Abstract
An extracellular β‐glucosidase (E.C. 3.2.1.21), induced by cellulose in the mycelial form of human pathogen fungus Sporothrix schenckii, was purified to homogeneity using hydroxyapatite (HAp) adsorption chromatography in batch and Sephacryl S200‐HR size exclusion chromatography. The molecular mass of the purified enzyme was estimated to be 197 kDa by size exclusion chromatography with a subunit of 96.8 kDa determined by SDS/PAGE. The β‐glucosidase exhibited optimum catalytic activity at pH 5.5/45 °C and was relatively stable for up to 24 h at 45 °C. Isoelectric focusing displayed an enzyme with a pI value of 4.0. Its activity was inhibited by Fe2+ but not by any other ions or chelating agents. K m and V max values of the purified enzyme were 0.012 mm and 2.56 nmol·min−1·mg−1, respectively, using 4‐methylumbelliferyl β‐D‐glucopyranoside (4‐MUG) as the substrate and 44.14 mm and 22.49 nmol·min−1·mg−1 when p‐nitrophenyl β‐D‐glucopyranoside (p‐NPG) was used. The purified β‐glucosidase was active against cellobioside, laminarin, 4‐MUG, and p‐NPG and slightly active against 4‐methylumbelliferyl β‐D‐cellobioside and p‐nitrophenyl β‐D‐cellobioside but did not hydrolyze 4‐methylumbelliferyl β‐D‐xyloside, 4‐methylumbelliferyl β‐D‐galactopyranoside nor 4‐methylumbelliferyl α‐D‐glucopyranoside. In addition, the enzyme showed transglycosylation activity when it was incubated along with different oligosaccharides. Whether the transglycosylation and cellulase activities function in vivo as a mechanism involved in the degradation of cellulolytic biomass in the saprophytic stage of S. schenckii remains to be determined.
Keywords: biochemical characterization, purification, Sporothrix schenckii, β‐glucosidase
Abbreviations
- 4‐MU
4‐methylumbelliferyl
- 4‐MUC
4‐methylumbelliferyl β‐D‐cellobioside
- 4‐MUG
4‐methylumbelliferyl β‐D‐glucopyranoside
- HAp
hydroxyapatite
- p‐NPC
p‐nitrophenyl β‐D‐cellobioside
- p‐NPG
p‐nitrophenyl β‐D‐glucopyranoside
Sporothrix schenckii is a dimorphic fungus within the family Ophiostomataceae able to infect humans and other mammals 1, 2. It is part of a species complex named Sporothrix schenckii complex along with S. globosa, S. brasiliensis, S. schenckii sensu stricto, S. luriei, S. albicans, S. mexicana, S. brunneoviolacea, and S. pallida 3, 4. This fungus is widely distributed in nature and grows as a saprophyte on dead or decomposing matter and also shows association with plants 5, 6. As other filamentous fungi, it is able to degrade biomass and organic matter due to its protein secretion system 7, 8, 9. One of the principal polymers and the main structural component of plant cell wall is the cellulose, a linear polymer of β‐(1,4)‐d‐glucose residues which can be hydrolyzed by different cellulases produced by several microorganisms in order to obtain glucose. One of the enzymes secreted by filamentous fungus 10, 11 and other microorganisms 12, 13 helping to degrade the cellulose is the β‐glucosidase. This enzyme releases glucose by acting on cello‐oligosaccharides originated by the action of other enzymes of the cellulolytic enzyme complex. Up to date, the most studied enzymes on S. schenckii are the proteinases. In the yeast parasitic form, it was determined that the proteolytic activity by different species of Sporothrix 14 and two proteinases were purified 15. Hydrolytic activity of different enzymes such as acid phosphatase 16, dextranase 17, catalase, urease, and gelatinase 14 in S. schenckii has been also detected.
Most of the studies performed on this fungus have been focused on its yeast phase due to its medical importance 1, 18, 19, therefore, its saprophytic stage has not been extensively studied and little is known about its metabolism in their saprophytic stage. In order to get an approach to its physiology and its poorly known saprophytic stage, we report the purification and characterization of the first extracellular enzyme from S. schenckii involved in the degradation of cellulolytic biomass.
Experimental procedures
Strains and culture conditions
Sporothrix schenckii, strain EH‐206 kindly provided by Dra. Conchita Toriello (UNAM, Unidad de Micología, Mexico), was maintained on Yeast extract Peptone Dextrose agar (YPD) at 28 °C. For cellulase production, 1 × 106 conidia per mL of S. schenckii were inoculated in Erlenmeyer flasks containing Mathur's medium: 10 mm MgSO4·7H2O; 20 mm KH2PO4; 35 mm l‐glutamic acid and 0.2% glucose, supplemented with 1% or 2% cellulose (Sigmacell, type 101; Sigma, St. Louis, MO, USA); final pH 4.5, and incubated at 28 °C with continuous shaking (120 r.p.m.). For enzymatic activity induction, we used a 125‐mL Erlenmeyer flask containing 40 mL of medium and 2‐L Erlenmeyer flasks with 600 mL of medium for the β‐glucosidase purification.
Sample preparation
For protein quantification and activity test, the samples were prepared as described below. After incubation, cultures were filtered through Whatman # 1 filter paper, the cell‐free growth medium was freeze dried and dissolved in a small volume of deionized water. The samples were centrifuged at 3000 g for 10 min to remove remaining cellulose. Low molecular weight metabolites and salts were removed by eluting the supernatant through a column (1.0 × 10 cm) of Bio‐Gel P‐10 with 10 mm phosphate buffer, pH 7.0. This sample, filtered and concentrated cell‐free extract, was denominated CFCF, fractions with β‐glucosidase activity were stored at 4 °C until the protein purification and quantification.
Induction of β‐glucosidase activity
To establish the optimal conditions for cellulolytic activities like induction, we performed growth kinetics and measured the β‐glucosidase activity at different times ranging from 12, 24, 48, 72, and 120 h, using 2% cellulose as the sole carbon source or glucose at the same concentration as a control. Fungal growth was measured as mycelium protein since residual cellulose present in the medium interfered with dry weight quantitation.
Purification of β‐glucosidase
β‐glucosidase purification was carried out at 4 °C. Sporothrix schenckii was grown during a period of 3 days, 3 L of medium was used as the starting material for enzyme purification. The desalted and concentrated sample was applied on 1 mL of hydroxyapatite (HAp) adsorption chromatography in batch, equilibrated with 10 mm phosphate buffer, pH 7, and eluted with a discontinuous phosphate buffer gradient. Fractions with positive activity were freeze dried, resuspended in 1 mL of deionized water, and eluted with 50 mm phosphate buffer, pH 7 in a Sephacryl S200‐HR size exclusion chromatography column (1 × 119 cm, 93.5 mL). Fractions with β‐glucosidase activity were pooled and used for biochemical characterization.
Enzyme assay
β‐glucosidase activity was measured by a fluorometric method using 4‐MUG (Sigma) as substrate 20. Reaction mixture consisting of 4‐MUG (5 μm), enzyme fraction, and 100 mm citrate buffer, pH 5.0, in a final volume of 200 μL were incubated at 45 °C. After 1 h, the reaction was stopped with 2.5 mL of 0.5 m Na2CO3 buffer, pH 10.4 and the 4‐methylumbelliferone (4‐MU) released was measured in a Perkin–Elmer LS‐5B luminescence spectrometer with excitation and emission set at 350 and 440 nm, respectively. β‐glucosidase activity was expressed in terms of specific activity as nmol of 4‐MU·min−1·mg−1 protein. To determine the specificity of the purified enzyme, its activity was tested against p‐NP‐glycosides, using p‐NPG and p‐NPC (Sigma) as substrates 21. Briefly, the enzyme fraction, the substrate at 5 mm final concentration and 100 mm citrate buffer, pH 5.0 in a final volume of 200 μL were mixed and incubated for 1 h at 45 °C. The reaction was stopped with 2.5 mL of 0.5 m Na2CO3 buffer, pH 10.4 and the p‐NP released was measured at 405 nm. The specific activity was expressed as nmol of p‐NP liberated per minute per milligram of protein. The purified enzyme was also tested against the natural substrates, cellobiose, and laminarin (Sigma). In this case the activity was assayed using the 3,5‐dinitrosalicylic acid (DNS) method for reducing sugar analysis using glucose as the standard 22. The reaction containing the purified enzyme, 50 μL of 1% substrate in 100 mm citrate buffer, pH 5.0 was carried out at 45 °C for 1 h. 300 μL of DNS reagent was added and the samples were boiled for 10 min. The color developed was measured at 540 nm. The enzyme activity was defined as the nmol of glucose liberated per minute per milligram of protein.
Protein quantitation
Protein concentration was estimated by the Bicinchoninic acid method 23 and Lowry's method 24 using bovine serum albumin as standard. Protein in the column eluates was monitored by measuring A280.
Determination of molecular mass by gel filtration
The molecular mass of the native protein was estimated by gel filtration on Sephacryl S200‐HR column, calibrated with: thyroglobulin (M r 669 000), β‐amylase (M r 200 000), ADH (M r 150 000), bovine serum albumin (M r 66 000), carbonic anhydrase (M r 29 000), cytochrome c (M r 12 000), and cyanocobalamin (M r 1350) as standard proteins (Sigma).
pI determination
The pI was determined using the Rotofor® System by Bio‐Rad according to the manufacturer instructions using Bio‐Lyte® ampholytes in the pH range 3–10.
Effects of metal ions and chelating agents on the enzyme activity
Effects of divalent metal ions and chelating agents on the β‐glucosidase activity were also evaluated. These were studied by determining the activity of the purified β‐glucosidase toward 4‐MUG in the presence of cations or chelant agents at different concentration (2.5–10 mm). The experiment was conducted in triplicate.
Thermostability
The thermostability of the purified enzyme was determined by incubating the enzyme in 100 mm sodium citrate buffer, pH 5 at 45 °C for different time periods (0–24 h). The remaining β‐glucosidase activity was measured as described above. The experiment was conducted in triplicate.
Determination of kinetic parameters
The Michaelis–Menten kinetics parameters (V max and K m) of hydrolysis of 4‐MUG and p‐NPG by the purified enzyme were determined by prisma 7 software using nonlinear regression with different substrate concentrations of the substrates (0–15 μm and 0–17.5 mm for 4‐MUG and p‐NPG, respectively) in 100 mm sodium citrate buffer, pH 5 at 45 °C.
Transglycosylase activity
A reaction mixture consisting of 48 μg of the purified enzyme, 0.02% sodium azide, 100 mm citrate buffer, pH 5.0, and 2.5 μg of cello‐oligosaccharides of different length (cellobiose, cellotriose, and cellotetraose, Seikagaku America, Associates of Cape Cod, Inc., MA, USA), in a final volume of 400 μL, were incubated at 45 °C. After 10 h of incubation, 1 mL of cold acetone was added and the samples were incubated at −20 °C for 30 min. Samples were centrifuged at 10 000 g for 10 min and the acetone from the recovered supernatant was evaporated. The samples were freeze dried and resuspended in the mínimum volume.
Hydrolytic products from cello‐oligosaccharides were analyzed by TLC using a silica gel TLC plate (Silica gel 60 F254; Merck Millipore, Darmstadt, Alemania) in a solvent system of butanol : 2‐propanol : water (3 : 12 : 4, by vol.) and the released sugars were visualized using a silver stain 25. Standard sugars: glucose (G1) at 1 μg, cellobiose (G2) at 2 μg, cellotriose (G3) at 4 μg, and cellotetraose (G4) at 4 μg.
SDS/PAGE
Purified protein pattern was analyzed through a 7% SDS/PAGE. The protein was stained with Flamingo™ fluorescent stain (Bio Rad Mexico, CDMX, Mexico). Relative molecular mass (M r) was calculated by comparison with the migration of the standard proteins (Bio‐Rad, SDS/PAGE Broad Range, 161–0317).
Zymogram analysis
For detection of β‐glucosidase activity through zymogram techniques a 7%, SDS/PAGE or native PAGE, were performed at 4 °C. In both conditions, the sample was incubated for 30 min at 37 °C in sample buffer. At the end of the electrophoresis, gels were rinsed 2× in water and 3× in 100 mm sodium citrate buffer, pH 5, for 5 min each. This was followed by incubation for 1 h at 45 °C in the presence of 4‐MUG (5 μm) as substrate. The zymogram gel was visualized under UV light to detect fluorescence due to the 4‐MU released.
Results
Growth kinetics and secreted β‐glucosidase activity
Sporothrix schenckii showed a higher growth rate when the medium was supplemented with glucose than cellulose (Fig. 1A), possibly due to cellulose crystalline nature that makes its degradation more complex, diminishing the fungus growth. The amount of extracellular protein when the fungus was grown in the presence of cellulose was four times higher than when glucose was added as the sole carbon source, suggesting the secretion of different enzymes in order to degrade the polysaccharide.
Figure 1.
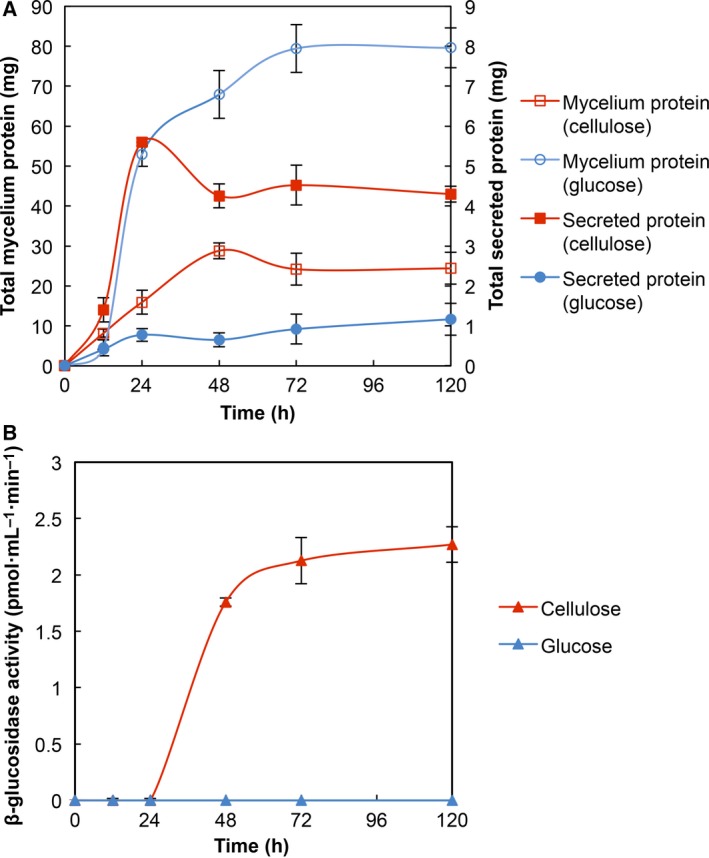
Growth kinetics of the mycelium form of Sporothrix schenckii and secreted β‐glucosidase activity. (A) Growth and secretion of protein by S. schenckii in the presence of 2% glucose ( , mycelium protein;
, mycelium protein;  , secreted protein) or under induction conditions with 2% cellulose (
, secreted protein) or under induction conditions with 2% cellulose ( , mycelium protein;
, mycelium protein;  , secreted protein). (B) Secreted β‐glucosidase activity in the presence of 2% glucose (
, secreted protein). (B) Secreted β‐glucosidase activity in the presence of 2% glucose ( ) or 2% cellulose (
) or 2% cellulose ( ).
).
The extracellular β‐glucosidase activity was determined by using 4‐MUG as substrate and measured as described in Experimental procedures. It was detected after 24 h of incubation and showed the highest activity at 72 h remaining constant through 120 h (Fig. 1B). This behavior suggests the action of previous enzymes such as endoglucanases and cellobiohydrolases acting on the cellulose to generate oligosaccharides which can be subsequently hydrolyzed by β‐glucosidase.
Purification of S. schenckii β‐glucosidase
Previous to the purification, zymogram gels were conducted to confirm the presence of β‐glucosidases and to estimate their molecular weight when the fungus was incubated in cellulose as the sole carbon source. Under semidenaturated conditions, we observed two bands with molecular weights of 204.9 and 97.2 kDa (Fig. 2A) while in native‐zymogram we obtained only the high molecular weight band (Fig. 2B).
Figure 2.
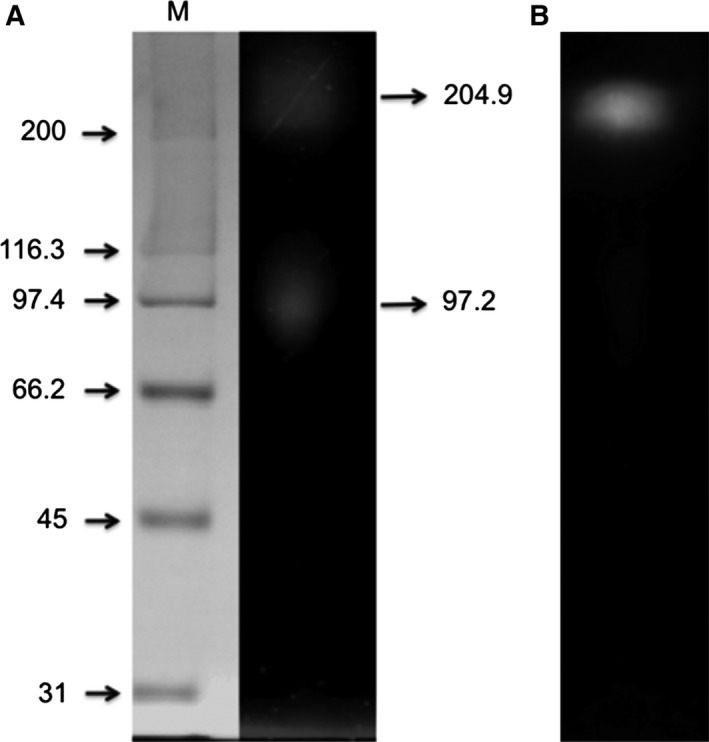
Zymogram of the β‐glucosidase activities from the CFCF (cell‐free concentrated filtered) recovered after 3 days of incubation. (A) Denaturated‐zymogram and (B) native‐zymogram. M, molecular weight standards.
The enzyme was purified to homogeneity in a two‐step protocol. Previous to its adsorption in hydroxyapatite resin (HAp), the sample was treated as described in methods. The enzymatic activity in CFCF did not interact with the HAp resin and it was recovered in the initial eluted fractions. Contaminant proteins were removed with 10 mm and 1 m phosphate buffer, pH 7. Fractions with β‐glucosidase activity were concentrated and applied to Sephacryl S200‐HR size exclusion chromatography. β‐glucosidase activity was recovered in a single symmetric enzymatic activity peak (fractions 39–43) with an estimated M r of 197 kDa and undetectable protein measured at A280 (Fig. 3). Fractions with positive activity were pooled and concentrated to determine its purity and to performe its biochemical characterization. Figure 4 shows the SDS/PAGE analysis (Fig. 4A) of the purified enzyme where only one protein band is exhibited with a calculated M r of 96.8 kDa and a zymogram under native conditions (Fig. 4B). A summary of the purification results is given in Table 1. Due to culture interferences to determine protein, the calculation of purification, was made after the remotion of salts. The enzyme was purified to homogeneity with a yield of 50.2% and a specific activity of 800.80 U·mg·protein−1.
Figure 3.
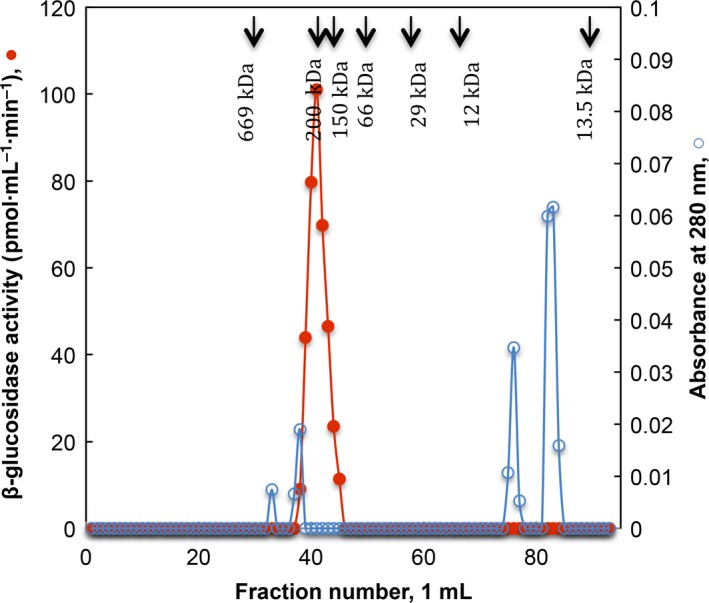
Sephacryl S‐200 HR gel filtration of β‐glucosidase activity. Profile elution of β‐glucosidase activity ( ) and protein (
) and protein ( ). Elution position of standard proteins is indicated with the arrows.
). Elution position of standard proteins is indicated with the arrows.
Figure 4.
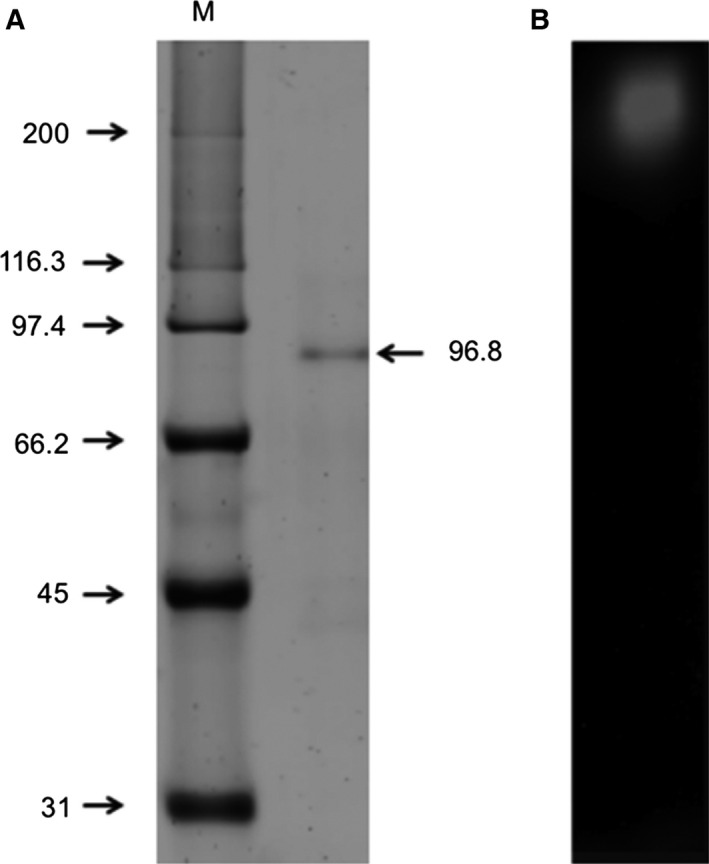
SDS/PAGE and zymogram of the purified β‐glucosidase. (A) Purified enzyme stained with Flamingo (Bio‐Rad) and (B) native‐zymogram. M, molecular weight standards.
Table 1.
Summary of the purification of Sporothrix schenckii β‐glucosidase. 1 unit (U) = 1 nmol methylumbelliferone released from methylumbelliferyl‐β‐D‐glucoside per min
| Purification steps | Total protein (mg) | Total activity (U) | Specific activity (U·mg·protein−1) | Fold purification | Yield (%) |
|---|---|---|---|---|---|
| Bio‐Gel P‐10 | 6.15 | 1.531 | 0.249 | 1.0 | 100.00 |
| HAp (in batch) | 4.40 | 1.225 | 0.278 | 1.1 | 80.01 |
| Sephacryl S200‐HR | 0.96 | 0.769 | 0.801 | 3.2 | 50.20 |
Biochemical characterization of β‐glucosidase
The β‐glucosidase activity was measured at different temperatures and pH values (Fig. 5). The optimum temperature determined for the purified enzyme was 45 °C (data not shown) and it was relatively stable when incubated at 45 °C for 24 h, retaining 60% of its initial activity (Fig. 6). Maximum enzyme activity was at pH 4.5–5.5 with citrate, acetate, or citrate‐phosphate buffers and no activity was observed at pH values above 7.5 using TRIS‐HCl and phosphate buffers (Fig. 5B). The pI value of the purified enzyme was determined with a preparative electrofocusing using a Rotofor (Bio‐Rad). The results showed a pI value in a range of 3.2–4.9 with the highest value at 4.0 (Fig. 7).
Figure 5.
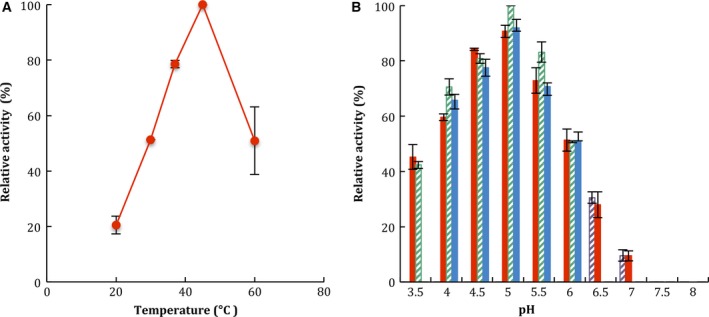
Effects of temperature (A) and pH (B) on β‐glucosidase activity from Sporothrix schenckii. To determine the effect of temperature on the activity, purified enzyme was incubated with 4‐MUG as substrate at 20–60 °C in 100 mm citrate buffer pH 5. To determine the effect of pH on the activity, purified enzyme was incubated with 4‐MUG as substrate in 100 mm following buffers: phosphate, pH 6.5–8.0 ( ), citrate‐phosphate, pH 3.5–8.0 (
), citrate‐phosphate, pH 3.5–8.0 ( ), citrate, pH 3.5–6.0 (
), citrate, pH 3.5–6.0 ( ), acetate, pH 4.0–6.0 (
), acetate, pH 4.0–6.0 ( ). Data are expressed as relative activity (%) where the maximum specific activity is 0.801 nmol of 4‐MU·mg−1·min−1. Values are means ± SD for three independent experiments.
). Data are expressed as relative activity (%) where the maximum specific activity is 0.801 nmol of 4‐MU·mg−1·min−1. Values are means ± SD for three independent experiments.
Figure 6.
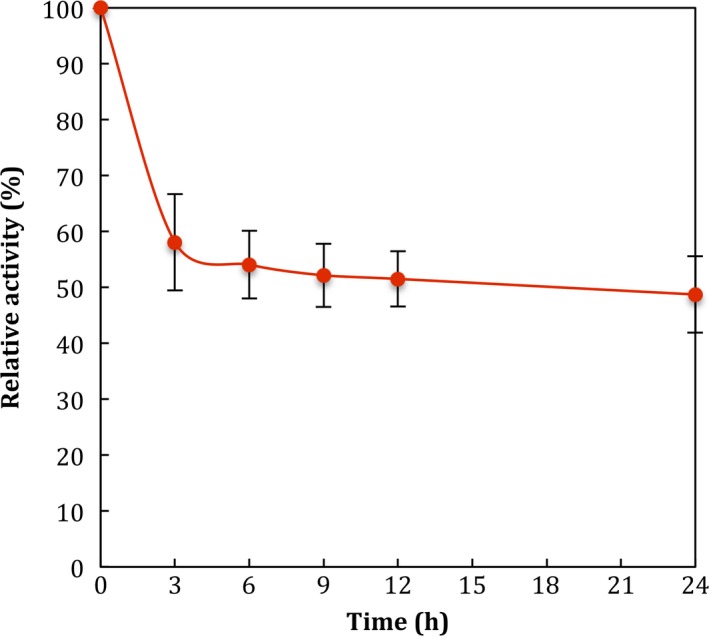
Effect of temperature on the stability of purified β‐glucosidase from Sporothrix schenckii.
Figure 7.
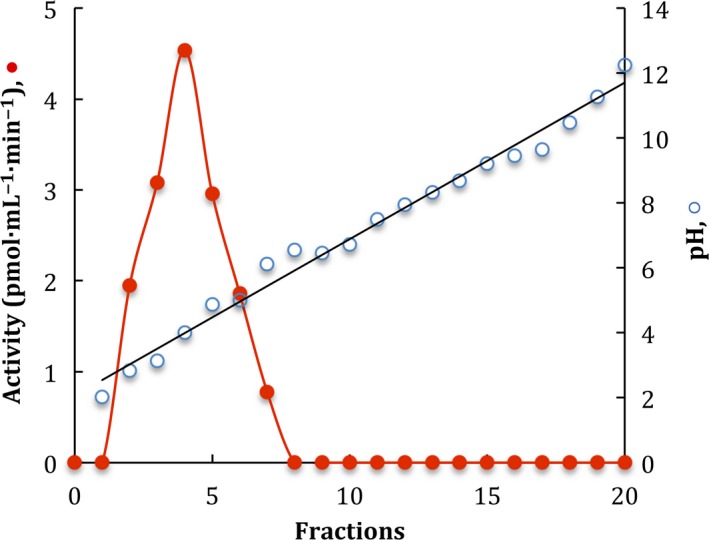
Isoelectrofocusing of the purified β‐glucosidase. Twenty fractions were collected and pH and β‐glucosidase activity ( ) were determined.
) were determined.
The effect of divalent ions and chelating agents were tested on the purified β‐glucosidase at different concentrations: 2.5, 5.0, and 10.0 mm (Table 2). The β‐glucosidase activity was only inhibited by Fe2+ addition and was not significantly influenced by any tested reagents.
Table 2.
Effect of divalent ions and chelating agents on β‐glucosidase activity. Values are means ± SD for three different experiments
| Metal ion | Relative activity (%)a | ||
|---|---|---|---|
| 2.5 mm | 5.0 mm | 10.0 mm | |
| None | 100 ± 0.2 | 100 ± 0.2 | 100 ± 0.2 |
| Ca2+ | 99 ± 1.9 | 99 ± 1.6 | 97 ± 1.1 |
| Co2+ | 99 ± 2.9 | 96 ± 4.9 | 94 ± 1.8 |
| Cu2+ | 93 ± 4.0 | 96 ± 5.5 | 93 ± 5.8 |
| Fe2+ | 72 ± 1.6 | 40 ± 1.3 | 19 ± 2.4 |
| Mg2+ | 95 ± 5.1 | 94 ± 0.6 | 96 ± 1.3 |
| Mn2+ | 98 ± 1.8 | 97 ± 2.1 | 95 ± 4.2 |
| EDTA | 98 ± 2.2 | 98 ± 1.5 | 96 ± 3.0 |
| EGTA | 95 ± 3.5 | 97 ± 4.5 | 97 ± 3.9 |
100% activity corresponds to 0.801 nmol·min−1·mg−1 protein.
According to nonlinear regression, Michaelis–Menten enzyme kinetics calculated were, the K m value for p‐NPG (44.14 mm ± 6.11) was higher than K m for 4‐MUG (0.012 mm ± 0.001), whereas the V max values were 22.49 ± 0.28 and 2.56 ± 0.31 nmol·mg−1·min−1 for p‐NPG and 4‐MUG, respectively (data not shown).
Substrate specificity was assayed using 4‐MU‐glycosides, p‐NP‐glycosides, and saccharides as substrates under the standard assay conditions (Table 3). Purified β‐glucosidase showed 2.2× higher specific activity on cellobioside than laminarin and 7.2× higher specific activity on p‐NPG than 4‐MUG. It also evidenced low activity on the cellobiose derivates, 4‐MUC, and p‐NPC. No activity was detected on 4‐MU‐β‐D‐xyloside or 4‐MU‐β‐D‐galactopyranoside.
Table 3.
Substrate specificity of the purified enzyme
| Substrate | Specific activity (nmol·mg−1·min−1) |
|---|---|
| Synthetic substrates | |
| 4MU‐β‐D‐glucoside | 0.75 ± 0.010 |
| 4MU‐β‐D‐cellobioside | 0.04 ± 0.006 |
| 4MU‐β‐D‐xyloside | N.D. |
| 4MU‐β‐D‐galactoside | N.D. |
| p‐Nitrophenyl β‐D‐glucopyranoside | 5.40 ± 0.038 |
| p‐Nitrophenyl β‐D‐cellobioside | 0.53 ± 0.081 |
| Saccharides | |
| Cellobiose | 107.41 ± 14.87 |
| Laminarin | 59.78 ± 9.40 |
N.D., not detected. Depending on the substrate, activities were determined by measuring the release of either 4‐MU, p‐NP, or glucose as described in Experimental procedures. The concentration of synthetic substrates was 5 μm (4‐MU substrate) and 5 mm (p‐NP substrates), whereas the concentration of saccharides was 1% (w/v). The specific activity is expressed as nmol of substrate hydrolyzed per minute per mg of protein. Values are means ± SD for three independent experiments.
In order to evaluate the hydrolysis on cellodextrins by the purified β‐glucosidase, we performed a TLC (Fig. 8). The enzyme was able to release glucose from cellobiose > cellotetraose > cellotriose. Apparently, glucose was the unique product from the hydrolysis of cellobiose (Fig. 8, line 2). When cellotriose (Fig. 8, line 3) was used as substrate, a strong signal of a probable tetrasaccharide and two diffuse spots, corresponding to glucose and a possible disaccharide, were showed. Using cellotetraose (Fig. 8, line 4) as substrate, only two spots appeared, one comparable to glucose mobility and other with a relative mobility lower than a tetrasaccharide. Additionally, when the enzyme was incubated in the presence of glucose (Fig. 8, line1), a glucose dimer appeared, suggesting transglycosylation activity. A similar effect was observed when cellotriose and cellotetraose were used in the assay (Fig. 8, line 3 and 4), spots with lower mobility were visualized, indicating the formation of a bigger oligosaccharide.
Figure 8.
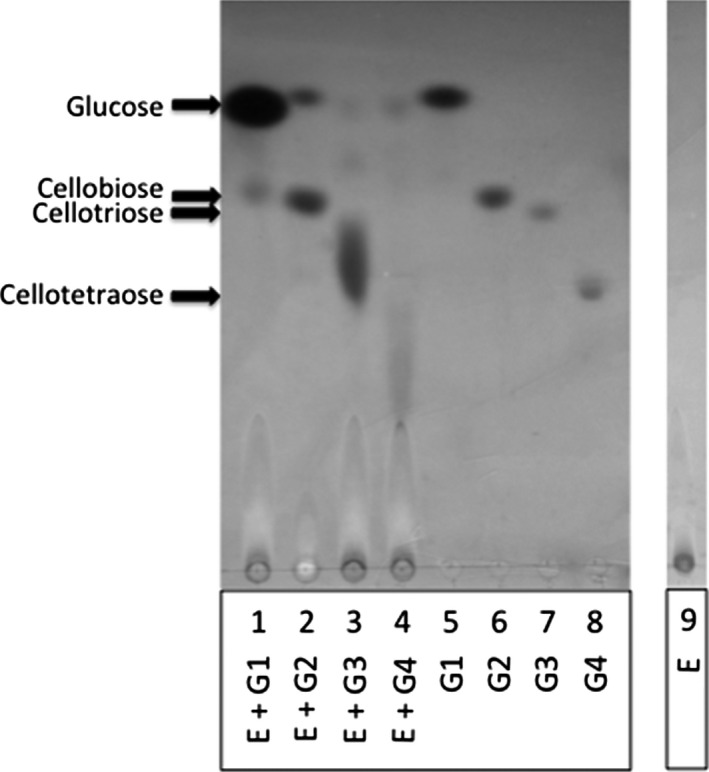
Thin‐layer chromatography separation of the released cellodextrins when purified enzyme was incubated with glucose (E + G1), cellobiose (E + G2), cellotriose (E + G3) or cellotetraose (E + G4). Reaction mixtures were stopped after 10 h. Glucose [G1 (1 μg), line 5], cellobiose [G2 (2 μg), line 6], cellotriose [G3 (4 μg), line 7] and cellotetraose [G4 (4 μg), line 8] were used as standards and purified β‐glucosidase as control (E, line 9).
Discussion
Despite being a human pathogenic fungus, S. schenckii has a saprophytic stage and it is phylogenetically close to another phytopathogenic and nonpathogenic fungus which secretes different hydrolytic enzymes 7, 8, 9. Recently, the characterization of 36 different isolates of S. schenckii complex based on the hydrolysis of 11 substrates 26 demonstrated that S. schenckii possesses a battery of extracellular lytic enzymes which may help it to obtain necessary nutrients for its development and maintenance during its saprophytic phase.
In the present work, we confirmed that S. schenckii produces and secretes enzymes capable of degrading cellulose, one of the main cell wall component of organic matter in which it develops. This fungus, in the presence of cellulose as the principal carbon source, produced considerable amounts of β‐glucosidase. It is known that this enzyme acts mainly on cellobiose and it is needed for an efficient degradation of cellulosic biomass as it reduces the inhibition of cellobiohydrolases by decreasing the product accumulation. β‐glucosidase has been well characterized in another fungus: opportunistic such as Aspergillus spp. 27, 28, phytopathogenic such as Fusarium oxysporum, 29 and saprophytic such as Neurospora crassa 8, 30, 31.
Under the tested growth conditions, S. schenckii secreted low quantity of protein. As part of this secreted proteins, we detected β‐glucosidase activity. The induction pattern of β‐glucosidase activity showed an increase in early stationary phase (72 h) and remains constant throughout the tested time (120 h). Similar results were shown in Aspergillus niger 32. This result is in contrast with other cellulases reported in other fungi, where the highest cellulolytic activity is expressed after 9–10 days of growth 33, 34, 35. When S. schenckii was grown in glucose, lower amount of protein was secreted and β‐glucosidase activity was not detected, however, it does not dismiss the possibility that low levels of constitutive enzyme could be present and cellulase induction could require its own basal expression as it has been previously reported in T. reesei 36. In addition of the secreted hydrolytic enzymes, the fungus produced a metabolic product that turned the culture medium yellow when it was grown in the presence of cellulose. Since this dye interfered with protein determination, the CFCF was eluted in Bio‐Gel P‐10 in order to remove it. After this filtration step, another two chromatographies were performed to purify to homogeneity a β‐glucosidase from S. schenckii with a 50% yield and 3.2‐fold of purification. Purified fungal β‐glucosidases present a wide diversity regarding the yield and the fold purification. While some reports had described the purification to homogeneity of fungal β‐glucosidases with high yields 11, 29 apparently, an overall characteristic of this enzymes is the low recovered rate 37, 38, 39 and the low fold purification 40, 41, suggesting its probable lability. In Penicillium italicum and Penicillium simplicissimum, an enzymatic activity recovery rate and fold purification has been reported similar to those obtained in the present study 42, 43.
The enzyme purified after filtration on Sephacryl S200‐HR was applied to an SDS/PAGE and the electrophoretic profile showed a single band with an estimated molecular weight of 96.8 kDa. The native molecular weight of the enzyme (197 kDa) determined by gel filtration on size exclusion chromatography suggests that the purified enzyme has a dimeric conformation, as observed in N. crassa intracellular β‐glucosidase 44. We were not able to obtain the denatured zymogram of the purified enzyme (data not shown), probably due to its lability as an unprotected single protein. Nevertheless, when the CFCF zymogram was performed under denatured conditions, two bands were exhibited (204.9 and 97.2 kDa), one of them with a similar molecular weight to the one displayed in the native zymogram of the purified protein. This result confirms the secretion of a dimeric protein with β‐glucosidase activity induced by cellulose. In other β‐glucosidases from other microorganisms, it was was demonstrated that the oligomeric conformation is not necessary for enzymatic activity 45. In contrast to these results 46, a barley β‐D‐glucan exohydrolase with β‐D‐glucosidase activity was reported and it was suggested that space at the interface between the two domains of the homodimeric protein is probably the active site of the enzyme.
Purified β‐glucosidases isolated from different microorganisms present a variability with respect to their physicochemical properties. Reported fungal β‐glucosidases display optimal pH values between 3 and 6 and are active in a broad range of temperatures from 40 to 60 °C 47. Sporothrix schenckii‐purified β‐glucosidase exhibit an optimal temperature and pH of 45 °C and 5.0, respectively. Similar values have been reported from other ascomycetes, such as N. crassa 48 and Xylaria regalis 37. Divalent cations, such as Ca2+, Mg2+, Co2+, and Mn2+ can affect the enzyme activity of β‐glucosidases by activating 37, 41 or inhibiting them 49. We only observed an inhibitory effect in the presence of 10 mm Fe2+ and no other effect with the tested reagents. Similar results were reported from purified β‐glucosidase from the wood‐decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm 39.
Interestingly when the thermostability of the enzyme was determined, the enzyme retained 60–50% of its initial activity after 3 h of incubation at 45 °C and it was maintained until 24 h of incubation. Although there is a considerable decrease in the enzyme activity during the first 3 h, we suppose that after initial incubation time, the conformation of the protein is no longer affected during the tested times. β‐glucosidases studies carried out in Melanocarpus sp. 40 and A. niger 11 also showed a significant decrease in β‐glucosidase activity when enzymes were incubated for short periods of time (60 min) at different temperatures ranging from 40 to 70 °C.
The K m and V max values from S. schenckii β‐glucosidase revealed that the enzyme had a 3678‐fold higher affinity to 4‐MUG than p‐NPG and can hydrolyze 8.8 times faster the former than the latter. The highest hydrolytic activity of the isolated β‐glucosidase was observed against glucose derivatives (4‐MU and p‐NP derivatives) as substrates, detecting only low activity against cellobiose derivatives. Activity was also detected when natural substrates such as cellobiose and laminarin were tested. This suggests the possibility that the purified enzyme could hydrolyze short‐chain cello‐oligosaccharides and other types of linkage (β‐1,3) as previously shown in other β‐glucosidases isolated from different microorganisms 29, 50, 51. This assumption was confirmed by TLC experiments, where the hydrolysis of cello‐oligosaccharides of different length by the purified β‐glucosidase was proved. Beside the detected oligosaccharides hydrolysis, new spots corresponding to higher molecular weight oligosaccharides appeared, suggesting transglycosylation activity in addition to the hydrolytic activity. Results from substrate specificity analysis confirmed the nature of this enzyme as a β‐glucosidase and proved that it can also catalyze both hydrolysis and transglycosylation reactions as reported in A. fumigatus, A. niger, A. oryzae, Magnaporthe grisea, N. crassa, and Penicillium brasilianum 52, 53.
In order to determine if this enzyme is present in another strain of the S. schenckii complex, Sporothrix brasiliensis was grown under similar conditions and β‐glucosidase activity was determined using 4‐MUG as substrate. Specific activity was four times lower than the one secreted by S. schenckii (data not shown). These data are consistent with a previous report which demonstrates the presence of a secreted β‐glucosidase activity in S. schenckii, S. brasiliensis, and S. albicans strains using the semiquantitative API‐ZYM commercial kit system (BioMérieux, Marcy‐l’Étoile, France). In contrast, this activity was not found when it was measured in S. mexicana and S. globosa supernatants using the same methodology 26.
To our knowledge, this work represents the first report of the purification of a β‐glucosidase secreted by the human pathogen fungus S. schenckii growth in a complex polysaccharide. The reported purified protein and other secreted enzymes, which have not been identified yet, could provide the ability to S. schenckii to grow as a saprophytic fungus. Detailed analysis such as molecular cloning and expression data are needed in order to give us better insights into the saprophytic phase of this fungus.
Author contributions
AHG, AFM, PPN, and JCVC planned the experiments; AHG, AFM, and JCVC performed the experiments; AHG, AFM, PPN, and JCVC analyzed the data; AHG and JCVC wrote the manuscript.
Acknowledgements
This work was supported by Universidad de Guanajuato (ref. 434/2014) and by Consejo Nacional de Ciencia y Tecnología (CONACyT), México (scholarship granted to AHG). We thank Franco B and Martínez Reyes JE for the proofreading of the manuscript.
References
- 1. Bastos de Lima Barros M, de Almeida Paes R and Schubach AO (2011) Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev 24, 633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson MDD and Warnock DW (2012) Sporotrichosis In Fungal Infection: Diagnosis and Management, 4th edn p. 352 Wiley‐Blackwell, Chichester. [Google Scholar]
- 3. López‐Romero E, Reyes‐Montes M, Pérez‐Torres A, Ruiz‐Baca E, Villagómez‐Castro JC, Mora‐Montes HM, Flores‐Carreón A and Toriello C (2011) Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol 6, 85–102. [DOI] [PubMed] [Google Scholar]
- 4. Sasaki AA, Fernandes GF, Rodrigues AM, Lima FM, Marini MM, Feitosa LDS, De Melo Teixeira M, Felipe MSS, Da Silveira JF and De Camargo ZP (2014) Chromosomal polymorphism in the Sporothrix schenckii complex. PLoS One 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flournoy DJ, Mullins JB and McNeal RJ (2000) Isolation of fungi from rose bush thorns. J Okla State Med Assoc 93, 271–274. [PubMed] [Google Scholar]
- 6. Mehta KIS, Sharma NL, Kanga AK, Mahajan VK and Ranjan N (2007) Isolation of Sporothrix schenckii from the environmental sources of cutaneous sporotrichosis patients in Himachal Pradesh, India: results of a pilot study. Mycoses 50, 496–501. [DOI] [PubMed] [Google Scholar]
- 7. de Vries RP and Visser J (2001) Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev 65, 497–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tian C, Beeson WT, Iavarone AT, Sun J, Marletta MA, Cate JHD & Glass NL (2009) Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa . Proc Natl Acad Sci USA 106, 22157–22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmoll M and Schuster A (2010) Biology and biotechnology of Trichoderma . Appl Microbiol Biotechnol 87, 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H, Hayn M and Esterbauer H (1992) Purification and characterization of two extracellular β‐glucosidases from Trichoderma reesei . Biochim Biophys Acta 1121, 54–60. [DOI] [PubMed] [Google Scholar]
- 11. Gong G, Zheng Z, Liu H, Wang L, Diao J, Wang P and Zhao G (2014) Purification and characterization of a β‐glucosidase from Aspergillus niger and its application in the hydrolysis of geniposide to genipin. J Microbiol Biotechnol 24, 788–794. [DOI] [PubMed] [Google Scholar]
- 12. Haq IU, Khan MA, Muneer B, Hussain Z, Afzal S, Majeed S, Rashid N, Javed MM and Ahmad I (2012) Cloning, characterization and molecular docking of a highly thermostable β‐1,4‐glucosidase from Thermotoga petrophila . Biotechnol Lett 34, 1703–1709. [DOI] [PubMed] [Google Scholar]
- 13. Linton SM and Greenaway P (2004) Presence and properties of cellulase and hemicellulase enzymes of the gecarcinid land crabs Gecarcoidea natalis and Discoplax hirtipes . J Exp Biol 207, 4095–4104. [DOI] [PubMed] [Google Scholar]
- 14. Almeida‐Paes R, de Oliveira LC, Oliveira MME, Gutierrez‐Galhardo MC, Nosanchuk JD and Zancopé‐Oliveira RM (2015) Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex . Biomed Res Int 2015, 212308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsuboi R, Sanada T, Takamori K and Ogawa H (1987) Isolation and characterization of extracellular proteinases from Sporothrix schenckii . J Bacteriol 169, 4104–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnold WN, Sakai KH and Mann LC (1987) Selective inactivation of an extra‐cytoplasmic acid phosphatase of yeast‐like cells of Sporothrix schenckii by sodium fluoride. J Gen Microbiol 133, 1503–1509. [DOI] [PubMed] [Google Scholar]
- 17. Arnold WN, Nguyen TB and Mann LC (1998) Purification and characterization of a dextranase from Sporothrix schenckii . Arch Microbiol 170, 91–98. [DOI] [PubMed] [Google Scholar]
- 18. Lopes‐Bezerra LM (2011) Sporothrix schenckii cell wall peptidorhamnomannans. Front Microbiol 2, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliveira DC, Markus Lopes PG, Spader TB, Mahl CD, Tronco‐Alves GR, Lara VM, Santurio JM and Alves SH (2011) Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol 49, 3047–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuranda MJ and Robbins PW (1987) Cloning and heterologous expression of glycosidase genes from Saccharomyces cerevisiae . Proc Natl Acad Sci USA 84, 2585–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deshpande MV, Eriksson KE and Pettersson LG (1984) An assay for selective determination of exo‐1,4‐beta‐glucanases in a mixture of cellulolytic enzymes. Anal Biochem 138, 481–487. [DOI] [PubMed] [Google Scholar]
- 22. Miller GL (1959) Use of dinitrosaiicyiic acid reagent for determination of reducing sugar. Anal Chem 31, 426–428. [Google Scholar]
- 23. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ and Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150, 76–85. [DOI] [PubMed] [Google Scholar]
- 24. Lowry OH, Roebrough NJ, Farr AL and Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193, 265–275. [PubMed] [Google Scholar]
- 25. Han NS and Robyt JF (1998) Separation and detection of sugars and alditols on thin layer chromatograms. Carbohydr Res 313, 135–137. [Google Scholar]
- 26. Oliveira DC, de Loreto ES, Mario DAN, Lopes PGM, Neves LV, da Rocha MP, Santurio JM and Alves SH (2015) Sporothrix schenckii complex: susceptibilities to combined antifungal agents and characterization of enzymatic profiles. Rev Inst Med Trop Sao Paulo 57, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dan S, Marton I, Dekel M, Bravdo BA, He S, Withers SG & Shoseyov O (2000) Cloning, expression, characterization, and nucleophile identification of family 3, Aspergillus niger β‐glucosidase. J Biol Chem 275, 4973–4980. [DOI] [PubMed] [Google Scholar]
- 28. Liu D, Zhang R, Yang X, Zhang Z, Song S, Miao Y and Shen Q (2012) Characterization of a thermostable β‐glucosidase from Aspergillus fumigatus Z5, and its functional expression in Pichia pastoris X33. Microb Cell Fact 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christakopoulos P, Goodenough PW, Kekos D, Macris BJ, Claeyssens M and Bhat MK (1994) Purification and characterisation of an extracellular β‐glucosidase with transglycosylation and exo‐glucosidase activities from Fusarium oxysporum . Eur J Biochem 224, 379–385. [DOI] [PubMed] [Google Scholar]
- 30. Romero M, Aguado J, González L and Ladero M (1999) Cellulase production by Neurospora crassa on wheat straw. Enzyme Microb Technol 25, 244–250. [Google Scholar]
- 31. Wu W, Hildebrand A, Kasuga T, Xiong X and Fan Z (2013) Direct cellobiose production from cellulose using sextuple beta‐glucosidase gene deletion Neurospora crassa mutants. Enzyme Microb Technol 52, 184–189. [DOI] [PubMed] [Google Scholar]
- 32. Sohail M, Siddiqi R, Ahmad A and Khan SA (2009) Cellulase production from Aspergillus niger MS82: effect of temperature and pH. N Biotechnol 25, 437–441. [DOI] [PubMed] [Google Scholar]
- 33. Amadioha AC (1993) Production of cellulolytic enzymes by Rhizopus oryzae in culture and Rhizopus‐Infected tissues of potato tubers. Mycologia 85, 574–578. [Google Scholar]
- 34. Magnelli P, Ramos AM and Forchiassin F (1996) Factors influencing cellulase production Saccobolus saccoboloides . Mycologia 88, 249–255. [Google Scholar]
- 35. Acosta‐Rodríguez I, Piñón‐Escobedo C, Zavala‐Páramo MG, López‐Romero E and Cano‐Camacho H (2005) Degradation of cellulose by the bean‐pathogenic fungus Colletotrichum lindemuthianum. Production of extracellular cellulolytic enzymes by cellulose induction. Antonie Van Leeuwenhoek 87, 301–310. [DOI] [PubMed] [Google Scholar]
- 36. Carle‐Urioste JC, Escobar‐Vera J, El‐Gogary S, Henrique‐Silva F, Torigoi E, Crivellaro O, Herrera‐Estrella A and El‐Dorry H (1997) Cellulase induction in Trichoderma reesei by cellulose requires its own basal expression. J Biol Chem 272, 10169–10174. [DOI] [PubMed] [Google Scholar]
- 37. Wei D, Kirimura K, Usami S and Lin T (1996) Purification and characterization of an extracellular β‐glucosidase from the wood‐grown fungus Xylaria regalis . Curr Microbiol 33, 297–301. [DOI] [PubMed] [Google Scholar]
- 38. Riou C, Salmon JM, Vallier MJ, Günata Z and Barre P (1998) Purification, characterization, and substrate specificity of a novel highly glucose‐tolerant β‐glucosidase from Aspergillus oryzae . Appl Environ Microbiol 64, 3607–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karnchanatat A, Petsom A, Sangvanich P, Piaphukiew J, Whalley AJS, Reynolds CD and Sihanonth P (2007) Purication and biochemical characterization of an extracellular β‐glucosidase from the wood‐decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm. FEMS Microbiol Lett 270, 162–170. [DOI] [PubMed] [Google Scholar]
- 40. Kaur J, Chadha BS, Kumar BA, Kaur GS & Saini HS (2007) Purification and characterization of β‐glucosidase from Melanocarpus sp. MTCC 3922. Electron J Biotechnol 10, 260–270. [DOI] [PubMed] [Google Scholar]
- 41. Chen HL, Chen YC, Lu MY, Chang JJ, Wang HT, Ke HM, Wang TY, Ruan SK, Wang TY, Hung KY et al (2012) A highly efficient β‐glucosidase from the buffalo rumen fungus Neocallimastix patriciarum W5. Biotechnol Biofuels 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park AR, Hong JH, Kim JJ and Yoon JJ (2012) Biochemical characterization of an extracellular β‐glucosidase from the fungus, Penicillium italicum, isolated from rotten citrus peel. Mycobiology 40, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bai H, Wang H, Sun J, Irfan M, Han M, Huang Y, Han X and Yang Q (2013) Production, purification and characterization of novel beta glucosidase from newly isolated Penicillium simplicissimum H‐11 in submerged fermentation. EXCLI J 12, 528–540. [PMC free article] [PubMed] [Google Scholar]
- 44. Yazdi M, Khosravi A, Nemati M and Motlagh N (2003) Purification and characterization of two intracellular β‐glucosidases from the Neurospora crassa mutant cell‐1. World J Microbiol Biotechnol 19, 79–84. [Google Scholar]
- 45. McAndrew RP, Park JI, Heins RA, Reindl W, Friedland GD, D'haeseleer P, Northen T, Sale KL, Simmons BA & Adams PD (2013) From soil to structure, a novel dimeric β‐glucosidase belonging to glycoside hydrolase family 3 isolated from compost using metagenomic analysis. J Biol Chem 288, 14985–14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Varghese J, Hrmova M and Fincher G (1997) Three‐dimensional structure of a barley β‐D‐glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 7, 179–190. [DOI] [PubMed] [Google Scholar]
- 47. Coughlan MP (1985) The properties of fungal and bacterial cellulases with comment on their production and application. Biotechnol Genet Eng Rev 3, 39–109. [Google Scholar]
- 48. Eberhart BM, Beck RS and Goolsby KM (1977) Cellulase of Neurospora crassa . J Bacteriol 130, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karnaouri A, Topakas E, Paschos T, Taouki I and Christakopoulos P (2013) Cloning, expression and characterization of an ethanol tolerant GH3 β‐glucosidase from Myceliophthora thermophila . PeerJ 1, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uchiyama T, Miyazaki K and Yaoi K (2013) Characterization of a novel β‐glucosidase from a compost microbial metagenome with strong transglycosylation activity. J Biol Chem 288, 18325–18334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baba Y, Sumitani JI, Tani S and Kawaguchi T (2015) Characterization of Aspergillus aculeatus β‐glucosidase 1 accelerating cellulose hydrolysis with Trichoderma cellulase system. AMB Express 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seidle HF and Huber RE (2005) Transglucosidic reactions of the Aspergillus niger family 3 β‐glucosidase: qualitative and quantitative analyses and evidence that the transglucosidic rate is independent of pH. Arch Biochem Biophys 436, 254–264. [DOI] [PubMed] [Google Scholar]
- 53. Bohlin C, Praestgaard E, Baumann MJ, Borch K, Praestgaard J, Monrad RN and Westh P (2013) A comparative study of hydrolysis and transglycosylation activities of fungal β‐glucosidases. Appl Microbiol Biotechnol 97, 159–169. [DOI] [PubMed] [Google Scholar]


