Abstract
The effect of twenty-first-century climate change on biodiversity is commonly forecast based on modelled shifts in species ranges, linked to habitat suitability. These projections have been coupled with species–area relationships (SAR) to infer extinction rates indirectly as a result of the loss of climatically suitable areas and associated habitat. This approach does not model population dynamics explicitly, and so accepts that extinctions might occur after substantial (but unknown) delays—an extinction debt. Here we explicitly couple bioclimatic envelope models of climate and habitat suitability with generic life-history models for 24 species of frogs found in the Australian Wet Tropics (AWT). We show that (i) as many as four species of frogs face imminent extinction by 2080, due primarily to climate change; (ii) three frogs face delayed extinctions; and (iii) this extinction debt will take at least a century to be realized in full. Furthermore, we find congruence between forecast rates of extinction using SARs, and demographic models with an extinction lag of 120 years. We conclude that SAR approaches can provide useful advice to conservation on climate change impacts, provided there is a good understanding of the time lags over which delayed extinctions are likely to occur.
Keywords: conservation prioritization, extinction risk, metapopulation, species–area relationship, species distribution model
1. Introduction
The impact of twenty-first-century climate change on biodiversity is typically framed in terms of potential shifts in species distributions predicted using bioclimatic envelope models (BEMs), from which local-to-regional scale range contractions can be inferred [1]. When combined with species–area relationships (SAR), BEMs can also be used to forecast, albeit indirectly, the number of species committed to extinction (i.e. imminent plus delayed extinctions) from human-induced climate change [2]. However, it remains unclear over what time scales these extinction rates will be realized, following the loss of climatically suitable areas and associated habitat degradation [3]. Answering this problem will help to set conservation priorities more efficiently [4], but it requires explicit demographic models that capture systematic and stochastic population-level factors.
Combined BEM−SAR predictions assume an extinction debt, where extinctions can occur with a substantial delay due to stochastic and deterministic processes [5]. Estimating the duration of this extinction debt is an important goal because forecast species losses might never occur if there is time for adverse climate conditions to be reversed through strong policy-based strategies to mitigate greenhouse gases. It is also relevant for ‘triage’-based conservation prioritization [6]. If long time scales for extinction debt exist for some species, the likelihood that these extinctions can be averted through active on-ground management increases, and other species in more immediate need could be targeted for early conservation intervention.
This is a particularly relevant issue to the climate-sensitive forests of the Australian Wet Tropics (AWT); ongoing research in this region suggests a high vulnerability to future range changes for many taxa [7], especially amphibians. Indeed, modelling has forecast that more than 35% of frogs in the region could be committed to extinction by 2050 due to the loss of climatically suitable areas and associated habitat degradation [2]. Here we link population dynamics (modelled as generic life histories) to bioclimatic and habitat models using coupled climate-demographic simulations [8] to determine whether extinction debt from climate change for frogs in the AWT is likely to occur over decades or centuries. We compare our results to combined BEM–SAR predictions of extinction risk, with and without matrix calibration [9].
2. Material and methods
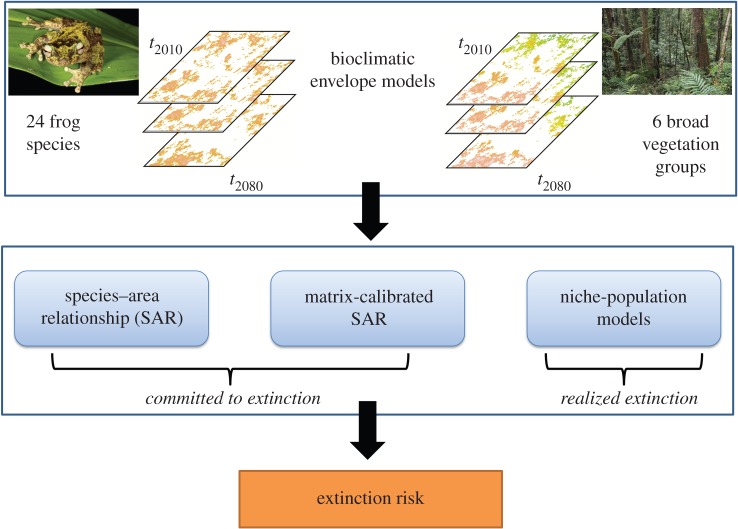
We used BEMs previously fitted to occurrence data for 24 species of native frogs and six broad vegetation groups in the AWT (covering the northeast coastline and adjacent hinterland of Queensland) [10] to project changing suitability surfaces through to 2080 under a business-as-usual emission scenario and a policy-based-intervention emission scenario (RCP8.5 and RCP4.5, respectively). We used coupled ecological niche-population models (NPMs) [8] to combine these projections with two classes of metapopulation and dispersal dynamics—characterizing stream- and forest-breeding frog life histories. We used SARs to determine the relationship between rainforest area and species richness in the AWT and to provide a broad-brush prediction of the number of species likely to go regionally extinct as a result of climate-driven losses of primary habitat [4]. We compared this classical approach with more sophisticated matrix-calibrated SARs, to incorporate the ability of some species to survive in non-preferred habitat [9].
Projected extinction rates from statistical and simulation models were based on forecast climate and habitat loss at a 10 km grid-cell resolution. The stochastic NPMs were used to simulate extinction risk directly, from 2010 to 2200. Climate change affected suitability annually until 2080 based on multi-model ensemble averaged forecasts; for the subsequent 120 years, the impact of climate change was fixed at 2080 levels. We also modelled a baseline ‘no climate change’ scenario, where habitat suitability was fixed to 2010 levels throughout the projection period. We established which parameters had the greatest influence on estimated extinction risk using a sensitivity analysis, and tested the modelled influence of an Allee effect on extinction rates (see figure 1 and the electronic supplementary material, Methods for further details). The data on which this paper is based are available in [10].
Figure 1.
Structure and flow of methods used to estimate extinction risk from future climate change for frogs in the Australian Wet Tropics. Bioclimatic envelope models were used to project changing suitability surfaces for 24 frog species and six vegetation groups between 2010 and 2080. These projections were combined with (i) species–area relationship (SAR) models, with and without matrix calibration, to predict the number of species committed to extinction due to climate change and (ii) metapopulation (niche-population) models to calculate the number of eventual (realized) extinctions due to climate change.
3. Results
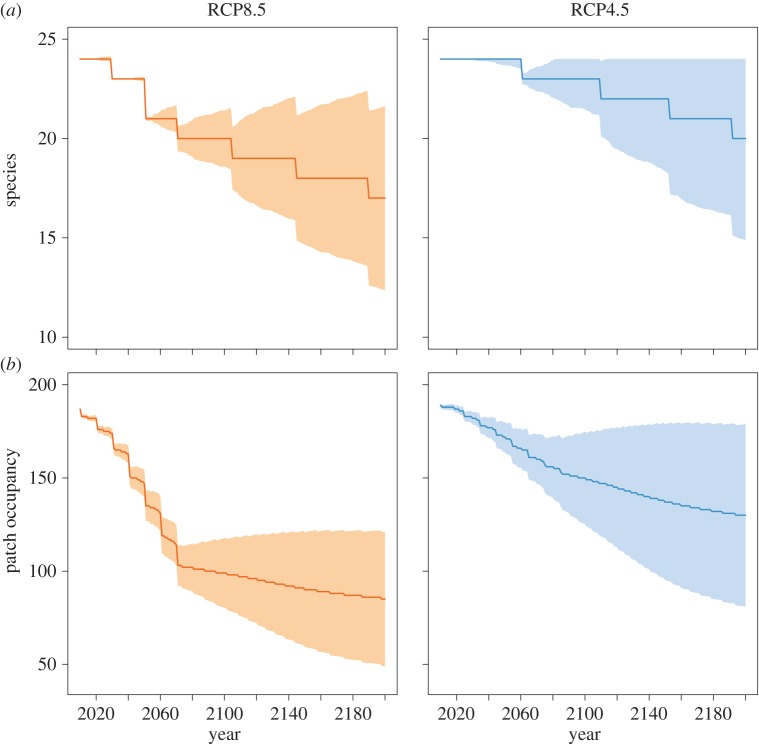
Climate change is forecast to reduce the average range size of all frog species modelled and to cause a mix of imminent and delayed extinctions in the AWT (figure 2). Coupled NPMs, simulated under a business-as-usual (RCP8.5) emission scenario, project that four species (±1 s.d. = 3–5 species) face imminent extinction by 2080, with a further two and three species likely to face delayed extinctions, occurring by 2150 and 2200, respectively (table 1). While extinction risk was lower under a policy-based (RCP4.5) scenario (table 1), there was still some evidence for delayed extinctions—although the uncertainty bounds (±1 s.d.) included zero extinctions. These results were affected only marginally by a 10-fold increase in the strength of the Allee effect being simulated (electronic supplementary material, figure S1).
Figure 2.
Imminent and delayed extinction risk for frogs in the Australian Wet Tropics. Forecast number of species persisting (a) and average number of patches of occupied habitat (b) using NPMs according to a business-as-usual (RCP8.5; orange) and policy-based intervention (RCP4.5; blue) greenhouse-gas-emission scenario. Species extinctions and patch loss after 2080 are delayed responses to the disappearance of climatically suitable areas and associated habitat degradation. Shaded areas show uncertainty bounds (±1 s.d. from the mean) due to demographic-based estimates.
Table 1.
Forecast extinction risk for frogs in the Australian wet tropics. Forecast number of extinctions and per cent decline in expected minimum abundance (change in EMA relative to 2080) in 2080, 2150 and 2200 using niche-population models (NPM). Forecast number of species committed (comm.) to extinction for 2080 using species–area relationships, with matrix calibration (MC-SAR) and without (SAR). Forecasts are for business-as-usual (RCP8.5) and policy-based-intervention (RCP4.5) greenhouse-gas-emission scenarios. Numbers in brackets are ±1 s.d., reflecting uncertainty due to demographic-based parameter estimates. EMA is a continuous metric integrating the risks of declines and extinction risk. The uncertainty bounds for change in EMA are calculated based on change from ±1 s.d. in EMA in 2080. Note that mean prediction error for the fit of the SAR model to the observed relationship between species richness and rainforest area was ±3 species (see electronic supplementary material, Methods).
| model | metric | RCP8.5 |
RCP4.5 |
||||
|---|---|---|---|---|---|---|---|
| 2080 | 2150 | 2200 | 2080 | 2150 | 2200 | ||
| NPM | |||||||
| extinctions | 4 (3–5) | 6 (3–9) | 7 (2–12) | 1 (0–2) | 2 (0–6) | 4 (0–9) | |
| change in EMA | — | 20 (8–55) | 25 (10–69) | — | 9 (4–34) | 13 (5–47) | |
| MC-SAR | |||||||
| comm. to extinction | 5 | — | — | 3 | — | — | |
| SAR | |||||||
| comm. to extinction | 9 | — | — | 5 | — | — | |
Expected minimum abundance and patch occupancy continued to decline after 2080, providing more evidence that lagged effects of climate change on metapopulation dynamics span centuries (table 1 and figure 2). There was encouraging congruence between BEM–SAR forecasts of the number of species committed to extinction (imminent + delayed extinctions) in 2080 and NPM forecasts of the number of realized extinctions in 2200 (table 1). The predicted numbers of imminent extinctions for 2080 were similar for the matrix-calibrated BEM–SAR and the NPM under the RCP8.5 scenario (table 1). In the absence of climate change, two species were forecast to go extinct in the AWT after 2100. However, the uncertainty bounds were large and intersected zero (electronic supplementary material, figure S2). Average metapopulation occupancy remained relatively stable compared with forecasts under the RCP4.5 and RCP8.5 scenarios (electronic supplementary material, figure S2). Results from NPMs were most sensitive to variation in the estimates of frog survival (electronic supplementary material, table S1).
4. Discussion
Climate change this century is forecast to cause imminent and delayed extinctions for frogs in the AWT. We show that these delayed extinctions are likely to occur at time lags greater than 100 years, following the disappearance of climatically suitable areas and associated habitat loss. This has important implications for mitigating climate-driven biodiversity loss, because long lag times (decades to centuries) provide conservation practitioners with more time for intervention.
The widespread practice of coupling BEMs with SARs to infer extinctions due to climate change has been criticized on technical grounds [11], and more detailed (but data-demanding) mechanistic approaches have been offered as a solution [8]. However, until now, no study has compared the likelihood of inferred extinction rates from BEM–SAR approaches with more direct mechanistic estimates, to better understand the proportion of climate-driven extinctions that are delayed, and as importantly, the lag times for these delays. We have shown that inferences of projected extinction rates from BEM–SARs (with and without matrix calibration) for frogs of the wet tropics—a particularly sensitive taxon—are comparable with direct estimates of extinction rates from NPMs if extinction debt is assumed to transpire over an additional 120 years. Therefore, BEM–SAR approaches provide useful forecasts of extinction risk for frogs, if it is understood that many of these extinctions will not be immediate, but instead are likely to be delayed for over a century following climate-driven habitat loss. Only two of 24 species of frogs were forecast to go extinct in our study region by 2200 in the absence of climate change (and management intervention), after time periods of more than 100 years. The driving mechanism for these non-climate losses is likely to be historical reductions in range and abundance, including declines induced by the pathogenic fungal disease chytridiomycosis [12]. This conclusion is tentative, however, with large uncertainty bounds that overlap with zero (losses of species).
As triage becomes more ingrained in climate change-adaptation strategies [13], a better understanding of the lag times for delayed extinctions is vital. This is because lag times extend the periods available to avert forecast extinctions from climate change, through assisted colonization and habitat restoration [14]. Accordingly, more species should remain on the conservation radar for longer, if it is the case that most extinctions from climate change are delayed and this extinction debt is paid back over long time scales.
The generality of our present results on lag times for extinctions due to climate change are constrained confidently only to short-lived range-restricted species with low dispersal capacities. We might expect more protracted lag times for long-lived wide-ranging species—theoretically in the order of centuries to millennia [3]. Furthermore, amphibian declines in the twenty-first century are likely to occur as a result of multiple drivers of extinction [15]. We implicitly (partially) accounted for chytridiomycosis in our BEM–SAR and NPM forecasts of extinction risk by (i) modelling aspects of climate and post-chytridiomycosis frog occurrence and (ii) using post-chytridiomycosis habitat preferences (electronic supplementary material, Methods). This approach is reasonable because climatic conditions exert a strong influence on the distribution and prevalence of chytridiomycosis [12]. We do not consider future land conversion to agriculture a threat because the unfarmed areas in our study region comprise the protected Wet Tropics of Queensland UNESCO World Heritage Area. Lastly, we note that uncertainty in forecasts of extinction rates from NPMs remains high, possibly affecting conclusions regarding agreement between BEM–SAR approaches and the more mechanistic NPM estimates. This uncertainty would be reduced through improved estimates of demographic parameters (particularly survival) for frog populations in the AWT. Species–area relationships will be improved with better on-ground estimates of species richness and rainforest area.
We conclude that coupled BEM–SAR approaches can provide a valuable instrument for conservation, provided it is understood that most of the extinctions forecast using this method will not be immediate.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Cleo Bertelsmeier for helping calculate frog habitat suitability, and John Alroy and two anonymous referees for useful comments.
Authors' contributions
The design of this project involved all authors. J.V. generated the BEMs. D.A.F. coupled the BEMs to the metapopulation simulations and wrote the initial draft of the manuscript. All authors discussed the results and contributed to the writing of the final version of the manuscript. The authors agree to be accountable for all aspects of the work reported.
Data accessibility
Data used in this research are available from Williams et al. [10]: http://dx.doi.org/doi:10.1890/09-1069.1. Specific frog and vegetation models are available upon request.
Competing interests
The authors have no competing interests.
Funding
The research was funded by an ARC Future Fellowship (FT140101192) awarded to D.A.F. and by an ARC Discovery grant (DP1096427).
Reference
- 1.Araujo M, Peterson AT. 2012. Uses and misuses of bioclimatic envelope modelling. Ecology 93, 1527–1539. ( 10.1890/11-1930.1) [DOI] [PubMed] [Google Scholar]
- 2.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 3.Halley JM, Sgardeli V, Triantis KA. 2014. Extinction debt and the species–area relationship: a neutral perspective. Glob. Ecol. Biogeogr. 23, 113–123. ( 10.1111/geb.12098) [DOI] [Google Scholar]
- 4.Brooks TM, Pimm SL, Oyugi JO. 1999. Time lag between deforestation and bird extinction in tropical forest fragments. Conserv. Biol. 13, 1140–1150. ( 10.1046/j.1523-1739.1999.98341.x) [DOI] [Google Scholar]
- 5.Kuussaari M, et al. 2009. Extinction debt: a challenge for biodiversity conservation. Trends Ecol. Evol. 24, 564–571. ( 10.1016/j.tree.2009.04.011) [DOI] [PubMed] [Google Scholar]
- 6.Bottrill MC, et al. 2008. Is conservation triage just smart decision making? Trends Ecol. Evol. 23, 649–654. ( 10.1016/j.tree.2008.07.007) [DOI] [PubMed] [Google Scholar]
- 7.Williams SE, Bolitho EE, Fox S. 2003. Climate change in Australian tropical rainforests: an impending environmental catastrophe. Proc. R. Soc. Lond. B 270, 1887–1892. ( 10.1098/rspb.2003.2464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fordham DA, Akçakaya HR, Araújo MB, Keith DA, Brook BW. 2013. Tools for integrating range change, extinction risk and climate change information into conservation management. Ecography 36, 956–964. ( 10.1111/j.1600-0587.2013.00147.x) [DOI] [Google Scholar]
- 9.Koh LP, Lee TM, Sodhi NS, Ghazoul J. 2010. An overhaul of the species–area approach for predicting biodiversity loss: incorporating matrix and edge effects. J. Appl. Ecol. 47, 1063–1070. ( 10.1111/j.1365-2664.2010.01860.x) [DOI] [Google Scholar]
- 10.Williams SE, et al. 2010. Distributions, life-history specialization, and phylogeny of the rain forest vertebrates in the Australian Wet Tropics. Ecology 91, 2493 ( 10.1890/09-1069.1) [DOI] [Google Scholar]
- 11.Ibanez I, Clark JS, Dietze MC, Feeley K, Hersh M, LaDeau S, McBride A, Welch NE, Wolosin MS. 2006. Predicting biodiversity change: outside the climate envelope, beyond the species–area curve. Ecology 87, 1896–1906. ( 10.1890/0012-9658(2006)87[1896:PBCOTC]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 12.Woodhams DC, Alford RA. 2005. Ecology of Chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conserv. Biol. 19, 1449–1459. ( 10.1111/j.1523-1739.2005.004403.x) [DOI] [Google Scholar]
- 13.Lawler JJ. 2009. Climate change adaptation strategies for resource management and conservation planning. Ann. NY Acad. Sci. 1162, 79–98. ( 10.1111/j.1749-6632.2009.04147.x) [DOI] [PubMed] [Google Scholar]
- 14.Akcakaya HR, Butchart SHM, Watson JEM, Pearson RG. 2014. Preventing species extinctions resulting from climate change. Nat. Clim. Change 4, 1048–1049. ( 10.1038/nclimate2455) [DOI] [Google Scholar]
- 15.Hof C, Araujo MB, Jetz W, Rahbek C. 2011. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, 516–519. ( 10.1038/nature10650) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this research are available from Williams et al. [10]: http://dx.doi.org/doi:10.1890/09-1069.1. Specific frog and vegetation models are available upon request.