Abstract
The research was to appraise the utility of the patient-derived tumor xenografts (PDXs) as models of estrogen receptor positive (ER+HER2− and ER+HER2+) breast cancers. We compared protein expression profiles by Reverse Phase Protein Array (RPPA) in tumors that resulted in PDXs compared to those that did not. Our overall PDX intake rate for ER+ breast cancer was 9% (9/97). The intake rate for ER+HER2+ tumors (3/16, 19%) was higher than for ER+HER2− tumors (6/81, 7%). Heat map analyses of RPPA data showed that ER+HER2− tumors were divided into 2 groups by luminal A/B signature [protein expression of ER, AR, Bcl-2, Bim (BCL2L11), GATA3 and INPP4b], and this expression signature was also associated with the rate of PDX intake. Cell survival pathways such as the PI3K/AKT signaling and RAS/ERK pathways were more activated in the specimens that could be established as PDX in both classes. Expression of the ER protein itself may have a bearing on the potential success of an ER+ PDX model. In addition, HER2 and its downstream protein expressions were up-regulated in the ER+HER2+ patient tumors that were successfully established as PDX models. Moreover, the comparison of RPPA data between original and PDX tumors suggested that the selection/adaptation process required to grow the tumors in mice is unavoidable for generation of ER+ PDX models, and we identified differences between patient tumor samples and paired PDX tumors. A better understanding of the biological characteristics of ER+PDX would be the key to using PDX models in assessing treatment strategies in a preclinical setting.
Keywords: Patient-derived tumor xenografts (PDXs), Breast Cancer, ER+HER2−, ER+HER2+, Luminal A/B
1. Introduction
Patient-derived tumor xenografts (PDXs) are renewable tumor models engrafted in mice generated from fresh human tumors without prior in vitro exposure. They are being increasingly appreciated as better models in cancer research particularly for preclinical testing because they reflect patients’ tumor biology more accurately than cancer cell lines and allow an invaluable assessment of tumor evolution and adaptive response to therapy [1-4]. These models have been indicated to be biologically stable and better reflect patients’ tumors with regards to tumor heterogeneity, histopathology, gene expression, genetic mutations, and therapeutic response [5-8]. PDX models have been successfully applied to preclinical drug testing and biomarker identification in some breast cancer subtypes. However, recent studies from multiple research groups [9, 10] using PDXs questioned the practicality of PDX as completely accurate tools for developing personalized treatment strategies for the patients who donated the original tumors, so-called ‘avator’ or ‘ co-clinical trial approach’ [2].
Among breast cancer PDX models, estrogen receptor-positive (ER+) PDX lines have been difficult to establish, with a low average rate of engraftment reported (2-15%) [6, 8, 11, 12]. In this report, ER stands for ESR1, unless it is defined differently. With a low intake rate, established ER+ PDX lines might not be representative of the overall ER+ tumor population. Therefore, characterization of established ER+ PDXs is critical for the proper use of them as preclinical models to examine treatment outcome. Furthermore, tumor subtype would be very important, and based on HER2 expression, ER+ cancers can be further classified as ER+HER2− and ER+HER2+. Women with ER+HER2+ breast cancers have worse five-year overall survival and disease-free survival than women with ER+HER2-negative (HER2−) cancers [13, 14]. This suggests that ER+HER2+ cancers have a different biology from ER+HER2− cancers that requires treatment strategies selective to this double positive subtype [15]. The current first-line standard-of-care treatment for patients with ER+ tumors is anti-hormonal therapy; however, patients with ER+HER2+ tumors typically receive anti-HER2 therapy as first-line treatment. Recent studies suggest that crosstalk between the ER and HER2 genes may be important for growth of ER+HER2+ tumors, suggesting that a combination of endocrine therapy plus anti-HER2 therapy may offer a synergistic benefit in these patients, and be a superior treatment approach to existing therapies [16-18]. Presently, there are limited numbers of ER+HER2+ breast cancer cell lines for research. To the best of our knowledge, this is the first report to examine ER+HER2− PDX and ER+HER2+ PDX models separately. A better tailor treatment for women with ER+HER2+ breast cancers could result from characterization of relevant PDX models of the two types of ER+ tumors. While PDX models have been recognized to be powerful tools in cancer biology, generation of them remains empirical, without a total understanding of what are the key elements to assure the success of engraftment. Although preclinical data indicate that the rate of engraftment in PDX models serves as an independent predictor for poor outcome [5, 19], we hypothesize that molecular differences within the original human ER+HER2+ or ER+HER2− tumors may affect the tumor intake rate in NSG mice. To test this hypothesis, we generated PDX models using ER+HER2+ and ER+HER− patient tumor tissues and performed reverse phase protein array (RPPA) analysis to identify molecular pathways that may relate to the establishment of ER+ breast cancer PDX models in NSG mice. RPPA is a powerful tool to study functional proteomics, a high-throughput antibody-based technique to evaluate protein (including phosphoprotein) expression in signaling networks. Our study represents the first systematic attempt to determine the molecular features of ER+ tumors (including both ER+HER2− and ER+HER2+ classes) that impacts PDX engraftment. A better understanding of protein signatures of the PDX models in different classes in ER+ cancers is critical for more appropriate utilization to develop targeted treatments of different ER+ breast cancers. Another purpose of this report is to re-evaluate the value of ER+ PDX models in the prediction of treatment in a preclinical setting, based on findings from us and other research groups.
2. Materials and Methods
2.1. Patient samples
Primary breast tumor samples and metastatic samples (where applicable) were collected from patients with stage 1-4 breast cancer treated at City of Hope. Tumor samples were collected at the time of surgery or biopsy. Tumor samples were tested for ER status (+/−), progesterone receptor (PR) status (+/−) and HER2 status (+/−) by standard immunohistochemistry. For ER and PR immunostaining, tumors with <1% of positive cells were reported as ER− and PR-negative. HER2 immunohistochemistry testing was performed in accordance with American Society of Clinical Oncology (ASCO) College of American Pathologists (CAP) guidelines. Tumor samples were reported as negative (0 or 1+), equivocal (2+), or positive (3+). In cases of an equivocal result, FISH analysis was performed to detect amplification of HER2 genes. This study was approved by the City of Hope Institutional Review Board (IRB) and all patients provided written informed consent prior to tissue collection.
2.2. Reverse phase protein array (RPPA) analysis
Reverse phase protein array analysis was performed by the MD Anderson Cancer Center Functional Proteomics RPPA Facility. Only antibodies with a Pearson correlation coefficient between RPPA and western blotting of greater than 0.7 were used in the reverse phase protein array study. Antibodies with a single or dominant band on western blotting were further assessed by direct comparison to RPPA using cell lines with differential protein expression or modulated with ligands/inhibitors or siRNA for phospho- or structural proteins, respectively. Array slides were probed with 297 primary antibodies for human samples and 232 primary antibodies for PDX samples. Sixty five individual mouse antibodies and 1 rat antibody were eliminated from the analysis of xenograft samples. Cross-reaction was minimized by excluding these antibodies interacting with proteins of mouse or rat origin. Please see supplementary materials for detail procedures.
2.3. PDX models
For PDX models, tumor samples were processed within an hour after collection. Tumors were kept in medium (DMSO/high Glucose medium supplemented with 1 % penicillin/streptomycin) on ice. All processes were performed under sterile conditions. Tumors were cut into fragment size of 2 mm3 - 3mm3 using a scalpel or razor blade and placed in the medium with Matrigel (Corning, MA, USA) (1:1). Surgical site on mouse was depilated using hair removal cream and disinfected with Techni-Care (Care-Tech® Laboratories, Inc., MO, USA). Tumors were grafted into the inguinal 4th mammary fat pad of 6-8 week-old female NOD/SCID/interleukin-2 receptor gamma chain null (NSG) mice under isofloren anesthesia (n=4 mice per patient tumor sample). Detailed surgical procedures were described previously [20]. Since the endogenous levels of estrogen are sufficient, intact female NSG mice were used without estrogen supplementation for both establishment and maintenance. A PDX model was determined as “established” after the original xenograft (tumor size became approximately 500mm3, defined as P0) passed through two consecutive in vivo passages. We concluded as “not growing” when tumors were not palpable 4 months after inoculation. Tumor growth was measured using calipers and tumor volumes were calculated using the formula: 4/3π × r1 × r2 (r1 < r2) (0.125), where r1 is the smaller radius [21]. When tumor size became approximately 1000 mm3, mice were euthanized via CO2 asphyxiation to harvest tumors. Tumors were removed and flash-frozen in liquid nitrogen within 5-10 minutes of resection. Tumors were stored at −80°C until analysis. Samples that were sent for RPPA analysis were from early passage (up to P2). NSG mice were breed and housed at the City of Hope Animal Resources Center in ventilated cage racks with free access to water and food. Mice were maintained on a 12 h light/dark cycle. All institutional guidelines for animal care and use were followed. All animal research procedures used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at City of Hope. Facilities are credited by AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care), and operated according to NIH guidelines.
2.3. Statistical analysis
Data were normalized and log2 transformed. The limma package [22] in Bioconductor was used to identify differentially expressed proteins between groups: 1) Tumors which were successfully engrafted into NSG mice versus tumors were not successfully engrafted into NSG mice, 2) patient tumor samples versus paired PDX tumor samples, 3) ER+HER2− versus ER+HER2+ patients tumors. A robustly moderated t-statistic other than the ordinary t-statistic was computed for each protein, taking into consideration the sample size. Specifically, the standard errors were moderated across proteins (shrunk towards a common value using a simple Bayesian model). The same method was used to detect proteins differentially expressed between the patient tumor samples that successfully established PDX models and the patient tumor samples that failed to establish PDX models. Benjamini and Hochberg’s method [23] was used to compute the adjusted p-value to control the false discovery rate (FDR). Adjusted p-values <0.05 were considered statistically significant and all tests were two-sided. All statistical analyses were performed using R statistical software (version 3.2.0). The Principal Components Analysis (PCA) was used to group patient tumor samples and PDX tumor samples for the ER+HER2+ and ER+HER2− groups.
3. Results
3.1. Patients
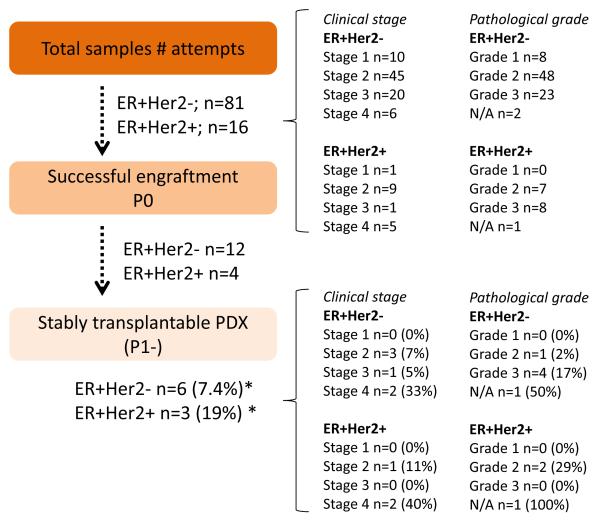
A total of 97 tumor specimens from ER+ patients were implanted into NSG mice to generate PDX models. Eighty one were ER+HER2− tumors and 16 were ER+HER2+ tumors. For ER+HER2− tumors, ten were invasive lobular carcinoma (ILC), two were metastatic carcinoma, and two were mucinous. Other tumors were invasive ductal carcinoma (IDC). For ER+HER2+ tumors, one was medullay breast cancer, one was inflammatory breast cancer, and one was metastatic carcinoma. The remaining 13 were IDC. Fig. 1 summarizes clinical stage and pathological grade information of 97 tumor specimens.
Fig. 1. Summary of ER+ PDX establishment.
Pathological grade was identified based on the Nottingham Histologic Score. Percentage was calculated by number of stably transplantable PDX / number of attempts. Overall, 9 ER+ PDX models were successfully established: 6 ER+HER2− and 3 ER+HER2+.
3.2. Establishment of PDX models
Overall, nine ER+ PDX models were successfully established from 97 tumor specimens: six ER+HER2− and three ER+HER2+. However, two of the ER+HER2− lines (COH-SC48 and COH-SC49) and one of the ER+HER2+ line (COH-GS4) were established after the RPPA analyses were performed, therefore those three samples were not available when we performed RPPA analyses. Therefore, four ER+HER2− and two ER+HER2+ PDX lines are included in the RPPA analysis. The patient and tumor characteristics underlying these nine PDX models are listed in Table 1 and images of immunohistochemical analysis of ER, PR, HER2 and H&E staining are shown in supplemental Fig. 1. The overall PDX intake rate for ER+ breast cancer PDX models was 9% (9/97). The intake rate for ER+HER2+ tumors (3/16, 19%) was higher than for ER+HER2− tumors (6/81, 7%). Overall, patient tumor samples of more advanced clinical stage had higher intake rates than those of less advanced clinical stage, and no PDX models were successfully established from Stage 1 patient tumor samples in either the ER+HER2+ or ER+HER2− breast cancer classes. Some tumor samples grew in mice but did not reach 1cm in diameter and others could not be passaged (i.e., 50% of ER+HER2− and 25% of ER+HER2− P0 tumors). Median time to first passage (P1) (from the time of inoculation in mice using patients samples to next passage) of nine successful ER+ PDX was 158 days. These results are summarized in Fig. 1. Nine ER+HER2− samples were lobular carcinoma; however, none of them could establish the PDX.
Table 1.
Characteristics of the patient tumors which were successfully established as PDX models
| PDX ID | ER | PR | HER2 | Age | Patient ethnicity | Clinical stage | Pathological grade |
Sample site |
|---|---|---|---|---|---|---|---|---|
| COH-GS1 | + | − | − | 71 | Hispanic | 2 | 3 | Breast |
| COH-GS2 | + | − | − | 63 | Hispanic | 3 | 3 | Breast |
| COH-SC7 | + | − | − | 63 | Caucasian | 4 | 2 | Chest wall |
| COH-GS3 | + | + | − | 52 | African-American | 4 | N/A | Brain |
| COH-SC1 | + | − | + | 34 | Caucasian | 2 | 2 | Breast |
| COH-SC31 | + | + | + | 72 | Caucasian | 4 | 3 | Chest wall |
| COH-SC48* | + | − | − | 53 | Caucasian | 2 | 3 | Breast |
| COH-SC49* | + | − | − | 67 | Caucasian | 2 | 3 | Breast |
| COH-GS4* | + | − | + | 37 | Caucasian | 4 | N/A | Brain |
2 of the ER+HER2− lines (COH-SC48 and COH-SC49) and 1 of the ER+HER2+ line (COH-GS4) were established after the RPPA analyses were performed. N/A; not applicable
3.3. RPPA analysis
To better understand the molecular features of our ER+ PDX models, we performed RPPA analysis to quantify relative expression levels of 297 cancer-related proteins and phosphoproteins using original tumor specimens from the six ER+ tumors (four ER+HER2− and two ER+HER2+) that were successfully established as PDX models, samples which were available when we performed RPPA, and 21 ER+ tumors that could not be engrafted into NSG mice. Twenty one tumors that did not establish as PDX models were chosen to match for tumor type (IDC or its metastases), clinical stage, and pathological grade of engrafted tumors: 7 ER+HER2+ tumor samples and 14 ER+HER− samples (Supplementary Table 1). In addition to original specimens, tumor samples from the six established ER+ PDX models (highlighted in Table 1) were also submitted for analysis.
3.3.1 RPPA analysis for ER+HER2− tumors
Results of unsupervised hierarchical clustering of RPPA data from 18 ER+HER2− tumors showed 2 distinguished clusters [cluster 1(C1) and cluster 2 (C2): n=11 and 7, respectively] (Fig. 2a). One ER+HER2− PDX was derived from tumors in the first cluster (C1), and three ER+HER2− PDX lines were in the second cluster (C2) (Fig. 2a). High expression of ER, PR, Androgen receptor (AR), Bcl-2, GATA3 and INPP4b define the luminal A cancers [24]. Tumors in cluster C1 expressed higher levels of ER, AR, Bcl-2 and GATA-3 proteins than those in C2, suggesting that tumors in C1 and C2 were luminal A and B subtypes, respectively (supplemental Fig. 2a). Importantly, higher expression of signaling molecules in the RAS/ERK pathway, PI3K/AKT pathway and mTOR pathway was found in C2 tumors, and lower expression of signaling molecules in 5′ AMP-activated protein kinase (AMPK) pathway (supplemental Fig. 2b and involved pathways are shown in Fig. 2b), implying that they are more proliferative than C1 tumors. This observation also supports that C2 tumors were luminal B subtype [24]. Agreeing with previous findings from RPPA analysis of luminal A and B tumors in The Cancer Genome Atlas (TCGA) network [24], C2 (luminal B) tumors expressed higher levels of cyclin D1, Myc, and FOXM1 (Supplemental Fig. 2c). Therefore, those expression results suggested that COH-SC7, COH-GS1, and COH-GS3 are luminal B PDXs. COH-GS2 is a luminal A PDX.
Fig. 2. Median centered unsupervised hierarchical clustering of results from Reverse Phase Protein Array (RPPA) analyses.
(a) Total of 18 samples of ER+HER2− patient tumor samples were analyzed. Results showed 2 distinguished clusters [cluster 1(C1): n=11 and cluster 2 (C2): n=7] for ER+HER2− tumors. (b) Summary of pathways which were changed in C2 compared to C1 in ER+HER2− patients tumors. (c) Comparison of protein expression between ER+HER2+ patient tumor samples that successfully generated PDX models (S; n=2) and ER+HER2+ patient tumor samples that failed to establish PDX models (F; n=7). (d) Human samples of ER+HER2− (n=18) labeled in black and ER+HER2− (n=9) in orange. Results showed 2 distinguished clusters [luminal A (C1) and B (C2): n=8 for each] for ER+HER2− tumors. Three ER+HER2− PDX’s were derived from tumors in the luminal B cluster. Protein profile (indicated by *) included the following proteins: ER, AR, Bcl-2, Bim (BCL2L11), GATA3 and INPP4b. ER+HER2+ tumors (labeled in orange color), except two, had similar protein expression signature to luminal A tumors, except region *. S; Tumors that were successfully engrafted into NSG mice, F; tumors that were not successfully engrafted into NSG mice. H; human tumor samples
We also compared protein expression between ER+HER2− original tumor samples that successfully generated PDX models (S, success; n=4) and ER+HER2− patient tumor samples that failed to establish PDX models (F, failure; n=14). Fourteen tumors that did not establish as PDX models were chosen to match for tumor type (IDC or its metastases), clinical stage, and pathological grade of engrafted tumors. The top 30 differentially expressed proteins (based on p-values) were included in ingenuity pathway analysis (IPA) analysis. The top two canonical pathways identified with differential expression between the S and F samples were the AMPK signaling pathway and PI3K/AKT signaling pathway (Table 2). The list of proteins is shown in Table 3 and Table 4.
Table 2.
Top 2 canonical pathways of protein expressed in original patients tumors which were successfully engrafted into NSG mice (S) compared to tumors were not successfully engrafted (F)
| p-value | Overlap | ||
|---|---|---|---|
|
S vs F (ER+HER2−)
| |||
| 1. AMPK Signaling | 1.43E-10 | 4.5% | 8/179 |
| 2. PI3K/AKT Signaling | 4.26E-10 | 5.7% | 7/123 |
|
| |||
|
S vs F (ER+HER2+)
| |||
| 1. PI3K/AKT Signaling | 2.54E-13 | 7.3% | 9/123 |
| 2. Endometrial Cancer Signaling | 1.79E-12 | 13.5% | 7/52 |
Table 3.
Top 5 of up regulated proteins in original patients tumors which were successfully engrafted into NSG mice (S) compared to tumors were not successfully engrafted (F), and in comparison between PDX tumors (PDX) and original patient tumors (Original)
| S vs F (ER+HER2−) | LogFC | p-value | adj.p-value |
|---|---|---|---|
| L1CAM | 2.01 | 0.002 | 0.178 |
| PTK2 | 1.22 | 0.016 | 0.777 |
| AKT | 1.01 | 0.031 | 0.451 |
| SERPINE1 | 0.77 | 0.003 | 0.185 |
| SLC16A4 | 0.74 | 0.009 | 0.307 |
|
| |||
| S vs F (ER+HER2+) | |||
|
| |||
| HER2 | 1.79 | 0.058 | 0.875 |
| DUSP4 | 1.44 | 0.081 | 0.875 |
| RAB25 | 1.08 | 0.053 | 0.875 |
| L1CAM | 1.03 | 0.030 | 0.875 |
| GAB2 | 0.77 | 0.055 | 0.875 |
|
| |||
| PDX vs Original (ER+HER2−) | |||
|
| |||
| RAB25 | 2.24 | <0.001 | 0.036 |
| ACC (pS79) | 1.85 | <0.001 | 0.046 |
| S6 (pS240) | 1.69 | <0.001 | 0.036 |
| MYH9 | 1.54 | 0.012 | 0.138 |
| SLC1A5 | 1.41 | 0.018 | 0.166 |
|
| |||
| PDX vs Original (ER+HER2+) | |||
|
| |||
| AKT | 2.51 | <0.001 | 0.020 |
| S6 (pS235) | 2.50 | 0.004 | 0.094 |
| EGFR | 1.85 | 0.002 | 0.058 |
| 4E-BP1 (pT37) | 1.71 | 0.010 | 0.140 |
| MYH11 | 1.54 | 0.001 | 0.058 |
Table 4.
Top 5 of down regulated proteins in original patients tumors which were successfully engrafted into NSG mice (S) compared to tumors were not successfully engrafted (F), and in comparison between PDX tumors (PDX) and paired original patient tumors (Original)
| S vs F (ER+HER2−) | LogFC | p-value | adj.p-value |
|---|---|---|---|
| ER | −1.74 | 0.022 | 0.398 |
| MYH9 | −1.36 | 0.016 | 0.398 |
| GATA3 | −1.13 | 0.031 | 0.451 |
| AR | −1.08 | 0.025 | 0.398 |
| ACC (pS79) | −0.87 | 0.056 | 0.563 |
|
| |||
| S vs F (ER+HER2+) | |||
|
| |||
| CDH1 | −1.21 | 0.119 | 0.875 |
| SRC (pY527) | −0.86 | 0.049 | 0.875 |
| RB1 | −0.65 | 0.109 | 0.875 |
| MAPK (pT202) | −0.63 | 0.111 | 0.875 |
| MAP2K1 | −0.54 | 0.095 | 0.875 |
|
| |||
| PDX vs Original (ER+HER2−) | |||
|
| |||
| ROCK1 | −2.05 | <0.001 | 0.036 |
| MYC | −1.70 | 0.002 | 0.063 |
| SOD2 | −1.59 | 0.007 | 0.136 |
| HSP70 | −1.54 | 0.008 | 0.136 |
| COL6A1 | −1.41 | 0.008 | 0.136 |
|
| |||
| PDX vs Original (ER+HER2+) | |||
|
| |||
| HSP70 | −1.75 | 0.005 | 0.094 |
| ROCK1 | −1.68 | <0.001 | 0.020 |
| AXL | −1.54 | 0.001 | 0.058 |
| PREX1 | −1.28 | 0.017 | 0.174 |
| CNST43 | −1.03 | 0.010 | 0.140 |
3.3.2. RPPA analysis for ER+HER2+ tumors
We compared protein expression between ER+HER2+ patient samples that successfully generated PDX models (S; n=2) and ER+HER2+ patient tumor samples that failed to establish PDX models (F; n=7). Unsupervised hierarchical clustering was shown in Fig. 2c. Expression of HER2 was higher in the two S samples compared to F samples (Table 3); HER2 and phosphorylated-HER2 (p-HER2) expression were shown in supplemental Fig. 3. The top 30 differentially proteins (based on p-values) were subjected to IPA analysis. The top two canonical pathways identified with differential expression between the S and F samples were the PI3K/AKT signaling pathway and the endometrial cancer signaling pathway (Table 2).
3.3.3. Comparison between ER+HER2− and ER+HER2+ patient’s tumor specimens
We also compared protein expression between the original ER+HER2− (n=18) (S; n=4 and F; n=14) and ER+HER2+ tumors (n=9) (S; n=2 and F; n=7) (Fig. 2d). As discussed above, based on protein expression signature, C1 and C2 are luminal A and B tumors, respectively. Interestingly, ER+HER2+ tumors (labeled in orange color), except two, had similar protein expression signature to luminal A tumors, except the region labeled by the * symbol in Fig. 2d *. Protein profile (region * in Fig. 2d) included the following proteins: Bcl-2, Bim (BCL2L11), AR, INPP4b, ER and GATA3. Two ER+HER2+ tumors (i.e., 16F and 19F) had protein expression signature resembling luminal B tumors. Unsupervised hierarchical clustering of RPPA data from 27 original specimens revealed that three ER+HER2− PDXs were derived from C2 (luminal B tumors). Three additional PDXs were in ER+HER2+ cluster, including one thought to be derived from a luminal A tumor (i.e., COH-GS2). Those proteins in region * were at lower levels in C2 and ER+HER2+ clusters. The significance of this observation is not fully understood, but suggesting that the tumors in these two clusters were more aggressive than C1 (luminal A) cluster.
3.4. Comparison between PDX tumor samples and paired patient tumor samples
We compared protein expression between the established PDX tumors (n=6) and the original tumor samples used to generate these models (n=6). Unsupervised hierarchical clustering identified that the PDXs and original tumor samples were generally separated, and majority of paired PDX and original tumors did not cluster together (Fig. 3a). The top 30 differentially expressed proteins (based on p-values) were included in IPA analyses comparing ER+HER2− patient and PDX tumor samples, and comparing ER+HER2+ patient and PDX tumor samples. In both HER2+ and HER2− tumors, the phosphorylated S6 protein were upregulated in PDX tumors compared to paired patient tumor samples (Table 3).
Fig. 3. Comparison of PDX and patients tumors.
Median centered unsupervised hierarchical clustering of results from RPPA analysis. H; human original samples, Px; PDX samples (a). Principal component analysis (PCA) of PDX and patients tissues of ER+HER2− tumors (b) and ER+HER2+ tumors (c) were also performed.
The PCA is shown in Fig. 3b,c. For ER+HER2− tumors (Fig. 3b), all four PDX tumors were clustered together, with limited overlap with the original patient specimens. Two ER+HER2+ PDX lines were also distinct from the original tumor specimens (Fig. 3c), especially when examined based on the first principal component (x-axis).
4. Discussion
Human tumors propagated in immune-compromised animals where tumor tissue excised at the time of surgery and immediately transplanted into immune deficient mice (PDX models) is an exciting technology. PDX models appear to maintain the genetic characteristics of their original tumors, and comprehensive genomic analyses have demonstrated that PDX models maintain the similar same overall global gene expression and activity as the source tumors [1, 12, 25-27]. Therefore these mouse models are thought to be powerful for preclinical studies of targeted therapeutic strategies and molecular analysis, thus bridging the gap between laboratory discoveries and clinical translation.
With the long term interest of our group in ER+ breast cancer, an effort was initiated three years ago to generate ER+ PDXs. The potential utility of ER+ PDX models is particularly exciting to better understand molecular differences between ER+HER2− and ER+HER2+ breast cancers, as the latter is a newly recognized class of breast cancer [28] with a unique biology that requires different treatment strategies.
In the current study, we characterized six ER+ PDX mouse models. We found that the intake rate of higher stage and/or grade tumors was greater than that of low-grade tumors, which agreed with previous studies, and our loss of several ER+ xenografts after early transplantation passage (P0 to P1) was also similar to what had been reported previously [1, 11]. It was reported that ductal carcinomas had higher intake rate compared to lobular carcinomas [11]; our sample number of lobular carcinoma was only ten and none of them established as PDX.
In our study, ER+HER2− tumors were lost more frequently during early passage than ER+HER2+ tumors, and our subtype analysis demonstrated that the intake rate of ER+HER2+ tumors was higher than that of ER+HER2− tumors; 19% versus 7%, respectively. This may be explained by the more aggressive clinical characteristics of ER+HER2+ breast cancer compared with ER+HER2− breast cancer and supports the hypothesis that ER+HER2− and ER+HER2+ tumors are biologically different. The intake rate might be higher if we waited longer than 4 months after the first inoculation. We classified as “not growing” when tumors were not palpable 4 months after inoculation because we were concerned that a late establishment of PDX could be affected by the mouse mammary gland microenvironment.
Breast cancers are classified in four molecularly distinct diseases with different features, clinical behavior and treatment response; basal like, HER2-enriched and luminal A and B subtypes. Luminal A tumors were characterized as having high ER expression and proliferation-related genes, whereas luminal B cancer has lower expression of ER and PR, in addition to higher expression of proliferation cluster genes [29]. In this study, we demonstrated that RPPA analysis is a cost-effective way to define luminal subtypes by examining the expression profiles of 297 important signaling proteins. We identified the unique groups comprised of 6 proteins [ER, AR, Bcl-2, Bim (BCL2L11), GATA3 and INPP4b] which significantly impact tumor intake in ER+ tumors. Our RPPA analysis showed that ER+HER2− tumors could be separated into 2 clusters (luminal A and B) and three ER+HER2− PDX’s were derived from tumors in the luminal B cluster. TCGAreported that high expression of ER, PR, AR, Bcl-2, GATA3 and INPP4b defined the luminal A cancers [24]. Interestingly, these unique protein groups were lower in ER+HER2+ tumor groups. These finding suggested that these unique 6 proteins [ER, AR, Bcl-2, Bim (BCL2L11), GATA3 and INPP4b] were not only an important signature to distinguish luminal A and B tumors, also this signature of original patient tumors has significant impact on ER+ tumors’ PDXs establishment. Moreover, IPA analysis showed that higher expression of signaling molecules in the RAS/ERK pathway and PI3K/AKT pathway and lower expression of signaling molecules in AMPK pathway were found in luminal B tumors. The PI3K signaling pathway is often activated through different molecular mechanisms in ER+ breast cancer, such as mutations and loss or amplification of genes encoding key components of the pathway. AKT is located downstream of the PI3K pathway, and its phosphorylation leads to cell growth, proliferation, and survival in ER+ breast cancers [30]. RAS/ERK pathway is the one of the most relevant MAP kinase pathway in breast cancer which transmits and amplifies signals for cell proliferation [31]. Acetyl Co-A Carboxylase (ACC) and pACC are both unregulated, and they are the established target of AMPK [32]. AMPK activation is known to inhibit mTOR pathway [33]. Reducing signaling of the AMPK pathway related to higher pathological grade and node metastasis [32]. Therefore our results confirm that luminal B tumors have significantly different protein expression profiles from luminal A tumors and are a more clinically aggressive type of ER+ tumors, this is corresponding to three ER+HER2− PDX’s derived from this subtype.
Further dissected RPPA analysis supported our main finding that expression of the ER protein itself may have a large bearing on the potential success of an ER+ PDX model; expression of the ER protein was significantly lower in ER+HER2− patient tumors that successfully established PDX models compared with ER+HER2− patient tumors that were unable to establish PDX models. GATA 3 regulated ER transcriptional activity in breast cancer cell lines [34] and expression of GATA3 was highly associated with ER expression [35]. Our analysis showed its decrease was also observed corresponding with ER decrease. It is also reported that engraftment rate was associated with the expression levels of ER [8, 36]. These suggest that ER expression may be a negative regulator of PDX intake in ER+HER2− breast cancer PDX models. This finding is supported by observations showing that low ER expression is a marker for cancer stem cells [37] and ER is a marker of human breast cancer differentiation [38]. In ER+HER2+ tumor samples, lower ER tumors had better tumor intake; in addition, we showed that HER2 expression was differentially regulated between ER+HER2+ patient tumors that successfully established ER+HER2+ PDX models and those that failed. This suggests that high HER2 expression in primary tumors may be a determining factor affecting the intake rate for ER+HER2+ PDX models. There are at least two types of clinically defined HER2+ tumors, HER2E mRNA-subtype/HER2+ and luminal-mRNA-subtype/HER2. HER2E-mRNA-subtype/HER2 tumors showed higher expression of FGFR4, EGFR, HER2 [24]. On the other hand, the luminal-mRNA-subtype/HER2 tumors showed higher expression of the luminal cluster of genes including GATA3, Bcl-2 and ER. In ER+HER2+ tumors, tumors with higher HER2 and lower ER have better intake rate, suggesting they were HER2E mRNA-subtype/HER2+.
It is important to compare our findings verse those from other laboratories. Charafe-Jauffret et al. showed that HER2 status was not a determining factor for successful tumor engraftment [8]. Li et al. showed that successful PDX rates were lower using ER+HER2− patient tumors compared to HER2+ tumors [39]. Manoir et al. reported that the intake rate using ER+HER2+ patient tumors was higher than the rate using ER+HER2− tumors [40]. Of these reports, only the study by Manior et al. analyzed the effect of HER2 status separately on ER+HER2− and ER+HER2+ tumors. Taken together with our results, HER2 expression may increase the intake rate of ER+ tumors, but ER expression may decrease the intake rate of HER2+ tumors. Therefore, ER+HER2+ tumors are probably more aggressive than ER+HER2− tumors, but less aggressive than ER−HER2+ tumors. These findings also suggest that a better understanding of the factors that drive successful ER+ PDX models will rely on analysis of the different ER+ classes (ER+HER2−; ER+HER+) separately. For example, our RPPA analysis has revealed that protein expression profile of ER+HER2+ tumors resembles more that of luminal A, not luminal B tumors, except the levels of those proteins in region * (Fig. 2d). The availability of ER+HER2+ PDX models will be critical to further define the molecular basis of this type of breast cancer and to test treatment modalities on them.
In our search for genes that increase the intake rate of ER+ PDX in NSG mice, we identified L1 cell adhesion molecule (L1CAM) as a potential predictive factor for tumor intake. This protein was increased in both ER+HER2− and ER+HER2+ tumors that successfully established PDX models, compared to the tumors that failed to establish PDX models. L1CAM is an adhesion molecule that functions in the development of the nervous system. It has also been shown to be a critical factor for cancer progression; and high L1CAM expression is a prognostic factor in multiple cancers [41]. In ovarian cancer mouse models, expression of L1CAM increased tumor growth of xenografted carcinomas (m130 and HEK293) in female nonobese diabetic/severe combined immunodeficient mice (NOD/SCID)[42] and NIH3T3 cells xenografted in CD1 nude male mice [43]. Other groups also have been reported, i.e, primary breast tumors that successfully generate PDXs have more ALDH-positive cells (CSCs) than tumors that failed to graft [7, 8]. VEGF and IL8 were significantly higher in the primary tumors that give rise to PDX compared to those that did not [40]. Moon et al, reported that PHLDA2 and TKT were the engraftment-related genes in triple negative breast cancer PDXs [19]. Those results suggested that expression of genes of original human tumors could impact intake rate of PDXs, definitive information will require the analysis of more samples for different subtypes, separately.
Since we have protein expression results of 6 established PDX tumors and 6 original tumor samples, we could determine how similar or different their molecular profiles were. Both results from PCA and unsupervised hierarchical clustering showed PDX tumors had specific protein expression patterns that suggest the PDX models were more similar to each other than to the original tumors samples. This phenomenon could be explained by 2 theories, 1) tumors evolve dynamically in order to adapt to grow in a different host, and 2) specific clones which had preference expression pattern could survive to be dominant. Recently, important findings were reported by Eirew et al. to address these questions [9]. They analyzed clonal dynamics at single cell resolution using copy-number alteration (CAN) and showed that the clones are mostly prelisting, and clones evolve dynamically in the host. All those findings, including ours, showed that PDX could have different protein expression patterns than original tumors. It is recognized that the selection/adaptation process required to grow the tumors in mice is unavoidable, and we identified differences between patient tumor specimens and paired PDX tumors. In order to identify the dominant expression pattern in PDX, we performed IPA analysis using RPPA results. Our results showed that the commonly changed canonical pathway in both ER+HER2− and ER+HER2+ PDX tumors that successfully grew in mice compared to the tumors that did not was the PI3K/AKT signaling pathway. In addition, the mTOR pathway was activated in both ER+HER2+ and ER+HER2− PDX tumors compared to paired human samples, evident in increased of S6 (pS235 or pS240) protein expression in PDX tumors compared to paired patient tumor samples. The mTOR pathway is dysregulated in breast cancer, which affects cell growth and tumor proliferation [44]. Therefore, these PDX models will be valuable to examine drugs targeting to PI3K/AKT/mTOR pathways. It is not surprising that we identified proteins and pathways responsible for increased tumor cell survival as dominant factors for tumor intake.
5. Conclusions
Based on our experience, ER+ PDX models probably could not be used to identify the treatment options for the patients who donated the tissue samples because of the following reasons: 1) ER+ tumors have a low intake rate, such that PDX could not be established from all patients; 2) it typically requires 4-6 months to establish transplantable PDXs; 3) the tumors that could establish PDXs had a different protein expression profile compared to patient tumors that could not be established and 4) the protein profile of established tumors was evolved/selected with changes in the microenvironment of the host. However, we would like to emphasize that PDXs with well characterized molecular profiles are significantly helpful in identifying new treatments for future patients who have similar molecular characteristics. For example, our PDX models express different levels of ER, allowing us to address the roles of ER using these models. Furthermore, there are not many ER+HER2+ cell lines that are available for in vitro studies, and our protein expression profiling has revealed that ER+HER2+ tumors are different from ER+HER2− tumors (both luminal A and B types); therefore, ER+HER2+ PDX models are powerful tools in predicting the translation of cancer treatment in a preclinical setting. In addition, the availability of both ER+HER2− and ER+HER2+ PDX models will allow us to test drugs selectively toward each ER+ breast cancer classes. Finally, our studies have revealed that ER+ PDXs have reduced expression of a group of six proteins. Such results are being validated using newly generated ER+ PDXs at City of Hope.
Supplementary Material
Highlights.
The intake rate of ER+HER2+ tumors is higher than that of ER+HER2− tumors.
The protein signature associated with the rate of ER+ PDX intake was identified.
Different protein expression was observed between patient tumor samples and paired PDX tumors.
PDX models are valuable in evaluating treatments in a preclinical setting.
Acknowledgements
The authors would like to thank Nicola Solomon, PhD, for assistance with writing and editing the manuscript. We also would like to thank Catherine Scher and Sylvana Salvatierrafor obtaining samples from COH tumor banks, and Tina Montgomery for immunohistochemical staining.
Funding
This work was supported by Panda Charitable Foundation. This work was also supported by the National Cancer Institute (P30 CA033572). The funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Marangoni E, Poupon MF. Patient-derived tumour xenografts as models for breast cancer drug development. Curr Opin Oncol. 2014;26(6):556–61. doi: 10.1097/CCO.0000000000000133. [DOI] [PubMed] [Google Scholar]
- [2].Hidalgo M, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lodhia KA, et al. Prioritizing therapeutic targets using patient-derived xenograft models. Biochim Biophys Acta. 2015;1855(2):223–34. doi: 10.1016/j.bbcan.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gao H, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318–25. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- [5].DeRose YS, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17(11):1514–20. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cottu P, et al. Modeling of response to endocrine therapy in a panel of human luminal breast cancer xenografts. Breast Cancer Res Treat. 2012;133(2):595–606. doi: 10.1007/s10549-011-1815-5. [DOI] [PubMed] [Google Scholar]
- [7].Kabos P, et al. Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat. 2012;135(2):415–32. doi: 10.1007/s10549-012-2164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Charafe-Jauffret E, et al. ALDH1-positive cancer stem cells predict engraftment of primary breast tumors and are governed by a common stem cell program. Cancer Res. 2013;73(24):7290–300. doi: 10.1158/0008-5472.CAN-12-4704. [DOI] [PubMed] [Google Scholar]
- [9].Eirew P, et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518(7539):422–6. doi: 10.1038/nature13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McAuliffe PF, et al. Ability to Generate Patient-Derived Breast Cancer Xenografts Is Enhanced in Chemoresistant Disease and Predicts Poor Patient Outcomes. PLoS One. 2015;10(9):e0136851. doi: 10.1371/journal.pone.0136851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marangoni E, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13(13):3989–98. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- [12].Landis MD, et al. Patient-derived breast tumor xenografts facilitating personalized cancer therapy. Breast Cancer Res. 2013;15(1):201. doi: 10.1186/bcr3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Onitilo AA, et al. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1-2):4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu VS, et al. From bench to bedside: What do we know about hormone receptor-positive and human epidermal growth factor receptor 2-positive breast cancer? J Steroid Biochem Mol Biol. 2015;153:45–53. doi: 10.1016/j.jsbmb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vici P, et al. Triple positive breast cancer: a distinct subtype? Cancer Treat Rev. 2015;41(2):69–76. doi: 10.1016/j.ctrv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- [17].Strasser-Weippl K, et al. Long-term hazard of recurrence in HER2+ breast cancer patients untreated with anti-HER2 therapy. Breast Cancer Res. 2015;17:56. doi: 10.1186/s13058-015-0568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goldhirsch A, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18(7):1133–44. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- [19].Moon HG, et al. Prognostic and functional importance of the engraftment-associated genes in the patient-derived xenograft models of triple-negative breast cancers. Breast Cancer Res Treat. 2015;154(1):13–22. doi: 10.1007/s10549-015-3585-y. [DOI] [PubMed] [Google Scholar]
- [20].DeRose YS, et al. Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol. 2013 doi: 10.1002/0471141755.ph1423s60. Chapter 14: p. Unit14 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–54. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- [22].Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. p. Article3. [DOI] [PubMed] [Google Scholar]
- [23].Benjamini Y.a.H., Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57(1):289–300. [Google Scholar]
- [24].Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choi SY, et al. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv Drug Deliv Rev. 2014;79-80:222–37. doi: 10.1016/j.addr.2014.09.009. [DOI] [PubMed] [Google Scholar]
- [26].Whittle JR, et al. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. 2015;17:17. doi: 10.1186/s13058-015-0523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nat Rev Cancer. 2015;15(5):311–6. doi: 10.1038/nrc3944. [DOI] [PubMed] [Google Scholar]
- [28].Howlader N, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):1–8. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ades F, et al. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J Clin Oncol. 2014;32(25):2794–803. doi: 10.1200/JCO.2013.54.1870. [DOI] [PubMed] [Google Scholar]
- [30].Zardavas D, Fumagalli D, Loi S. Phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway inhibition: a breakthrough in the management of luminal (ER+/HER2−) breast cancers? Curr Opin Oncol. 2012;24(6):623–34. doi: 10.1097/CCO.0b013e328358a2b5. [DOI] [PubMed] [Google Scholar]
- [31].Santen RJ, et al. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80(2):239–56. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- [32].Hadad SM, et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer. 2009;9:307. doi: 10.1186/1471-2407-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qi J, et al. Absent, small or homeotic 2-like protein (ASH2L) enhances the transcription of the estrogen receptor alpha gene through GATA-binding protein 3 (GATA3) J Biol Chem. 2014;289(45):31373–81. doi: 10.1074/jbc.M114.579839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Voduc D, Cheang M, Nielsen T. GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol Biomarkers Prev. 2008;17(2):365–73. doi: 10.1158/1055-9965.EPI-06-1090. [DOI] [PubMed] [Google Scholar]
- [36].Zhang X, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013;73(15):4885–97. doi: 10.1158/0008-5472.CAN-12-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kabos P, et al. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2011;128(1):45–55. doi: 10.1007/s10549-010-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Osborne CK, et al. Estrogen receptor, a marker for human breast cancer differentiation and patient prognosis. Adv Exp Med Biol. 1981;138:377–85. doi: 10.1007/978-1-4615-7192-6_23. [DOI] [PubMed] [Google Scholar]
- [39].Li S, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4(6):1116–30. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].du Manoir S, et al. Breast tumor PDXs are genetically plastic and correspond to a subset of aggressive cancers prone to relapse. Mol Oncol. 2014;8(2):431–43. doi: 10.1016/j.molonc.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Altevogt P, Doberstein K, Fogel M. L1CAM in human cancer. Int J Cancer. 2015 doi: 10.1002/ijc.29658. [DOI] [PubMed] [Google Scholar]
- [42].Gast D, et al. L1 augments cell migration and tumor growth but not beta3 integrin expression in ovarian carcinomas. Int J Cancer. 2005;115(4):658–65. doi: 10.1002/ijc.20869. [DOI] [PubMed] [Google Scholar]
- [43].Gavert N, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168(4):633–42. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Paplomata E, O'Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6(4):154–66. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.