 2006); (2) perirhinal cortex to entorhinal cortex (Pinto et al., 2006; Apergis-Shoute et al., 2007); (3) entorhinal cortex to hippocampus (Germroth et al., 1989; Melzer et al., 2012); (4) hippocampus to subicular and retrosplenial regions (Jinno et al., 2007; Miyashita and Rockland, 2007); and (5) subicular region to entorhinal cortex (Van Haeften et al., 1997). These interconnections have been termed the temporal lobe GABAergic “supernetwork” (Buzsaki and Chrobak, 1995). The finding of reciprocal interconnections between the amygdala and hippocampal region involving GABAergic LRNP neurons indicates that this temporal lobe GABAergic supernetwork includes the basolateral amygdala (Fig. 11). In addition, for both the hippocampus and amygdala, interconnections of these regions with the basal forebrain also involve GABAergic neurons, thus extending the supernetwork (Fig. 11).
Fig. 11.
Schematic diagram illustrating interconnections between the amygdala, hippocampal/parahippocampal areas, and basal forebrain that are mediated in part by GABAergic projection neurons, most of which also express SOM (shown in red). The series of glutamatergic projections starting at the entorhinal cortex and extending through the hippocampal formation, including the “trisynaptic circuit”, are indicated by black arrows (all other glutamatergic projections are not shown). GABAergic projections from the basal forebrain to the amygdala (from the substantia innominata and ventral pallidal regions; SI/VP) and hippocampus (from the medial septum) are shown in blue; these neurons also express PV. Numbers in the connections of the amygdala refer to the following studies: (1) McDonald and Zaric, 2015a; (2) McDonald et al., 2012; (3) Mascagni and McDonald, 2009 and McDonald et al., 2011; (4) Müller et al., 2012; (5) McDonald and Zaric, 2015b. See reviews by Jinno (2009) and Caputi and coworkers (2013) for the studies demonstrating the connections that are not designated by numbers. Modified from McDonald and Zaric, 2015b.
Only a small number of LRNPs are involved in most of the interconnections of the temporal lobe GABAergic supernetwork. For example, in our study of LERC-amygdalar projections we found that only 2% of LERC projection neurons projecting to the BLC were LRNP neurons, and they constituted only 2% of the total GABAergic population. These percentages are very similar to those exhibited by the perirhinal to LERC projection where 3% of perirhinal projection neurons were LRNP neurons, and they constituted 2% of the total GABAergic population (Apergis-Shoute et al., 2007). However, electron microscopic studies found that 12% of the axons involved in the latter projection formed symmetrical synapses typical of inhibitory inputs, which suggests that these perirhinal LRNP neurons have axons that branch extensively (Pinto et al., 2006). It will be of interest to perform similar quantitative ultrastructural analyses of the interconnections between the amygdala and hippocampal/parahippocampal region, as well as electrophysiological studies of these projections. However, previous limited ultrastructural analyses suggest that such extensive branching may not occur in amygalohippocampal interconnections involving GABAergic projection neurons (Pitkänen et al., 2002; Müller et al., 2011). It will also be important to determine the neuronal targets of these inhibitory projections in the amygdalar complex and hippocampal/parahippocampal region. Either pyramidal neurons (Pinto et al., 2006; Jinno et al., 2007) or nonpyramidal neurons (Van Haeften et al., 1997; Melzer et al., 2012) have been identified as targets of LRNP neurons in different hippocampal-parahippocampal interconnections.
Amygdalohippocampal interconnections are important for memory and learning
It is well established that the basolateral amygdala, especially the anterior portion of the basolateral nucleus (BLa), modulates the consolidation of memories of emotionally arousing experiences through its projections to other brain regions, including the hippocampal region (Packard et al., 1994; McGaugh, 2004). Studies in rats suggest that the modulatory effects on hippocampal-dependent memories depend on direct or indirect pathways connecting the BLa with the hippocampus (Packard et al., 1994) and entorhinal cortex (Roesler et al., 2002). Likewise, studies in humans indicate that amygdalohippocampal interactions are critical for the enhancement of declarative (explicit) memory of emotionally arousing events (Cahill and McGaugh, 1998; Phelps, 2004). Functional imaging studies in humans have shown that the formation of memories of emotional stimuli involves interactions of the basolateral amygdala with the entorhinal cortex and anterior portions of the hippocampus (Dolcos et al., 2004), and that these areas are also activated by retrieval of emotional memories (Dolcos et al., 2005).
Studies of synaptic plasticity in the rat have shed light on possible anatomical pathways mediating BLa modulation of memory consolidation. Long-term potentiation (LTP) is a candidate mechanism for memory formation, and there is compelling evidence for the involvement of amygdalohippocampal projections in the modulation of hippocampal LTP in the rat (Abe, 2001). Lesions or inactivation of the BLa or the basomedial nucleus (BM), but not the central nucleus, attenuated LTP in the dorsal (septal) part of dentate gyrus (dDG) induced by high-frequency stimulation of the medial perforant pathway (MPP; Ikegaya et al., 1994, 1995a, 1996a). Electrical stimulation of the BLa evoked synaptic potentials in the dDG of anesthetized rats (Ikegaya et al., 1996b), and high-frequency stimulation of the BLa or BM, but not the lateral nucleus, facilitated dDG LTP induced by MPP stimulation (Ikegaya et al., 1996b, 1995b; Akirav and Richter-Levin, 1999, Li and Richter-Levin, 2013). There is evidence that the modulatory effects of BLa and BM are via separate pathways, and that both involve increases in the excitability of dDG neurons (Ikegaya et al., 1996b; Abe, 2001).
Because there are no direct projections of BLa and BM to the dDG, these modulatory pathways must be polysynaptic. The inputs to the DG are limited and arise from the entorhinal cortex, supramammillary hypothalamic nucleus, medial septum, and brainstem monoaminergic nuclei (Johnston and Amaral, 2004). The BLC activates brainstem monoaminergic systems indirectly via the central nucleus; because lesions of the latter do not attenuate MPP-dDG LTP (see above) this would seem to rule out modulation by monoaminergic systems. Since the BLC has little or no projection to the supramammillary nucleus or medial septum, the entorhinal cortex is the most likely intermediary in the BLC to dDG pathway. Because the BLC to dDG pathway does not appear to involve the MPP from the MERC (Ikegaya et al., 1996b), the most likely origin is from layer 2 of the LERC via the lateral perforant pathway (LPP). Indeed, stimulation of the LPP, like BLa stimulation, enhances MPP-dDG LTP (Nakao et al., 2004). Moreover, electrophysiological studies by Finch and co-workers (1986) demonstrated that simulation in and near the BLC activated layer 2 neurons of the lateral part of the LERC that could be antidromically activated from the dDG. In addition, when these layer 2 LERC neurons were filled with HRP their axons could be traced to the DG (Finch et al, 1986). Anatomical investigations also support this interpretation. Studies of the topographical organization of the perforant pathways indicate that only layer 2 of the far lateral part of the LERC projects to the dDG (Dolorfo and Amaral, 1998). It is of interest that both the BLa and BM have dense projections to the lateral LERC. Although these projections target the deeper layers of the LERC, there are cells in these deeper layers that have axonal collaterals that arborize in layer 2 (Amaral and Witter, 1995). Another possible pathway to layer 2 of the LERC is through the parasubiculum. The parasubiculum is targeted by several nuclei of the BLC (Pikkarainen et al., 1999) and has a dense projection to layer 2 of both the LERC and MERC (Van Groen and Wyss, 1990). A third possible pathway from the BL to layer 2 of the LERC is via APIR (termed the postpiriform transition area, TR, by Petrovich et al., 2001), which has dense projections to layer 2 of the LERC (Fig. 6; Petrovich et al., 2001). Anterograde and retrograde tracing studies have shown that the rostral part of the BLp, which appears to be in range of the BL stimulation sites used by Ikegaya and coworkers, has strong projections to APIR (Petrovich et al., 2001; Santiago and Shammah-Lagnado, 2005). Thus, there are several possible amygdalar pathways to the dDG that may play a role in modulating MPP-dDG LTP. Given the especially robust direct projections of the amygdala to the VSub/VCA1, entorhinal cortex, and perirhinal cortex, it seems likely that LTP, and mnemonic functions, would be modulated by glutamatergic projections of the BLC to these areas as well.
Amygdalohippocampal interconnections are also critical for fear conditioning and extinction. Thus, studies using c-fos as a marker for neuronal activation demonstrated increased activation of lateral nucleus neurons projecting to the LERC during acquisition of cued fear conditioning (Majak and Pitkänen, 2003b), whereas optogenetic inhibition of glutamatergic projections from the BLa to the LERC disrupted the acquisition of contextual fear conditioning (Sparta et al., 2014). However, most studies have investigated the role of hippocampal projections to the amygdala in fear conditioning. Dorsal hippocampal lesions block fear conditioning to contextual conditioned stimuli, but not to simple conditioned stimuli like tones (Phillips and LeDoux, 1992; Maren and Fanselow. 1997; Maren et al., 1997). In contrast, lesions of the amygdala which involve the BLC block fear conditioning to both types of stimuli (Phillips and LeDoux, 1992; Maren and Fanselow, 1995). These data, as well as the results of more recent studies (e.g., Trifilieff et al., 2007; Ramirez et al., 2013; de Oliviera Coelho et al., 2013), suggest that the dorsal hippocampus transmits contextual information to the BLC needed for contextual fear conditioning. These findings have been confirmed and extended using optogenetic techniques. Thus, optogenetic inhibition of dorsal CA1 pyramidal neurons blocks acquisition of contextual fear conditioning, but not auditory tone-cued fear conditioning, and can reversibly block retrieval of contextual fear conditioning (Goshen et al., 2011). Consistent with previous lesion studies, optogenetic inhibition of BLa pyramidal neurons blocks acquisition of both contextual and cued fear conditioning (Goshen et al., 2011).
The output portions of the dorsal hippocampus (dorsal subiculum/CA1) have no direct projections to the BLC, but could activate the BLC via a relay in the LERC or perirhinal cortex (Room and Groenewegen, 1986; Van Groen et al., 1986; Witter et al., 1989), which have robust projections to the BLC (Figs. 4, 5). This proposed pathway is consistent with studies which have shown that large lesions of the entorhinal cortex, which also involved the ventral hippocampus, block contextual fear conditioning (Maren and Fanselow, 1995; Maren and Fanselow, 1997). Electrophysiological studies have shown that stimulation of the entorhinal cortex and VSub activate the BLp via glutamatergic projections, and that high frequency stimulation of the entorhinal cortex and VSub induces NMDA receptor-dependent LTP in the BLp (Maren and Fanselow, 1995). These data suggest that plasticity at these hippocampal-BLp synapses may be critical for contextual fear conditioning.
Fear extinction occurs when fear conditioned animals are exposed to a conditioned stimulus (e.g., tone) that is no longer paired with the associated unconditioned stimulus (e.g., foot shock). Fear extinction has been shown to be context-dependent such that animals subjected to extinction training in one context will still exhibit fear when presented with the conditioned stimulus in a different context. This is termed fear “renewal”. Given the importance of hippocampal inputs to the amygdala for contextual fear conditioning, it is not surprising that these inputs also play a critical role in fear extinction and renewal. Thus, inactivation of the dorsal hippocampus with muscimol impairs fear renewal when extinction is tested in a novel context (Corcoran and Maren, 2001). This impairment of fear renewal is associated with a decrease in the tone-evoked firing of individual neurons in the lateral nucleus that are associated with fear behavior (Hobin et al., 2003; Maren and Hobin, 2007). These data suggest that context-related suppression of fear behavior after extinction is mediated, in part, by inhibitory mechanisms in the lateral nucleus activated by the dorsal hippocampus. The anatomical pathway from the dorsal hippocampus to the lateral nucleus most likely involves a projection of the dorsal subiculum/CA1 to the perirhinal cortex or LERC (Room and Groenewegen, 1986; Van Groen et al., 1986; Witter et al., 1989), which in turn project to the lateral nucleus. Another possible route is through the medial prefrontal cortex (mPFC); the dorsal CA1 projects to the infralimbic cortex of the mPFC (Hoover and Vertes, 2007), which in turn has a strong projection to the lateral nucleus (McDonald et al., 1996).
It is also known that ventral hippocampal inactivation with muscimol can disrupt context-specific fear memory retrieval, which presumably depends on direct VSub/VCA1 projections to the BLC (Hobin and Maren, 2006). Moreover, fear renewal with conditioned stimulus presentation outside of the extinction context was associated with the activation of neurons in the VSub/VCA1 region and the mPFC, and lesions of either of these regions eliminated fear renewal (Orsini et al., 2011). These findings suggest that convergent input from the ventral hippocampus and mPFC to the amygdala are critical for contextual regulation of fear after extinction. The context-dependency of fear and extinction may involve the differential activation of “fear neurons” and “extinction neurons” in the basolateral nucleus, both of which are pyramidal cells (Herry et al., 2008). Fear neurons exhibit conditioned stimulus-evoked firing during and after fear conditioning, whereas extinction neurons exhibit increases in firing only after extinction training. Electrophysiological studies demonstrated that fear neurons, but not extinction neurons, in the basolateral nucleus receive input from the ventral hippocampus (Herry et al., 2008). Likewise, neurons in the lateral nucleus that are active during fear renewal (following extinction training in a novel context) receive much greater input from the ventral hippocampus than neurons active during retrieval of extinction memories in the extinction training context (Knapska et al., 2012).
Amygdalohippocampal synchronous oscillations and their role in memory and learning
Both the amygdala and hippocampus exhibit epochs of rhythmic, synchronized firing of large populations of neurons which create currents that constitute the electroencephalogram. These rhythmic oscillations, which emerge from the intrinsic properties of constituent neurons as well as network properties, are often synchronized in the basolateral amygdala and hippocampal/parahippocampal region because of amygdalohippocampal interconnections (Pape and Paré, 2010). Synchronous oscillations in the amygdalohippocampal network create recurring “time windows” in which synaptic interactions between these structures, including synaptic plasticity, is facilitated (Paré et al., 2002). Thus, as discussed below, it is not surprising that synchronous rhythmic amygdalohippocampal oscillations have been shown to be critical for consolidation of memories of emotional events, as well as fear conditioning and extinction.
Amygdalohippocampal oscillations occur at several different frequencies. The lateral nucleus and perirhinal cortex (PRC) exhibit highly synchronized slow oscillations (1 Hz) during slow wave sleep (SWS) and in anesthetized animals (Collins et al., 2001). The synchronization of activity in these areas is thought to involve the extensive reciprocal interconnections between the lateral nucleus and the perirhinal cortex (Figs. 2, 5, 6). Interestingly, the conduction velocities of axons interconnecting the lateral nucleus with more distant caudal levels of the PRC is greater than that of axons with shorter connections with more rostral levels of the PRC, thus providing synchronous activation of the entire PRC (Pelletier and Paré, 2002). This presumably facilitates processing of emotionally-salient sensory inputs by the lateral nucleus/PRC network. It has been suggested that rhythmic oscillations of the amygdalohippocampal network during sleep may be critical for emotional memory consolidation (Paré et al., 2002).
Theta rhythms (4-8 Hz) are also synchronized in the BLC and hippocampal region. In the basolateral nucleus pyramidal neurons and interneurons fire at opposite phases of entorhinal theta (Paré and Gaudreau, 1996). Neurons in the lateral nucleus of fear conditioned cats become synchronized in the theta band by tones that predict footshock (Paré and Collins, 2000). The lateral nucleus also exhibits theta activity in fear conditioned mice when they are subsequently exposed to the conditioned fear stimulus (Narayanan et al., 2007). Moreover, there is increased theta synchronization in the lateral nucleus and dorsal CA1 with retrieval of fear conditioning memories (Seidenbecher et al., 2003; Pape et al., 2005; Narayanan et al., 2007), but a decrease during fear extinction which remains low during extinction recall (Lesting et al., 2011). This synchronization of theta activity in the lateral nucleus and dorsal CA1 presumably requires indirect interconnections between these two structures. Since theta activity in the entorhinal cortex is typically synchronous with that in the hippocampus, the pathways interconnecting the lateral nucleus with the dorsal CA1 most likely involve an intermediate connection in the lateral part of the LERC (see above).
There is also evidence for a similar coupling of fast gamma oscillations (40-100 Hz) in the BLC, PRC, and LERC during appetitive learning. In a task in which a visual conditioned stimulus predicted a food reward it was found that learning of the association between the conditioned stimulus and the reward was associated with an increase in synchronization of 35-45 Hz activity in the BLC and parahippocampal cortices (Bauer et al., 2007). Moreover, this BLC-dependent synchronization has been shown to facilitate the transmission of sensory information from the neocortex to the hippocampus by increasing effective communication between the intervening perirhinal and entorhinal cortices (Paz et al., 2006).
Both anatomical and electrophysiological studies suggest that glutamatergic pyramidal neurons are the main cell type mediating amygdalohippocampal interconnections critical for synchronous oscillatory activity among these structures. However, it is possible that GABAergic long-range nonpyramidal neurons (LRNP neurons) may also play a role in coupling synchronized oscillations even though the overall percentage of these neurons involved in these interconnections is low. There is certainly compelling evidence that this is the case for GABAergic LRNP neurons interconnecting different hippocampal/parahippocampal regions, which are also found in low numbers (Buzsaki and Chrobak, 1995; Jinno, 2009; Caputi et al., 2013). It is of interest in this regard that the axons of hippocampal GABAergic LRNP neurons have a larger diameter and thicker myelin sheath than pyramidal cell axons, which suggests that rhythmic inhibitory inputs provided by these LRNP neurons are likely to precede the arrival of excitatory inputs to their targets, and thereby reset the excitability and oscillatory phase of their targets just before the slower rhythmic excitatory pyramidal cell inputs arrive (Jinno et al., 2007). Thus it is possible that GABAergic LRNP neurons interconnecting the BLC and hippocampal region are involved in the synchronization of a variety of oscillations, including theta, gamma, and slow oscillations, in these regions (Paré and Gaudreau, 1996; Paré et al., 2002; Bauer et al., 2007). These oscillations could entrain synchronous firing of amygdalar and hippocampal/parahippocampal pyramidal neurons, thus facilitating functional interactions between them including synaptic plasticity involved in mnemonic function.
Neuropsychiatric and neurological diseases affecting amygdalohippocampal regions/interconnections produce memory impairments
Alzheimer's disease
Alzheimer's disease (AD) is the most common memory disorder, affecting 11% of people older than 65, and 32% of people older than 85. Although there are neuropathological changes (neurofibrillary tangles and amyloid plaques) in all cortical areas, the density of atrophic neurons exhibiting neurofibrillary tangles is far greater in the medial temporal lobe, including the amygdala, hippocampus, and parahippocampal cortices (Van Hoesen et al., 1986; Arnold et al., 1991; Poulin and Zakzanis, 2002). The entorhinal cortex is generally the first medial temporal lobe area to undergo neuropathology, followed by the hippocampus, and then the amygdala (Braak et al., 1996). The amygdala in AD exhibits marked atrophy, and all amygdalar nuclei show plaques and tangles (Herzog and Kemper, 1980; Van Hoesen et al., 1986, Kromer Vogt et al., 1990; Cuénod et al., 1993; Poulin et al., 2011). There are discrepancies in different reports as to which amygdalar nuclei are most affected, which may be related to individual variability and/or stages of the disease. However, the general consensus is that the nuclei in the medial and ventral portions of the amygdala are most affected (i.e., nuclei in the human amygdala that correspond to the BLp, BM, and cortical nuclei of the rat) (Braak et al., 1996; Wright, 2009). As in the rat, these nuclei have dense interconnections with the hippocampal/parahippocampal region in primates (Amaral and Cowan, 1980; Aggleton, 1986; Amaral et al., 1992; Pitkänen et al., 2002).
Of considerable interest from the standpoint of amygdalohippocampal interconnections is that the areas of the hippocampal region that have the most direct interconnections with the amygdala are most affected in AD. Thus, the perirhinal cortex, entorhinal cortex, parasubiculum, subiculum, and CA1 have a much greater degree of neuropathology than CA3 and the dentate gyrus (Van Hoesen et al., 1986). These findings would predict that there would be impairment in the formation of memories of emotional events in AD. In an MRI study in patients with AD it was found that impairment of emotional event memory was proportional to the degree of amygdalar damage, but less so in relation to hippocampal damage (Mori et al., 1999). Likewise, in patients with Urbach-Wiethe disease, in which there is selective bilateral calcification of the amygdala, there are impairments in long-term declarative memory of emotional material, but no impairments in remembering neutral material (Markowitsch et al., 1994; Cahill et al., 1995; Adolphs et al., 1997). In addition to impairments in declarative emotional memory, there are also severe impairments in fear conditioning (nondeclarative emotional memory) in AD, presumably due to the amygdalar atrophy and neuropathology in this disease (Hamann et al., 2002).
Posttraumatic stress disorder (PTSD)
PTSD is thought to result from impairments in fear extinction and/or over-consolidation of fear memories due to dysfunction of amygdalar interactions with the hippocampus and mPFC (Rauch et al., 2003; Shin and Liberzon, 2010). It is characterized by several symptoms including intrusive re-experiencing of the traumatic event, avoidance of reminders of the event, and hyperarousal. PTSD effects approximately 10% of individuals exposed to traumatic events that involve death, or threat of death or serious injury. The pathogenesis of PTSD is thought to be a fear conditioning process marked by amygdalar hyperarousal (Rauch et al., 2003). It is usually also associated with decreased activity of the mPFC, resulting in diminished extinction, and decreased hippocampal/parahippocampal activity, resulting in over-generalization of fear to non-threatening stimuli and the inability to distinguish safe and threatening contexts (Lanius et al., 2002, 2003; Rauch et al., 2003).
The deficits seen with decreased hippocampal/parahippocampal activity, and often decreased hippocampal volumes (Rauch et al., 2003; Shin and Liberzon, 2010), in PTSD are consistent with functional imaging studies which have demonstrated that fear conditioning and extinction in humans is associated with activation of both the amygdala and the hippocampal/parahippocampal cortices (Milad et al., 2007; Alvarez et al., 2008; Lang et al., 2009; Lonsdorf et al., 2014). Moreover, correlated hippocampal/amygdalar activity was seen with extinction of contextual fear conditioning, suggesting that amygdalohippocampal interconnections were involved (Lang et al., 2009). Not surprisingly, therefore, patients with PTSD exhibit impaired contextual processing during fear extinction (Rougemont-Bücking et al., 2011) and impaired fear renewal when tested for extinction in a different context from the extinction context (Garfinkel et al., 2014). The findings of these investigations in humans are in agreement with studies in rodents indicating that the hippocampal/parahippocampal region is important for distinguishing safe versus threatening contexts and can regulate the activity of “fear neurons” in the amygdala. Since context-dependent relapse of extinguished fear in PTSD patients is common with exposure therapies (Bouton, 2002), blocking fear renewal via modulation of amygdalohippocampal interconnections may be a useful therapeutic intervention in this condition.
Temporal Lobe Epilepsy (TLE)
Partial onset epilepsies are the most common form of seizures in adults and TLE is the most common form of partial epilepsy (Tellez-Zenteno and Hernandez-Ronquillo, 2012). Most cases of TLE appear to involve dysregulation of amygdalo-hippocampal function, since partial resection of the temporal lobe, including hippocampus and amygdala virtually eliminates seizures in more than 80% of patients (Dodrill et al., 1986; Walczak et al., 1990). The central role of amygdala and hippocampus in TLE is also evident in depth electrode recordings which show participation of these brain regions in 70-80% of seizures (Quesney, 1986; So et al., 1989). The integrity of hippocampus and amygdala is critical for proper memory function and so it has been proposed that in addition to contributing to seizures, dysfunction of these structures in TLE is responsible for cognitive deficits in TLE patients (Abrahams et al., 1999; Ploner et al., 2000; Guerreiro et al., 2001). Indeed, TLE is commonly associated with cognitive impairment, most frequently deficits in declarative and spatial memory (Hermann et al., 2006; 2007; Baxendale et al., 2010). In addition, deficits in emotional enhancement of declarative memory are seen in TLE patients, presumably due to “a specific deficit in the emotion-driven encoding enhancement mediated by the amygdala-hippocampus loop” (Müller et al., 2009). Moreover, deficits in emotional enhancement of declarative memory (Ahs et al., 2010) and fear conditioning (LaBar et al., 1995) are seen in TLE patients after unilateral anterior temporal lobectomy that involves the amygdala and hippocampal region.
The central role for both the amygdala and hippocampal formation in TLE is based on the heavy interconnections within and between these regions. Seizure activity in one region rapidly recruits the other to participate in the seizure. This pathological neuronal activity is associated with neuronal loss in both hippocampus and amygdala. Accordingly, histopathological and imaging studies have shown amygdalar damage (Margerison and Corsellis, 1966; Van Paesshen et al., 1996) that often appears in combination with hippocampal damage in TLE (Cendes et al., 1993; Bronen et al., 1995; Bernasconi et al., 2003; Goldberg et al., 2014). While hippocampal atrophy in patients with TLE has been well described (for review see Lado et al., 2002), the distribution of damage in different nuclei of human amygdala is less well understood. However, studies in rat seizure models have provided more detailed insight. In post-status epilepticus TLE models the amygdalar nuclei showing the greatest damage are the deep layers of the anterior cortical and medial nuclei, the ventromedial lateral nucleus, the posterior basolateral nucleus, the ventral basolateral nucleus, the posterior basomedial nucleus, and the posterolateral cortical nucleus (Tuunanen et al., 1996). These findings suggest that amygdalohippocampal interconnections involving these nuclei would be most affected in TLE.
Only one study to date has investigated pathway specific damage in amygdalohippocampal projections during TLE (Kemppainen and Pitkanen, 2004). This study used FG to examine monosynaptic amygdalar projections to the VSub/VCA1 in rats made epileptic using kainic acid. The study reported that a large projection originating in the lateral division of the amygdalohippocampal area and the posteromedial and posterolateral cortical nuclei had disappeared in TLE in parallel with severe neuronal loss in these nuclei (Kemppainen and Pitkanen, 2004). This projection provides a pathway by which emotionally tagged sensory input can modulate hippocampal processing. Loss of this pathway in TLE might lead to deficits in processing the emotional significance of hippocampal-dependent memories.
The ventromedial lateral nucleus is also severely damaged in TLE and projections from this region to the VSub/VCA1 were correspondingly reduced (Kemppainen and Pitkanen, 2004). The lateral nucleus is critical for auditory and contextual fear conditioning (LeDoux, 1995). Destruction of the amygdalo-hippocampal pathway originating from this nucleus may contribute to TLE-associated behavioral impairments in fear conditioning in animal studies (Botterill et al., 2014).
Another prominent projection from the amygdala to the VSub/VCA1 originates in the posterior basolateral nucleus (Pikkarainen et al., 1999; Petrovich et al., 2001; Kemppainen and Pitkanen, 2004). While the lateral portion of this nucleus is damaged in TLE, the medial portion is well preserved (Kemppainen and Pitkanen, 2004). Thus, unlike other amygdalohippocampal pathways, the projection from this nucleus to hippocampus remains intact in epilepsy. The basolateral nucleus has been proposed to play a primary role in seizure initiation (White and Price, 1993). The preservation of the pathway from the basolateral nucleus to the hippocampus in TLE may therefore serve to facilitate seizure spread and interictal bursting. A role for this pathway in epilepsy gains support from reports that the basolateral nucleus exhibits increased excitability, reduced inhibition, and a lowered seizure threshold in TLE (Niittykoski et al., 2004). The lowered seizure threshold in this region is, in part, caused by a severe loss of GABAergic nonpyramidal neurons (Tuunanen et al., 1996; Fritsch et al., 2009). As has been described in the hippocampal formation (Sloviter, 1987; Sperk et al., 1992), GABAergic somatostatin-containing (SOM+) nonpyramidal neurons in the BLC are more sensitive than other GABAergic nonpyramidal subgroups to seizure-induced damage (Tuunanen et al., 1997). Loss of GABA+/SOM+ neurons occurs in the medial part of the basolateral nucleus, despite the preservation of pyramidal projection neurons in this area (Tuunanen et al., 1997; Aroniadou-Anderjaska et al., 2008). The resulting loss of inhibition leads to enhanced excitability and responsiveness of BLC pyramidal projection neurons (Smith and Dudek, 1997). These observations suggest that even a few seizures originating in the amygdala could cause the loss of GABA+/SOM+ neurons in the basolateral nucleus which could then increase excitability in this important amygdalar projection nucleus and facilitate the spread of seizures from the damaged, epileptic amygdala to other brain regions. In addition, since some of the GABA+/SOM+ neurons in both the BLC and hippocampal/parahippocampal region are projection neurons involved in amygdalohippocampal interconnections, it seems likely that these neurons are also lost in TLE. The loss of these inhibitory projection neurons would be expected to facilitate the spread of seizures and alter synchronized oscillatory activity in amygdalohippocampal circuits critical for fear conditioning and emotional enhancement of declarative memory.
Conclusions
Neuroanatomical tract tracing studies have provided detailed descriptions of the interconnections, both direct and indirect, between distinct amygdalar nuclei and laminae in discrete areas of the hippocampal region. These interconnections are exceedingly complex and specific organizing principles are not yet apparent. Most of these interconnections involve projections of pyramidal neurons in one region to spines and distal dendrites of pyramidal neurons in the other region. However, a small number of GABAergic nonpyramidal neurons are also projection neurons. One important aspect of these interconnections which has not been thoroughly investigated is the extent to which specific interneuronal subpopulations are postsynaptic targets of the projections of both types of projection neurons. Because interneurons exhibit differential innervation of pyramidal neuronal compartments and other types of interneurons, knowledge of the subpopulations innervated should contribute to an understanding of information processing in the amygdalohippocampal network. In addition, since interneurons play an important role in the induction and maintenance of rhythmic oscillations critical for synaptic plasticity, knowledge of their connections within the amygdalohippocampal network is essential for understanding the generation of synchronous oscillations involved in emotional learning and memory.
Whereas some basic information is available regarding the role of distinct amygdalohippocampal interconnections in specific aspects of emotional learning and memory, much more needs to be learned about electrophysiological and behavioral aspects of these circuits before we can begin to understand their exact function. Knowledge of the neuroanatomy and neurotransmitters used by these interconnections combined with the use of optogenetic techniques to selectively activate/inactivate distinct components of amygdalohippocampal circuits in brain slices and conscious behaving animals will undoubtedly greatly advance our understanding of their participation in mnemonic function/dysfunction in health and disease.
Significance Statement.
This review discusses what is currently know about amygdalohippocampal interconnections, including their neuroanatomy and neurotransmitters, their functional role in learning and memory, and their involvement in mnemonic dysfunction in Alzheimer's disease, posttraumatic stress disorder (PTSD), and temporal lobe epilepsy. Knowledge of brain connections obtained from neuroanatomical studies has always guided subsequent electrophysiological and behavioral studies. The description of the functional neuroanatomy of amygdalohippocampal interconnections provided by this review should be useful for designing electrophysiological and behavioral investigations using recently developed optogenetic techniques to selectively activate/inactivate different components of amygdalohippocampal circuits in brain slices and conscious behaving animals.
Acknowledgments
The authors would like to thank Drs. Asla Pitkänen (University of Kuopio) and Larry Swanson (University of Southern California) for permission to use their illustrations for Figures 2 and 6, respectively, of this manuscript. We also thank Dr. Marlene Wilson (University of South Carolina) for comments on an earlier version of this manuscript.
Grant Support: National Institutes of Health Grant R01MH104638
Literature Cited
- Abe K. Modulation of hippocampal long-term potentiation by the amygdala: a synaptic mechanism linking emotion and memory. Jpn J Pharmacol. 2001;86:18–22. doi: 10.1254/jjp.86.18. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Morris RG, Polkey CE, Jarosz JM, Cox TCS, Graves M, Pickering A. Hippocampal involvement of spatial and working memory: a structural MRI analysis of patients with unilateral mesial temporal lobe sclerosis. Brain Cogn. 1999;41:39–65. doi: 10.1006/brcg.1999.1095. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exp Brain Res. 1986;64:515–526. doi: 10.1007/BF00340489. [DOI] [PubMed] [Google Scholar]
- Ahs F, Kumlien E, Fredrikson M. Arousal enhanced memory retention is eliminated following temporal lobe resection. Brain Cogn. 2010;73:176–179. doi: 10.1016/j.bandc.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Priming stimulation in the basolateral amygdala modulates synaptic plasticity in the rat dentate gyrus. Neurosci Lett. 1999;270:83–86. doi: 10.1016/s0304-3940(99)00488-7. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmicheal ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol. 1980;189:573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. Second Edition Academic Press; San Diego: 1995. pp. 443–494. [Google Scholar]
- Apergis-Schoute J, Pinto A, Paré D. Muscarinic control of long-range GABAergic inhibition within the rhinal cortices. J Neurosci. 2007;27:4061–4071. doi: 10.1523/JNEUROSCI.0068-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78:102–116. doi: 10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, Paz R, Paré D. Gamma oscillations coordinate amygdalo-rhinal interactions during learning. J Neurosci. 2007;27(35):9369–9379. doi: 10.1523/JNEUROSCI.2153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazelot M, Bocchio M, Kasugai Y, Fischer D, Dodson PD, Ferraguti F, Capogna M. Hippocampal Theta Input to the Amygdala Shapes Feedforward Inhibition to Gate Heterosynaptic Plasticity. Neuron. 2015;87:1290–1303. doi: 10.1016/j.neuron.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale S, Heaney D, Thompson PJ, Duncan JS. Cognitive consequences of childhood-onset temporal lobe epilepsy across the adult lifespan. Neurology. 2010;75:705–711. doi: 10.1212/WNL.0b013e3181eee3f0. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- Botterill JJ, Fournier NM, Guskjolen AJ, Lussier AL, Marks WN, Kalynchuk LE. Amygdala kindling disrupts trace and delay fear conditioning with parallel changes in Fos protein expression throughout the limbic brain. Neurosci. 2014;265:158–171. doi: 10.1016/j.neuroscience.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J. Pattern of brain destruction in Parkinson's and Alzheimer's diseases. J Neural Transm. 1996;103:455–90. doi: 10.1007/BF01276421. [DOI] [PubMed] [Google Scholar]
- Bronen RA, Fulbright RK, Kim JH, Spencer SS, Spencer DD, Al-Rodham NFR. Regional distribution of MR findings in hippocampal sclerosis. Am. J. Neuroradiol. 1995;16:1193–1200. [PMC free article] [PubMed] [Google Scholar]
- Brothers LA, Finch DM. Physiological evidence for an excitatory pathway from entorhinal cortex to amygdala in the rat. Brain Res. 1985;359:10–20. doi: 10.1016/0006-8993(85)91407-6. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Caputi A, Melzer S, Michael M, Monyer H. The long and short of GABAergic neurons. Curr Opin Neurobiol. 2013;23:179–186. doi: 10.1016/j.conb.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Cendes F, Anderman F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, Olivier A, Anderman E, Robitaille Y, Lopes-Cendes I, Peters T, Melanson D. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: a MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- Colino A, Fernández de Molina A. Electrical activity generated in subicular and entorhinal cortices after electrical stimulation of the lateral and basolateral amygdala of the rat. Neuroscience. 1986;19:573–580. doi: 10.1016/0306-4522(86)90282-4. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pelletier JG, Paré D. Slow and fast (gamma) neuronal oscillations in the perirhinal cortex and lateral amygdala. J Neurophysiol. 2001;85:1661–1672. doi: 10.1152/jn.2001.85.4.1661. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuénod CA, Denys A, Michot JL, Jehenson P, Forette F, Kaplan D, Syrota A, Boller F. Amygdala atrophy in Alzheimer's disease. An in vivo magnetic resonance imaging study. Arch Neurol. 1993;50:941–945. doi: 10.1001/archneur.1993.00540090046009. [DOI] [PubMed] [Google Scholar]
- de Oliveira Coelho CA, Ferreira TL, Soares JC, Oliveira MG. Hippocampal NMDA receptor blockade impairs CREB phosphorylation in amygdala after contextual fear conditioning. Hippocampus. 2013;23:545–551. doi: 10.1002/hipo.22118. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Wilkus RJ, Ojemann GA, Ward AA, Wyler AR, van Belle G, Tamas L. Multidisciplinary prediction of seizure relief from cortical resection surgery. Ann. Neurol. 1986;20:2–12. doi: 10.1002/ana.410200103. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–26231. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398:25–48. [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch DM, Wong EE, Derian EL, Chen XH, Nowlin-Finch NL, Brothers LA. Neurophysiology of limbic system pathways in the rat: projections from the amygdala to the entorhinal cortex. Brain Res. 1986. 1986;370:273–284. doi: 10.1016/0006-8993(86)90482-8. [DOI] [PubMed] [Google Scholar]
- French SJ, Hailstone JC, Totterdell S. Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res. 2003;981:160–167. doi: 10.1016/s0006-8993(03)03017-8. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Rogawski MA, Braga MF. Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis. Neuroscience. 2009;163:415–429. doi: 10.1016/j.neuroscience.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34:13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germroth P, Schwerdtfeger WK, Buhl EH. GABAergic neurons in the entorhinal cortex project to the hippocampus. Brain Res. 1989;494:187–192. doi: 10.1016/0006-8993(89)90162-5. [DOI] [PubMed] [Google Scholar]
- Goldberg H, Weinstock A, Bergsland N, Dwyer MG, Farooq O, Sazgar M, Poloni G, Treu C, Weinstock-Guttman B, Ramanathan M, Zivadinov R. MRI segmentation analysis in temporal lobe and idiopathic generalized epilepsy. BMC Neurol. 2014;14:131. doi: 10.1186/1471-2377-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Guerreiro CAM, Jones-Gotman M, Andermann F, Bastos A, Cendes F. Severe amnesia in epilepsy: causes, anatomopsychological considerations, and treatment. Epil. Behav. 2001;2:224–246. doi: 10.1006/ebeh.2001.0167. [DOI] [PubMed] [Google Scholar]
- Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer's disease. Neuropsychologia. 2002;40:1187–1195. doi: 10.1016/s0028-3932(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, Bell B. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. 2007;13:12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Kemper TL. Amygdaloid changes in aging and dementia. Arch Neurol. 1980;37:625–629. doi: 10.1001/archneur.1980.00500590049006. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hübner C, Bosch D, Gall A, Lüthi A, Ehrlich I. Ex vivo dissection of optogenetically activated mPFC and hippocampal inputs to neurons in the basolateral amygdala: implications for fear and emotional memory. Front Behav Neurosci. 2014;8:64. doi: 10.3389/fnbeh.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. Attenuated hippocampal long-term potentiation in basolateral amygdala-lesioned rats. Brain Res. 1994;656:157–164. doi: 10.1016/0006-8993(94)91377-3. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. Requirement of basolateral amygdala neuron activity for the induction of long-term potentiation in the dentate gyrus in vivo. Brain Res. 1995a;671:351–354. doi: 10.1016/0006-8993(94)01403-5. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neurosci Res. 1995b;22:203–207. doi: 10.1016/0168-0102(95)00894-7. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. The basomedial and basolateral amygdaloid nuclei contribute to the induction of long-term potentiation in the dentate gyrus in vivo. Eur J Neurosci. 1996a;8:1833–1839. doi: 10.1111/j.1460-9568.1996.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. Dentate gyrus field potentials evoked by stimulation of the basolateral amygdaloid nucleus in anesthetized rats. Brain Res. 1996b;718:53–60. doi: 10.1016/0006-8993(95)01465-9. [DOI] [PubMed] [Google Scholar]
- Ino T, Matsuzaki S, Shinonaga Y, Ohishi H, Ogawa-Meguro R, Mizuno N. Direct projections of non-pyramidal neurons of Ammon's horn to the amygdala and the entorhinal cortex. Neurosci Lett. 1990;115:161–166. doi: 10.1016/0304-3940(90)90448-i. [DOI] [PubMed] [Google Scholar]
- Insausti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus. 1997;7:146–183. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsáki G, Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S. Structural organization of long-range GABAergic projection system of the hippocampus. Front Neuroanat. 2009;3:13. doi: 10.3389/neuro.05.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Amaral DG. Hippocampus. In: Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford University Press; New York: 2004. pp. 455–498. [Google Scholar]
- Jolkkonen E, Miettinen R, Pitkänen A. Projections from the amygdalo-piriform transition area to the amygdaloid complex: a PHA-L study in rat. J Comp Neurol. 2001;432:440–465. doi: 10.1002/cne.1113. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D (28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Jolkkonen E, Pitkänen A. Projections from the posterior cortical nucleus of the amygdala to the hippocampal formation and parahippocampal region in rat. Hippocampus. 2002;12:735–755. doi: 10.1002/hipo.10020. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Damage to the amygdalo-hippocampal projection in temporal lobe epilepsy: a tract-tracing study in chronic epileptic rats. Neuroscience. 2004;126:485–501. doi: 10.1016/j.neuroscience.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, Pieprzyk M, Cymerman IA, Werka T, Sheng M, Maren S, Jaworski J, Kaczmarek L. Functional anatomy of neural circuits regulating fear and extinction. Proc Natl Acad Sci U S A. 2012;109:17093–17098. doi: 10.1073/pnas.1202087109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Smialowska M, Eriksson LG, Chanpalay V, Davies S. Origin of the neuropeptide Y innervation of the rat retrohippocampal region. Neurosci Lett. 1986;65:287–292. doi: 10.1016/0304-3940(86)90276-4. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol. 1977. 1977;172:723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- Kromer Vogt LJ, Hyman BT, Van Hoesen GW, Damasio AR. Pathological alterations in the amygdala in Alzheimer's disease. Neuroscience. 1990;37:377–385. doi: 10.1016/0306-4522(90)90408-v. [DOI] [PubMed] [Google Scholar]
- Lado FA, Laureta EC, Moshé SL. Seizure-induced hippocampal damage in the mature and immature brain. Epileptic Disord. 2002;4:83–97. [PubMed] [Google Scholar]
- Lang EJ, Paré D. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience. 1998;83:877–889. doi: 10.1016/s0306-4522(97)00420-x. [DOI] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 2009;29:823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu. Rev. Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala and emotion: a view through fear. In: Aggleton JP, editor. The Amygdala. Oxford University Press; Oxford: 2000. pp. 289–310. [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Richter-Levin G. Priming stimulation of basal but not lateral amygdala affects long-term potentiation in the rat dentate gyrus in vivo. Neuroscience. 2013;246:13–21. doi: 10.1016/j.neuroscience.2013.03.059. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Haaker J, Kalisch R. Long-term expression of human contextual fear and extinction memories involves amygdala, hippocampus and ventromedial prefrontal cortex: a reinstatement study in two independent samples. Soc Cogn Affect Neurosci. 2014;9:1973–1983. doi: 10.1093/scan/nsu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Majak K, Pitkänen A. Projections from the periamygdaloid cortex to the amygdaloid complex, the hippocampal formation, and the parahippocampal region: a PHA-L study in the rat. Hippocampus. 2003a;13:922–942. doi: 10.1002/hipo.10134. [DOI] [PubMed] [Google Scholar]
- Majak K, Pitkänen A. Activation of the amygdalo-entorhinal pathway in fear-conditioning in rat. Eur J Neurosci. 2003b;18:1652–1659. doi: 10.1046/j.1460-9568.2003.02854.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JAN. Epilepsy and the temporal lobes: a clinical, electroencephalographic and neuropathological study of the brain in epilepsy with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Calabrese P, Würker M, Durwen HF, Kessler J, Babinsky R, Brechtelsbauer D, Heuser L, Gehlen W. The amygdala's contribution to memory--a study on two patients with Urbach-Wiethe disease. Neuroreport. 1994;5:1349–1352. [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, Muly EC, Rainnie DG, McDonald AJ. Immunohistochemical characterization of parvalbumin-containing interneurons in the monkey basolateral amygdala. Neuroscience. 2009;158:1541–1550. doi: 10.1016/j.neuroscience.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Parvalbumin-immunoreactive neurons and GABAergic neurons of the basal forebrain project to the rat basolateral amygdala. Neuroscience. 2009;160:805–812. doi: 10.1016/j.neuroscience.2009.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J. Comp. Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala. Wiley-Liss; New York: 1992a. pp. 67–96. [Google Scholar]
- McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Res. Bull. 1992b;28:179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Augustine JR. Neuropeptide Y and somatostatin-like immunoreactivity in neurons of the monkey amygdala. Neuroscience. 1995;66:959–982. doi: 10.1016/0306-4522(94)00629-j. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1997;77:445–459. doi: 10.1016/s0306-4522(96)00478-2. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann. N.Y. Acad. Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Mc Donald AJ, Betette R. Parvalbumin containing neurons in the rat basolateral amygdala: morphology and colocalization of calbindin D-28k. Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and gamma-aminobutyric acid in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Is there an amygdala and how far does it extend? : An anatomical perspective. Ann. N.Y. Acad. Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. Postsynaptic targets of GABAergic basal forebrain projections to the basolateral amygdala. Neuroscience. 2011;183:144–159. doi: 10.1016/j.neuroscience.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Zaric V. Subpopulations of somatostatin-immunoreactive non-pyramidal neurons in the amygdala and adjacent external capsule project to the basal forebrain: evidence for the existence of GABAergic projection neurons in the cortical nuclei and basolateral nuclear complex. Front Neural Circuits. Jul 24. 2012;6:46. doi: 10.3389/fncir.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Zaric V. GABAergic somatostatin-immunoreactive neurons in the amygdala project to the entorhinal cortex. Neuroscience. 2015a;290:227–242. doi: 10.1016/j.neuroscience.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Zaric V. Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience. 2015b;294:82–100. doi: 10.1016/j.neuroscience.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello LE, Tan AM, Finch DM. Convergence of projections from the rat hippocampal formation, medial geniculate and basal forebrain onto single amygdaloid neurons: an in vivo extra- and intracellular electrophysiological study. Brain Res. 1992;587:24–40. doi: 10.1016/0006-8993(92)91425-e. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Rockland KS. GABAergic projections from the hippocampus to the retrosplenial cortex in the rat. Eur J Neurosci. 2007;26:1193–1204. doi: 10.1111/j.1460-9568.2007.05745.x. [DOI] [PubMed] [Google Scholar]
- Mori E, Ikeda M, Hirono N, Kitagaki H, Imamura T, Shimomura T. Amygdalar volume and emotional memory in Alzheimer's disease. Am J Psychiatry. 1999;156:216–222. doi: 10.1176/ajp.156.2.216. [DOI] [PubMed] [Google Scholar]
- Müller NG, Wohlrath B, Kopp UA, Lengler U. Emotional content does not interfere with verbal memory in patients with temporal lobe epilepsy. Epilepsy Behav. 2009;15:367–371. doi: 10.1016/j.yebeh.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Müller M, Faber-Zuschratter H, Yanagawa Y, Stork O, Schwegler H, Linke R. Synaptology of ventral CA1 and subiculum projections to the basomedial nucleus of the amygdala in the mouse: relation to GABAergic interneurons. Brain Struct Funct. 2012;217:5–17. doi: 10.1007/s00429-011-0326-9. [DOI] [PubMed] [Google Scholar]
- Nakao K, Matsuyama K, Matsuki N, Ikegaya Y. Amygdala stimulation modulates hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:14270–14275. doi: 10.1073/pnas.0405709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo-hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Niittykoski M, Nissinen J, Penttonen M, Pitkänen A. Electrophysiologic changes in the lateral and basal amygdaloid nuclei in temporal lobe epilepsy: an in vitro study in epileptic rats. Neuroscience. 2004;124:269–281. doi: 10.1016/j.neuroscience.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Onteniente B, Tago H, Kimura H, Maeda T. Distribution of gamma-aminobutyric acid-immunoreactive neurons in the septal region of the rat brain. J Comp Neurol. 1986;248:422–430. doi: 10.1002/cne.902480310. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci U S A. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Narayanan RT, Smid J, Stork O, Seidenbecher T. Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus. 2005;15:874–880. doi: 10.1002/hipo.20120. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Dong J, Gaudreau H. Amygdalo-entorhinal relations and their reflection in the hippocampal formation: generation of sharp sleep potentials. J Neurosci. 1995;15:2482–2503. doi: 10.1523/JNEUROSCI.15-03-02482.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Paz R, Pelletier JG, Bauer EP, Paré D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nat Neurosci. 2006;9:1321–1329. doi: 10.1038/nn1771. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Paré D. Uniform range of conduction times from the lateral amygdala to distributed perirhinal sites. J Neurophysiol. 2002;87:1213–1221. doi: 10.1152/jn.00623.2001. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdala. In: Aggleton JP, editor. The Amygdala. Oxford University Press; Oxford: 2000. pp. 31–116. [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kelly JL, Amaral DG. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus. 2002;12:186–205. doi: 10.1002/hipo.1099. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud-Pechoux S, Baulac M, Clemenceau S, Samson S, Pierrot-Deseilligny C. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb. Cortex. 2000;10:1211–1216. doi: 10.1093/cercor/10.12.1211. [DOI] [PubMed] [Google Scholar]
- Poulin P, Zakzanis KK. In vivo neuroanatomy of Alzheimer's disease: evidence from structural and functional brain imaging. Brain Cogn. 2002;49:220–225. [PubMed] [Google Scholar]
- Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC. Amygdala atrophy is prominent in early Alzheimer's disease and relates to symptom severity. Psychiatry Res. 2011;194:7–13. doi: 10.1016/j.pscychresns.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Bjorklund A, Hokfelt T, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Vol. 5. Elsevier Science Publishers; Amsterdam: 1987. pp. 279–388. [Google Scholar]
- Quesney LF. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27(Suppl 2):27–45. doi: 10.1111/j.1528-1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann. N.Y. Acad. Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Roesler R, Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. Eur J Neurosci. 2002;15:905–910. doi: 10.1046/j.1460-9568.2002.01924.x. [DOI] [PubMed] [Google Scholar]
- Room P, Groenewegen HJ. Connections of the parahippocampal cortex. I. Cortical afferents. J Comp Neurol. 1986;251:415–450. doi: 10.1002/cne.902510402. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17:227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago AC1, Shammah-Lagnado SJ. Afferent connections of the amygdalopiriform transition area in the rat. J Comp Neurol. 2005;489:349–371. doi: 10.1002/cne.20637. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–350. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol. 1999;406:299–328. doi: 10.1002/(sici)1096-9861(19990412)406:3<299::aid-cne2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Enhanced population responses in the basolateral amygdala of kainate-treated, epileptic rats in vitro. Neurosci. Lett. 1997;222:1–4. doi: 10.1016/s0304-3940(97)13326-2. [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré JF, Paré D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- So N, Gloor P, Quesney LF, Jones-Gotman M, Olivier A, Andermann F. Depth electrode investigations in patients with bitemporal epileptiform abnormalities. Ann Neurol. 1989;25:423–431. doi: 10.1002/ana.410250502. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology. 2011;60:765–773. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Smithuis J, Stamatakis AM, Jennings JH, Kantak PA, Ung RL, Stuber GD. Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Front Behav Neurosci. 2014;8:129. doi: 10.3389/fnbeh.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Marksteiner J, Gruber B, Bellmann R, Mahata M, Ortler M. Functional changes in neuropeptide Y- and somatostatin-containing neurons induced by limbic seizures in the rat. Neuroscience. 1992;50:831–846. doi: 10.1016/0306-4522(92)90207-i. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. J Comp Neurol. 2010;518:1670–1687. doi: 10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Téllez-Zenteno JF, Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:630853. doi: 10.1155/2012/630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P1, Calandreau L, Herry C, Mons N, Micheau J. Biphasic ERK1/2 activation in both the hippocampus and amygdala may reveal a system consolidation of contextual fear memory. Neurobiol Learn Mem. 2007;88:424–434. doi: 10.1016/j.nlm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Tóth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience. 1992;49:793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkänen A. Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur J Neurosci. 1996;8:2711–2725. doi: 10.1111/j.1460-9568.1996.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkänen A. Decrease in somatostatin-immunoreactive neurons in the rat amygdaloid complex in a kindling model of temporal lobe epilepsy. Epilepsy Res. 1997;26:315–27. doi: 10.1016/s0920-1211(96)00900-x. [DOI] [PubMed] [Google Scholar]
- Van Groen T, van Haren FJ, Witter MP, Groenewegen HJ. The organization of the reciprocal connections between the subiculum and the entorhinal cortex in the cat: I. A neuroanatomical tracing study. J Comp Neurol. 1986. 1986;250:485–497. doi: 10.1002/cne.902500407. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990;518:227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- Van Haeften T, Wouterlood FG, Jorritsma-Byham B, Witter MP. GABAergic presubicular projections to the medial entorhinal cortex of the rat. J Neurosci. 1997;17:862–874. doi: 10.1523/JNEUROSCI.17-02-00862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen GW, Hyman BT, Damasio AR. Cell-specific pathology in neural systems of the temporal lobe in Alzheimer's disease. Prog Brain Res. 1986;70:321–335. doi: 10.1016/s0079-6123(08)64313-7. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, Connelly A, Johnson CL, Duncan JS. The amygdala and intractable temporal lobe epilepsy: a quantitative magnetic resonance imaging study. Neurology. 1996;47:1021–1031. doi: 10.1212/wnl.47.4.1021. [DOI] [PubMed] [Google Scholar]
- Walczak TS1, Radtke RA, McNamara JO, Lewis DV, Luther JS, Thompson E, Wilson WP, Friedman AH, Nashold BS. Anterior temporal lobectomy for complex seizures: evaluation, results, and long-term follow-up in 100 cases. Neurology. 1990;40:413–418. doi: 10.1212/wnl.40.3_part_1.413. [DOI] [PubMed] [Google Scholar]
- White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. II: The effects of focal deactivation. J. Neurosci. 1993;13:4810–4830. doi: 10.1523/JNEUROSCI.13-11-04810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Wright CI. The human amygdala in normal aging and Alzheimer's Disease. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. The Guilford Press; New York: 2009. pp. 382–405. [Google Scholar]