Figure 1. MUC16/MUC16C interacts with β-catenin.
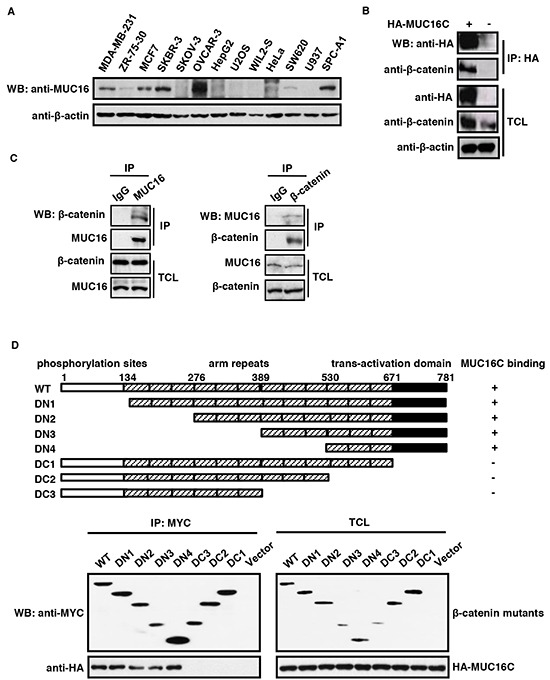
A. Expression profile of MUC16 in different cell lines. The cell lysates were analyzed by Western blot with the self-made mouse anti-MUC16 (40 μg input) or mouse anti-β-actin (8 μg input) antibody respectively. B. Ectopically expressed MUC16C interacts with endogenous β-catenin. HeLa cells were transfected with pcDNA3.3-HA-MUC16C or the empty vector as a control. At 24 h post-transfection, cells were lysed and subjected to immunoprecipitation with mouse anti-HA antibody, followed by Western blot with mouse anti-HA and rabbit anti-β-catenin antibodies separately. C. MUC16 and β-catenin interact with each other in vivo. Lysates of SKBR-3 cells were subjected to immunoprecipitation with the control IgG, rabbit anti-β-catenin and mouse anti-MUC16 antibodies respectively. The precipitates were then detected with indicated antibodies. D. C-terminus of β-catenin is essential for its interaction with MUC16. For the domain mapping experiment, structures of deletion mutants of β-catenin are shown on the top of the panel. Functional domains of β-catenin are indicated above the schema; the remaining fragments of each deletion mutant are shown in the diagram. HEK293T cells were co-transfected with pcDNA3.3-HA-MUC16C and different MYC-β-catenin mutants or the empty vector as a control. At 24 h post-transfection, cells were lysed and subjected to immunoprecipitation with rabbit anti-MYC antibody, followed by Western blot with mouse anti-HA or anti-MYC antibody.
