Abstract
Purpose
The study aims to investigate the role of 18F-alfatide positron emission tomography/computed tomography (PET/CT) in predicting the short-term outcome of concurrent chemoradiotherapy (CCRT) in patients with advanced non-small cell lung cancer (NSCLC).
Methods
Eighteen patients with advanced NSCLC had undergone 18F-alfatide PET/CT scans before CCRT and PET/CT parameters including maximum and mean standard uptake values (SUVmax/SUVmean), peak standard uptake values (SUVpeak) and tumor volume (TVPET and TVCT) were obtained. The SUVmax of tumor and normal tissues (lung, blood pool and muscle) were measured, and their ratios were denoted as T/NT (T/NTlung, T/NTblood and T/NTmuscle). Statistical methods included the Two-example t test, Wilcoxon rank-sum test, Receiver-operating characteristic (ROC) curve analysis and logistic regression analyses.
Results
We found that SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle were higher in non-responders than in responders (P = 0.0024, P = 0.016, P < 0.001, P = 0.003, P = 0.004). According to ROC curve analysis, the thresholds of SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle were 5.65, 4.46, 7.11, 5.41, and 11.75, respectively. The five parameters had high sensitivity, specificity and accuracy in distinguishing non-responders and responders. Multivariate logistic regression analyses showed that T/NTlung was an independent predictor of the short-term outcome of CCRT in patients with advanced NSCLC (P = 0.032).
Conclusions
18F-alfatide PET/CT may be useful in predicting the short-term outcome of CCRT in patients with advanced NSCLC.
Keywords: Non-small cell lung cancer, Concurrent Chemoradiotherapy, 18F-alfatide, PET/CT, Integrin αvβ3
Background
Lung cancer is the leading cause of cancer death worldwide, and non-small cell lung cancer (NSCLC) accounts for 85 % [1]. One-third of patients with newly diagnosed NSCLC have been advanced and not amenable for curative resection. Concurrent chemoradiotherapy (CCRT) represents the standard of therapy protocol for patients with advanced NSCLC who have good performance status and no significant weight loss [2]. Even with the standard therapy, one-third of these patients still experience local failure [3]. Thus, it’s important to find an effective predicting tool to select patients who are likely to benefit from the treatment. This may help to personalize the treatment in NSCLC patients by avoiding ineffective CCRT and continuing the primary treatment in responding patients.
The tumor node metastasis (TNM) staging system is considered the most important prognostic tool [4], but TNM staging does not correspond to biological aggressiveness and fails to explain the wide variation of the outcomes in patients within the same stage [5]. Various techniques including all kinds of molecular imaging had been developed to predict the tumor response to therapy.
Angiogenesis, the growth of new blood vessels from preexisting vessels, is an essential step in tumor development and metastasis. It is widely accepted that the imaging of tumor angiogenesis can be used not only for the early detection of cancers but also for monitoring treatment outcomes [6]. Integrin αvβ3 has been shown to play an important role in angiogenesis and up-regulated obviously in various types of tumor cells and the activated endothelial cells of tumor angiogenesis [7–9]. Because the arginine-glycine-aspartic acid (RGD) tripeptide sequence can bind to integrin αvβ3 with high affinity and specificity [10, 11], RGD PET/CT (positron emission tomography/computed tomography) may be helpful to evaluate tumor angiogenesis. A novel one-step labeled integrin αvβ3-targeting PET probe, 18F-AlF-NOTA-PRGD2 (denoted as 18F-alfatide) has been proved to be safe [12] and can identify lung cancer clearly with desirable image contrast [13]. We had performed a pilot clinical study in which 18F-alfatide PET/CT parameters could predict the tumor sensitivity to CCRT in patients with glioma [14]. Therefore, we think 18F-alfatide PET/CT might be a potential tool for predicting the short-term outcome of CCRT in patients with advanced NSCLC.
In this clinical study, we aim to investigate whether 18F-alfatide PET/CT parameters could be used as a classifier for predicting the short-term outcome of CCRT in patients with advanced NSCLC.
Materials and methods
Patients
Eighteen patients with advanced NSCLC were enrolled in this study (Table 1). There were 14 males and four females with median age of 62 (range: 45–85). All patients had given informed consent to participate in this study, which was approved by the ethics committee of Shandong Cancer Hospital affiliated to Shandong University and met the following inclusion criteria: (1) advanced NSCLC diagnosed by histological and imaging examination such as CT or 18F-fluorodeoxyglucose (FDG) PET/CT (stage IIIA, IIIB or IV); (2) Karnofsky performance status (KPS) ≥70; (3) had measurable primary tumors according to Response Evaluation Criteria in Solid Tumors (RECIST). All patients were ready to undergo CCRT without undergoing surgery, chemotherapy or radiotherapy for thoracic tumors formerly, and they had the 18F-alfatide PET/CT scans before CCRT.
Table 1.
Clinicopathological features of the patients with advanced NSCLC
| Characteristics | Number of cases (%) |
|---|---|
| Age | 62 ± 12.04 |
| < 65 | 10 |
| ≥ 65 | 8 |
| Sex | |
| Male | 14 (78) |
| Female | 4 (22) |
| Stage | |
| IIIA | 6 (33) |
| IIIB | 6 (33) |
| IV | 6 (33) |
| Pathological type | |
| Adenocarcinoma | 8 (44) |
| Squamous cell carcinoma | 9 (50) |
| Other | 1 (6) |
| RECIST | |
| Complete response | 1 (6) |
| Partial response | 8 (44) |
| Stable disease | 8 (44) |
| Progressive disease | 1 (6) |
CCRT
Patients were treated with chemoradiotherapy in a concurrent regimen. An intensity-modulated radiotherapy technique (IMRT) or three-dimensional conformal RT (3D-CRT) was delivered to all patients with megavoltage equipment (6 MV). RT was given as the conventionally fractionated regimen, 1.8 to 2.0 Gy for five days per week, and the total dose administered to patients ranged from 56 to 66 Gy (median dose, 60 Gy). RT was planned based on a CT scan performed for planning purposes, the gross tumor volume (GTV) included the primary tumor and involved lymph nodes, and the planning target volume (PTV) included the GTV plus a margin of 1.0–1.5 cm. All patients were treated with two cycles of chemotherapy with a cisplatin/docetaxel or a cisplatin/pemetrexed region during RT and the first cycle of chemotherapy was applied on day 1 of RT. Two to four additional cycles of chemotherapy were needed every 3 weeks after RT.
PET scanning
The simple lyophilized kit for labeling PRGD2 peptide was purchased from Jiangsu Institute of Nuclear Medicine, and the synthesis process was carried out by reference to previous study [13]. The radiochemical purity of the 18F-alfatide exceeded 95 %, and its specific radioactivity exceeded 37 GBq (1,000 mCi)/μmol. Patients were not requested to fast and to confirm blood glucose levels. After injected with18F-alfatide (214.38 ± 19.8 MBq) intravenously, they needed to rest for approximately 60 min. Scanning was performed with an integrated in-line PET/CT system (Discovery LS; GE Healthcare). PET images were performed from the head to the thigh, and the spiral CT component was performed with an x-ray tube voltage peak of 140 kV, 80 mA, a 6:1 pitch, a slice thickness of 4.25 mm, and a rotation speed of 0.8 s per rotation. A full-ring dedicated PET scan of the same axial range followed. The patients were in normal shallow respiration during image acquisition. The images were attenuation-corrected with the transmission data from CT. The attenuation-corrected PET images, CT images, and fused PET/CT images, displayed as coronal, sagittal, and transaxial slices, were viewed on a Xeleris workstation (GE Healthcare). The 18F-alfatide PET/CT scans were performed within 7 days before the start of CCRT.
Image analysis
Two experienced nuclear medicine physicians assessed the 18F-alfatide PET/CT images visually, referring to PET fusion and CT images, until consensuses were reached. Acquired 18F-alfatide PET/CT data was transferred into the workstation in the DICOM format. The radiotracer concentration in the regions of interest (ROI) was normalized to the injected dose per kilogram of the patients’ body weight to derive the standardized uptake values (SUVs). The SUVs were calculated according to the following formula: [measured activity concentration (Bq/mL) × body weight (g)]/injected activity (Bq).
PET/CT parameters such as maximum and mean standard uptake values (SUVmax and SUVmean) and tumor volume (TVPET) were generated using a vendor-provided automated contouring program. Peak standard uptake values (SUVpeak) were defined as the average SUV in a 1 cm3 sphere surrounding the voxel with the highest activity. We outlined the healthy lung with the position and volume similarly to the primary tumor to obtain the maximal activity of lung background. In addition, the maximal activity of 1 cm3 within the aortic arches and erector spinae were measured. Then the ratios of primary tumor and normal tissues based on SUVmax were calculated, denoted as T/NT (T/NTlung, T/NTblood and T/NTmuscle). In addition, tumor volumes were also measured by the CT images of PET/CT images, donated as TVCT.
Response evaluation
Short-term outcome was assessed at 4 weeks after CCRT (56–66 Gy RT and 4–6 cycles of chemotherapy) according to the revised RECIST criteria (v.1.1) using chest CT. According to RECIST criteria, the responders included the patients with an outcome of complete response (CR) or partial response (PR); the patients who had an outcome of stable disease (SD) or progressive disease (PD) were classified as the non-responders.
Statistical analysis
All statistical tests were performed with SPSS 17.0 and MedCalc 11.0.1.0. Statistical significance was assumed for P values less than 0.05 and all P values were 2-tailed. Eighteen patients were classified as responders and non-responders according to the revised RECIST criteria (v.1.1). Quantitative data for SUVmax, SUVpeak, SUVmean, TVPET, TVCT and T/NT (T/NTlung, T/NTblood and T/NTmuscle) were expressed as mean ± standard deviation (SD) or median (interquartile range). Two-sample t tests and Wilcoxon rank-sum tests were used to compare the PET/CT parameters between responders and non-responders. SUVmax, SUVpeak and T/NT and multiple clinical variables such as age, stage, and histopathology were tested by logistic regression analyses to identify the relationships between these variables and the short-term outcomes. Receiver-operating characteristic (ROC) curve analysis was used to achieve the thresholds with the maximum Youden index and determine the diagnostic accuracy of 18F-alfatide PET/CT parameters in identifying the responders and non-responders.
Results
Tumor response
Eighteen patients with advanced NSCLC had undergone 18F-alfatide PET/CT scans. Nine patients were classified as responders (52 %), including one complete response, eight partial responses, and nine patients were classified as non-responders (48 %) including eight stable disease and one progressive disease.
Correlations between 18F-alfatide PET/CT parameters and tumor response
SUVmax, SUVpeak, SUVmean, TV (TVPET and TVCT) and T/NT (T/NTlung, T/NTblood and T/NTmuscle) are listed in Table 2. The single data of SUVmax, SUVpeak and SUVmean are presented in Table 3. The differences of SUVmean, TVPET and TVCT between responders and non-responders were not significant in statistics (3.14 ± 0.17 vs. 3.76 ± 0.24, P = 0.05, 15,872 (27,232) vs. 55,296 (68,864), P = 0.07 and 32,856 (39,664) vs. 53,558 (90,508), P = 0.59). SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle were significantly higher in non-responders than in responders (7.61 ± 0.77 vs. 4.95 ± 0.61, P = 0.024, 6.22 ± 0.65 vs. 3.99 ± 0.51, P = 0.016, 8.31 ± 0.61 vs. 6.53 ± 0.78, P < 0.001, 6.77 ± 0.63 vs. 3.86 ± 0.0.57, P = 0.003 and 12.56 ± 0.73 vs. 7.87 ± 1.14, P = 0.004).
Table 2.
Parameters of pretreatment for18F-alfatide PET/CT scan
| Parameters | All patients | Non-responders | Responders | p |
|---|---|---|---|---|
| SUVmax | 6.28 ± 2.44 | 7.61 ± 0.77 | 4.95 ± 0.61 | 0.024 |
| SUVmean | 3.44 ± 0.69 | 3.76 ± 0.24 | 3.14 ± 0.17 | 0.05 |
| SUVpeak | 5.10 ± 2.06 | 6.22 ± 0.65 | 3.99 ± 0.51 | 0.016 |
| TVPET | 22,368 (62,480) | 55,296 (68,864) | 15,872 (27,232) | 0.07 |
| TVCT | 37,570 (61,028) | 53,558 (90,508) | 32,856 (39,664) | 0.59 |
| T/NTlung | 6.27 ± 2.5 | 8.31 ± 0.61 | 4.31 ± 0.48 | <0.001 |
| T/NTblood | 5.51 ± 2.56 | 6.77 ± 0.63 | 3.86 ± 0.57 | 0.003 |
| T/NTmuscle | 10.32 ± 3.53 | 12.56 ± 0.73 | 7.87 ± 1.14 | 0.04 |
Table 3.
The single data of SUVmax, SUVpeak and SUVmean
| patients | SUVmax | SUVpeak | SUVmean |
|---|---|---|---|
| 1 | 5.32 | 3.72 | 3.40 |
| 2 | 9.21 | 7.19 | 4.90 |
| 3 | 3.95 | 3.00 | 3.01 |
| 4 | 8.74 | 8.45 | 4.02 |
| 5 | 2.86 | 2.41 | 2.21 |
| 6 | 5.11 | 4.56 | 3.08 |
| 7 | 8.33 | 7.47 | 4.30 |
| 8 | 8.07 | 6.44 | 3.85 |
| 9 | 4.70 | 4.20 | 3.07 |
| 10 | 9.40 | 7.68 | 4.06 |
| 11 | 3.69 | 3.15 | 2.86 |
| 12 | 4.90 | 4.35 | 3.17 |
| 13 | 5.16 | 4.33 | 3.45 |
| 14 | 4.60 | 3.06 | 2.99 |
| 15 | 11.79 | 9.08 | 4.49 |
| 16 | 5.97 | 4.58 | 3.45 |
| 17 | 4.31 | 3.52 | 3.00 |
| 18 | 7.00 | 4.68 | 2.78 |
ROC curve analysis
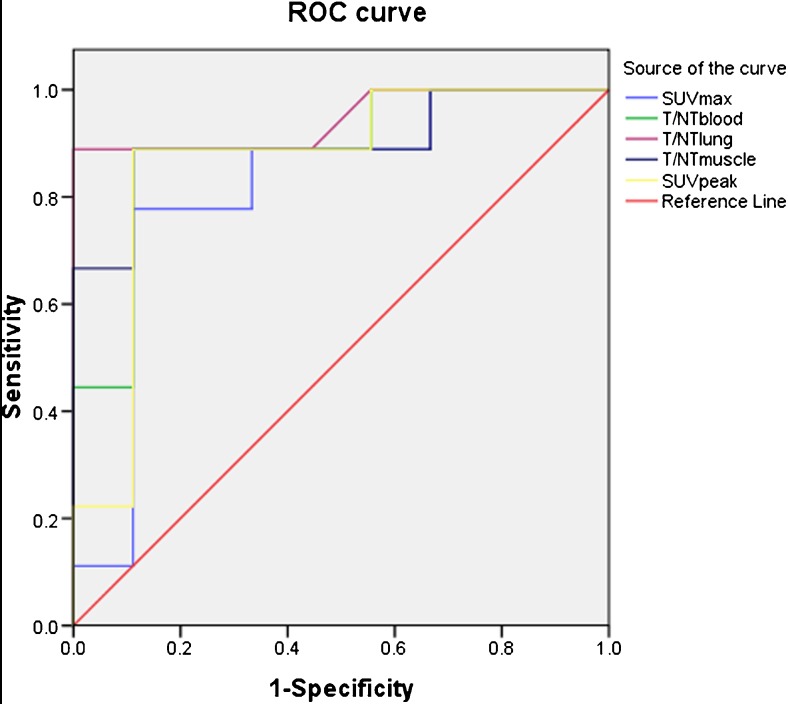
ROC curve analysis was performed to determine the diagnostic accuracy of the five parameters (SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle) in identifying responders. There were highly significant correlations between SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle of 18F-alfatide PET/CT and the short-term outcomes assessed by RECIST (P < 0.001) (Fig. 1). The AUC of T/NTlung (AUC = 0.944) were higher than SUVmax, SUVpeak, T/NTblood and T/NTmuscle (AUC = 0.815, 0.864, 0.889, 0.901) (Table 4), but the differences between them were not statistically significant (tested by MedCalc 11.0.1.0). According to ROC curve analysis, the thresholds of SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle were 5.65, 4.46, 7.11, 5.41, and 11.75, respectively. The sensitivity, specificity, and accuracy of SUVmax for predicting tumor response were 77.8, 88.9, and 83.3 %, respectively. The sensitivity, specificity, and accuracy of T/NTlung were 88.9, 100, and 94.4 %, respectively. The sensitivity, specificity, and accuracy of SUVpeak,T/NTblood and T/NTmuscle for predicting tumor response were all 88.9, 88.9, and 88.9 %, respectively (Table 5).
Fig. 1.
ROC curves of 18F-alfatide PET/CT parameters
Table 4.
Area under the curve of SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle for predicting tumor response
| Interval test result variable (s) | Area | SEa | Asymptotic sig.b | Asymptotic 95 % confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| SUVmax | 0.815 | 0.901 | 0.079 | 0.517 | 1 |
| SUVpeak | 0.864 | 0.096 | 0.009 | 0 | 1 |
| T/NTlung | 0.944 | 0.58 | 0.001 | 0 | 1 |
| T/NTblood | 0.889 | 0.081 | 0.005 | 0 | 1 |
| T/NTmuscle | 0.901 | 0.079 | 0.004 | 0 | 1 |
aUnder the nonparametric assumption
bNull hypothesis: true area = 0.5
Table 5.
The specificity, sensitivity, and accuracy of SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle for predicting tumor response
| Parameters | Threshold | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| SUVmax | 5.65 | 77.8 | 88.9 | 88.9 |
| SUVpeak | 4.46 | 88.9 | 88.9 | 88.9 |
| T/NTlung | 7.11 | 88.9 | 100 | 94.4 |
| T/NTblood | 5.41 | 88.9 | 88.9 | 88.9 |
| T/NTmuscle | 11.75 | 88.9 | 88.9 | 88.9 |
18F-alfatide PET/CT parameters compared to other predictors
Multiple clinical variables included patients’ age, stage, histopathology, and 18F-alfatide PET/CT parameters (SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle) were tested by binary logistic regression analyses. We did not take smoking into account because only four patients had never smoked. According to univariate analyses, all the five 18F-alfatide PET/CT parameters that could predict the short-term outcome of CCRT, patients’ age, stage, and histopathology failed. Multivariate analyses were performed when baseline characteristics and 18F-alfatide PET/CT parameters (SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle, respectively) were considered. The result showed that T/NTlung was a significant predictor of CCRT sensitivity (P = 0.032) based on binary logistic regression analyses. However, the SUVmax (P = 0.080), SUVpeak (P = 0.088), T/NTblood (P = 0.098) and T/NTmuscle (P = 0.060) were not predictive for the short-term outcome of CCRT.
Discussion
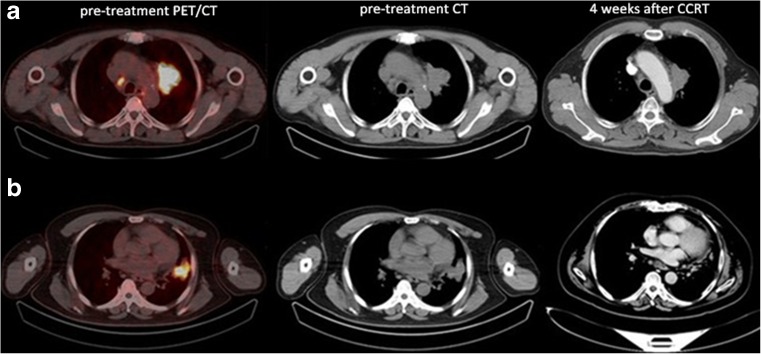
There are significant differences in CCRT responses among the advanced NSCLC patients, so the early prognosis of the sensitivity to CCRT is the premise of personalized treatment. The TNM staging system and histopathology are considered important prognostic tools of overall survival, but they have limited effect on the short-term outcomes. In this study, the results indicated that 18F-alfatide PET/CT may be useful in predicting the short-term outcome of CCRT in patients with advanced NSCLC (Fig. 2). SUVmax, SUVpeak, T/NTlung, T/NTblood and T/NTmuscle obtained from 18F-alfatide PET/CT were higher in non-responders than responders. Even in the multivariate logistic regression analyses, T/NTlung was still an independent predictor of CCRT sensitivity.
Fig. 2.
Two typical examples of 18F-alfatide PET/CT scans in patients with non-responding (a, T/NTlung = 8.88) and responding (b, T/NTlung = 6.47) tumors
PET is a non-invasive modality to evaluate specific molecular progress and a potential tool in the prediction of treatment response. Huang et al. found that the changes in SUV and metabolic tumor volume (MTV) obtained from 18F-FDG PET/CT of pre-treatment and intra-treatment CRT were significantly different between responders and non-responders in patients with locally advanced NSCLC (P = 0.002). However, the baseline parameters failed to differentiate the responders and the non-responders (all P > 0.05) [15]. A study showed that baseline 18F-fluorothymidine (FLT) PET achieved prediction of the treatment response in patients with lung cancer and non-Hodgkin lymphoma [16]. 18F-fluoromisonidazole (FMISO) PET, as an index of tissue oxygenation, can potentially aid in disease prognosis, given the leading role of hypoxia in radiation resistance [17]. Recently, our team had performed a pilot clinical study in which 18F-alfatide PET/CT parameters could predict the tumor sensitivity to CCRT in patients with glioma. Both baseline SUVmax and intra-treatment SUVmax showed correlations with response to CCRT, with the lesion volume change determined by MRI as the “gold” standard [14].
Why 18F-alfatide PET/CT is useful in prediction of the response to CCRT in patients with advanced NSCLC? 18F-alfatide PET/CT was known to be helpful to evaluate tumor angiogenesis, and angiogenesis was well recognized as an essential marker for tumor growth, invasion, and metastasis [18]. The integrin αvβ3 is up-regulated on the activated endothelial cells with tumor angiogenesis, and it can bind to 18F-alfatide with high affinity and specificity. Therefore, the 18F-alfatide uptake of tumor was potentially able to predict the responsiveness to CCRT in patients with advanced NSCLC. Similarly, Niu G and Chen X et al. commented that the responsiveness of glioma to CCRT may be partially due to the low malignancy indicated by the low SUV of 18F-alfatide PET/CT [19]. Besides, the differences between responders and non-responders to CCRT may be attributed to hypoxia. Neo-angiogenic vessels are often poorly perfused with low microvascular pressure, thus promoting blood stasis and hypoxia [20]. This can lead to suboptimal delivery of chemotherapy and also increases radio-resistance in tumors [21]. Therefore, the value of SUV obtained from 18F-alfatide PET/CT may be useful in predicting the short-term outcome of CCRT in patients with advanced NSCLC.
The results showed that SUVmean and TVPET were not different statistically significant between responders and non-responders. SUVmean and TVPET incorporate both tumor volume and metabolic activity, and they need accurate tumor contours. They could be easily affected by the setting threshold and the heterogenous uptake of the 18F-alfatide. That may be the reason why SUVmean and TVPET cannot respond to CCRT short-term outcome of the tumors as well as SUVmax.
As 18F-alfatide PET/CT was helpful to evaluate the tumor angiogenesis, it may be potential in acting as a predictive biomarker to select patients who will most likely benefit from a specific angiogenesis inhibitor, and to detect emerging resistance. Hopefully, more clinical studies are needed to reveal the value of 18F-alfatide PET/CT in therapy decisions and for therapy response monitoring in these diseases.
Conclusion
This study showed that 18F-alfatide PET/CT may be used to predict the short-term outcome of CCRT in patients with advanced NSCLC. With baseline SUVmax, SUVpeak and T/NT, patients’ screening may be performed to avoid unnecessary therapy. The number of patients included in this study is small and a further validation study is needed to test the potential of 18F-alfatide PET/CT in guiding treatment decisions. In addition, it is a pity that only two patients received both 18F-alfatide PET/CT and 18F-FDG PET/CT scans, and we will continue to compare the potential ability of 18F-FDG PET/CT and 18F-alfatide PET/CT for prediction of the responses to CCRT in patients with advanced NSCLC.
Acknowledgments
We thank the PET/CT staff and the technologists at our institute for their excellent support. All authors had read and approved the manuscript. This study was funded by the Natural Science Foundation of China (NSFC81172133, NSFC81372413), the special fund for Scientific Research in the Public Interest (201402011), and the Outstanding Youth Natural Science Foundation of Shandong Province (JQ201423).
Compliance with ethical standards
Funding
This study was funded by the Natural Science Foundation of China (NSFC81172133, NSFC81372413), the special fund for Scientific Research in the Public Interest (201402011), the projects of medical and health technology development program in Shandong province (2014WS0058), and the Outstanding Youth Natural Science Foundation of Shandong Province (JQ201423). No other potential conflicts of interest relevant to this article are reported.
Conflict of interest
The authors declare that there are no conflicts of interests.
Ethical standards
Our investigation of 18 patients was approved by the Shandong Cancer Hospital affiliated to Shandong University Ethical Committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study.
References
- 1.Manegold C, Thatcher N. Survival improvement in thoracic cancer: progress from the last decade and beyond. Lung Cancer. 2007;57(Suppl 2):S3–5. doi: 10.1016/S0169-5002(07)70420-8. [DOI] [PubMed] [Google Scholar]
- 2.Traynor AM, Schiller JH. Systemic treatment of advanced non-small cell lung cancer. Drugs Today (Barc) 2004;40:697–710. doi: 10.1358/dot.2004.40.8.850472. [DOI] [PubMed] [Google Scholar]
- 3.Aupérin A, Le PC, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–90. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 4.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima N, Kataoka M, Sugawara Y, Ochi T, Kiyoto S, Ohsumi S, et al. Volume-based parameters of 18F-fluorodeoxyglucose positron emission tomography/computed tomography improve disease recurrence prediction in postmastectomy breast cancer patients with 1 to 3 positive axillary lymph nodes. Int J Radiat Oncol Biol Phys. 2013;87:738–46. doi: 10.1016/j.ijrobp.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Niu G, Wu H, Chen X. Clinical application of radiolabeled RGD peptides for PET imaging of integrin αvβ3. Theranostics. 2016;6:78–92. doi: 10.7150/thno.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 8.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–8. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 9.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–5. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 10.Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci. 2009;122:165–70. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao S, Wu H, Li W, Zhao S, Teng X, Lu H, et al. A pilot study imaging integrin αvβ3 with RGD PET/CT in suspected lung cancer patients. Eur J Nucl Med Mol Imaging. 2015;42:2029–37. doi: 10.1007/s00259-015-3119-1. [DOI] [PubMed] [Google Scholar]
- 13.Wan W, Guo N, Pan D, Yu C, Weng Y, Luo S, et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–8. doi: 10.2967/jnumed.112.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Liu N, Gao S, Hu X, Zhao W, Tao R, et al. Can an 18F-ALF-NOTA-PRGD2 PET/CT scan predict treatment sensitivity to concurrent chemoradiotherapy in patients with newly diagnosed glioblastoma. J Nucl Med. 2016;57:524–9. doi: 10.2967/jnumed.115.165514. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Zhou T, Ma L, Sun H, Gong H, Wang J, et al. Standard uptake value and metabolic tumor volume of 18F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2011;38:1628–35. doi: 10.1007/s00259-011-1838-5. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann K, Buck AK, Schuster T, Junger A, Wieder HA, Graf N, et al. Predictive value of initial 18F-FLT uptake in patients with aggressive non-Hodgkin lymphoma receiving R-CHOP treatment. J Nucl Med. 2011;52:690–6. doi: 10.2967/jnumed.110.084566. [DOI] [PubMed] [Google Scholar]
- 17.Sachpekidis C, Thieke C, Askoxylakis V, Nicolay NH, Huber PE, Thomas M, et al. Combined use of (18)F-FDG and (18)F-FMISO in unresectable non-small cell lung cancer patients planned for radiotherapy: a dynamic PET/CT study. Am J Nucl Med Mol Imaging. 2015;5:127–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Bikfalvi A. Angiogenesis: health and disease. Ann Oncol. 2006;17(Suppl 10):x65–70. doi: 10.1093/annonc/mdl239. [DOI] [PubMed] [Google Scholar]
- 19.Niu G, Chen XRGDPET. From lesion detection to therapy response monitoring. J Nucl Med. 2016;57:501–2. doi: 10.2967/jnumed.115.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–58. [PubMed] [Google Scholar]
- 21.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–74. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]