Abstract
The painted lady, Vanessa cardui, is a migratory butterfly that performs an annual multi-generational migration between Europe and North Africa. Its seasonal appearance south of the Sahara in autumn is well known and has led to the suggestion that it results from extremely long migratory flights by European butterflies to seasonally exploit the Sahel and the tropical savannah. However, this possibility has remained unproven. Here, we analyse the isotopic composition of butterflies from seven European and seven African countries to provide new support for this hypothesis. Each individual was assigned a geographical natal origin, based on its wing stable hydrogen isotope (δ2Hw) value and a predicted δ2Hw basemap for Europe and northern Africa. Natal assignments of autumn migrants collected south of the Sahara confirmed long-distance movements (of 4000 km or more) starting in Europe. Samples from Maghreb revealed a mixed origin of migrants, with most individuals with a European origin, but others having originated in the Sahel. Therefore, autumn movements are not only directed to northwestern Africa, but also include southward and northward flights across the Sahara. Through this remarkable behaviour, the productive but highly seasonal region south of the Sahara is incorporated into the migratory circuit of V. cardui.
Keywords: Vanessa cardui, insect migration, isoscapes, deuterium, Sahara, tropical savannah
1. Introduction
Long-range insect migration is a timely and important topic in ecological research but one that is still in its infancy [1]. With a few exceptions [2], the small size of insects has prevented the use of exogenous markers to track their movements, which means that the most basic aspects of migration, the route itself and the distances covered, remain poorly known for most species. Fortunately, this difficulty is increasingly being overcome with the use of intrinsic markers such as stable isotopes [3,4].
In the Palaearctic, each year large numbers of insects undertake seasonal movements between Africa and Europe [5]. One such insect is the painted lady butterfly Vanessa cardui, which, through the succession of at least six generations, accomplishes a complete round-trip migration across most of Europe in spring and summer, and northern Africa in autumn and winter [6]. Although its general pattern of migration is known, many uncertainties regarding the distances covered by individual butterflies and the movements within Africa still exist. First, it has never been shown that the butterflies appearing south of the Sahara in autumn (sometimes in great numbers) have a European origin [6,7]. Second, although it is believed that northwestern Africa (Maghreb) is colonized in autumn by European migrants, both ground-level and radar observations of northward migration in Morocco and Mauritania in October–November also point to sub-Saharan origins [6].
Here, we present new evidence on both of these questions by means of stable isotope (δ2H) analysis, based on a comprehensive collection of butterflies across southern Europe, North Africa and South-Saharan Africa. Our data conclusively show that, in autumn, some European butterflies reach the tropical savannah south of the Sahara, where they are known to breed [7]. We also show that some of their offspring migrate northwards and cross the Sahara to breed in the Maghreb. These complex movements across the Sahara give a new dimension to our understanding of long-distance insect migration between Africa and Europe.
2. Material and methods
(a). Butterfly collection
We gathered 334 butterflies from seven European and seven African countries around the Mediterranean, and from an extensive area south of the Sahara (figure 1). Butterflies were mainly collected in 2014 and, additionally, between 2009 and 2013 (electronic supplementary material, table S1). European samples were obtained in spring, summer and autumn (i.e. the period comprising northward and southward migrations and summer breeding), while African samples were mainly obtained from October to December (i.e. the period of colonization of North Africa and the region south of the Sahara, and the start of local emergences).
Figure 1.
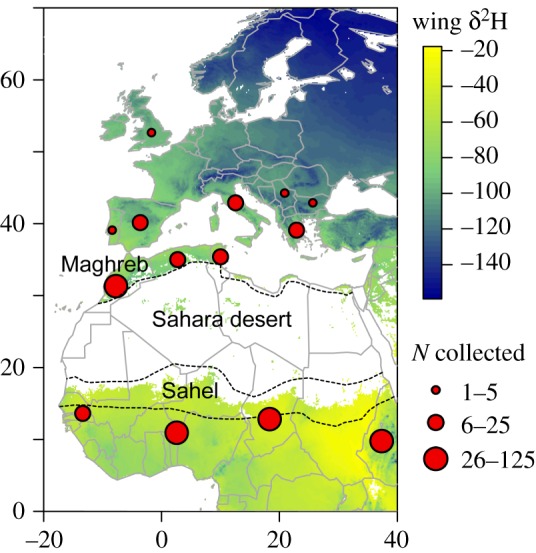
Sample locations and sizes, superimposed on the isoscape of estimated δ2Hw for the wings of painted ladies in Europe and Africa.
For each butterfly, wing condition was scored from 1 (fresh) to 5 (extremely worn). We assumed that categories 1 and 1.5 corresponded to recent local emergences not having undertaken migratory flights yet, and therefore these were excluded from analyses on the natal origin of potential migrants (see the electronic supplementary material, figure S1 and methods, for examples of wing wear categories and the rationale behind our assumptions).
(b). Stable isotope analysis and natal assignments to potential migrants
Non-exchangeable δ2H values from wing chitin were obtained using the comparative equilibration method [8]. All δ2H results were reported in per mil (‰) deviations from the VSMOW-SLAP standard scale (see the electronic supplementary material for details).
Prior to any isotopic assignment, δ2H values from all samples were arranged into five groups using a k-means clustering analysis [9]. These clusters explained 92% of the variation and represented the potential groups of natal origin. The five natal areas were related to eastern-central Europe (Group 1, centroid δ2H = –111‰, n = 35), western-central Europe, southern Europe and Maghreb (Group 2, δ2H = –92‰, n = 116), Maghreb and Mediterranean Islands (Group 3, –80‰, n = 96), western Africa (Group 4, δ2H = –65‰, n = 60), and central and eastern Africa (Group 5, δ2H = –39‰, n = 27) (see the electronic supplementary material, figure S2).
To assign natal origins to potential migrants, a geospatial natal assignment method was used to link butterfly wing δ2H values (δ2Hw) to well-known spatial hydrological hydrogen isotopic distribution (isoscapes) in precipitation (δ2Hp) of Europe and northern Africa [10]. The δ2Hp isoscape [11] was then converted to a spatially explicit butterfly wing isoscape by using a calibration relationship determined for known-origin butterflies across the western Palaearctic [12]. Probability density surfaces were obtained using the complete individual spatial probability surface (no odds ratio used). All calculations and modelling were analysed in R [13].
3. Results and discussion
Stable hydrogen isotopes confirmed that the seasonal population shift of V. cardui between Europe and Africa is the result of long-distance migration by successive generations (i.e. multi- or transgenerational migration; [14]). Butterflies collected in southern Europe showed a temporal decline in δ2Hw values, from –81 ± 17‰ in April–May to –100 ± 19‰ in July (r = 0.49, p < 0.01). This is explained by the replacement of a spring population of northward migrants having developed as larvae in North Africa, by a summer population dominated by European local emergences. Butterflies collected in Africa showed an opposite trend (r = 0.40, p < 0.01), from –86 ± 9‰ in early October to –60 ± 17‰ in late November. This trend is in accordance with first arrivals of European migrants in October, followed by less negative values as their offspring emerged in the following month.
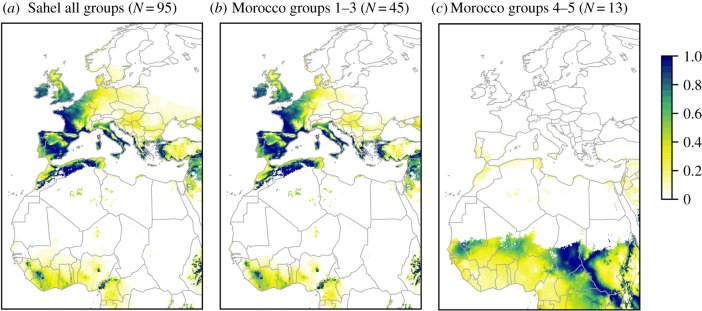
Natal assignments of autumn migrants collected south of the Sahara revealed long-distance movements most probably starting in southern and central Europe (natal cluster groups 2–3 mostly, 6% from group 1, 7% from group 4; figure 2a). Although the mountains in the Maghreb also appeared as a potential natal area, field observations indicate that densities in the region are very low until the arrival of European migrants in October. This means that summer breeding in Maghreb mountains is at most a local phenomenon and cannot explain the origin of most butterflies appearing south of the Sahara in autumn. Although migration between Europe and the African tropical savannah had already been suggested [6,7,15], our analyses represent the first empirical confirmation of this phenomenon. Depending on the exact origin of the butterflies, these flights could exceed 4000 km. Such flights can probably only be accomplished by taking advantage of favourable winds [6,16].
Figure 2.
Assigned natal origins of painted ladies collected in autumn in the Sahel (a) and Morocco (b,c), with the corresponding number of butterflies analysed (N). Natal groups 1–5 were defined with a k-means clustering analysis (see §2). Colours depict the predicted probability (0–1) of natal origins of these migrants.
Samples from Maghreb revealed a mixed origin of migrants. Most individuals (78%) shared essentially the same European natal origins as those collected south of the Sahara (figure 2b). A smaller fraction (22%), however, appeared to originate in the Sahel (natal groups 4–5, figure 2c). Butterflies with a European origin showed a wider range of wing wear, including very worn individuals that were absent from the samples of Sahelian origin. The proportions of categories 2–3.5 versus categories 4–5 differed between these two geographical groups (χ2 = 4.371, p = 0.037), suggesting that European butterflies comprised a mixture of early and late migratory waves, while Sahelian butterflies only corresponded to more recent waves.
For a migratory insect, colonization of the Sahel and further south in autumn seems highly adaptive, as the whole region offers suitable breeding conditions coinciding with a short period of high productivity after the rainy season [7,17]. This also explains the 3500–4500 million birds migrating in autumn into this region, most of which depend on the seasonal insect populations [18].
However, strong seasonality also means that locally produced sub-Saharan generations of V. cardui experience rapid worsening of environmental conditions. Our data conclusively show that some butterflies migrate northwards across the Sahara, to colonize favourable areas in the Maghreb. This may seem surprising in a period when continuous southward-blowing dry winds (the so-called ‘Harmattan’) prevail in the Sahara [5]. However, autumn flights between western Sahel and the Maghreb also occur in other migrant insects moving down-wind (e.g. in swarms of the desert locust [19]), indicating that favourable conditions for northward migration across the Sahara still occur under some circumstances. Additional evidence comes from repeated observations of northward migrations of V. cardui in the south of Morocco in late autumn (C.S. 2009 and 2015, personal observation).
In conclusion, our results convincingly show that autumn migration by V. cardui entails extremely long flights of 4000 km or more from Europe to the south of the Sahelian belt, in addition to the well-known destination in northwestern Africa. Moreover, we confirm the existence of complex movements in Africa leading to the reinforcement of the autumn breeding population in the Maghreb by butterflies originating south of the Sahara. This information will prove essential to model population trends in Europe in relation to the weather conditions experienced by the African populations.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Oskar Brattström and an anonymous reviewer made helpful comments on the manuscript. Many people contributed to this work. See a complete list in the electronic supplementary material.
Data accessibility
Data supporting this article are included as part of the electronic supplementary material.
Authors' contributions
All authors conceived the study. G.T., R.V. and C.S. carried out the fieldwork. D.X.S., K.A.H. and C.S. analysed the data. C.S. drafted the manuscript and all authors edited and approved the final version of the manuscript. All authors agree to be held accountable for the content of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
K.A.H. was funded by an operating grant and D.X.S. by an NSERC visiting fellowship from Environment Canada. Funding to R.V. and G.T. was provided by the Committee for Research and Exploration of National Geographic (grant no. 9528-14) and by the Spanish Ministerio de Economía y Competitividad (CGL2013-48277-P). G.T. is supported by the Marie Curie Actions FP7-PEOPLE-2013-IOF (project 622716) and the grant BP-A00275 (AGAUR-Generalitat de Catalunya). Expeditions in Morocco were funded by Antoni Jonch Cooperació.
References
- 1.Chapman JW, Reynolds DR, Wilson K. 2015. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 18, 287–302. ( 10.1111/ele.12407) [DOI] [PubMed] [Google Scholar]
- 2.Wikelski M, Moskowitz D, Adelman JS, Cochran J, Wilcove DS, May ML. 2006. Simple rules guide dragonfly migration. Biol. Lett. 2, 325–329. ( 10.1098/rsbl.2006.0487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flockhart DT, Wassenaar LI, Martin TG, Hobson KA, Wunder MB, Norris DR. 2013. Tracking multi-generational colonization of the breeding grounds by monarch butterflies in eastern North America. Proc. R. Soc. B 280, 20131087 ( 10.1098/rspb.2013.1087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobson KA, Anderson RC, Soto DX, Wassenaar LI. 2012. Isotopic evidence that dragonflies (Pantala flavescens) migrating through the Maldives come from the Northern Indian subcontinent. PLoS ONE 7, e52594 ( 10.1371/journal.pone.0052594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedgley DE, Reynolds DR, Tatchell GM. 1995. Long-range insect migration in relation to climate and weather: Africa and Europe. In Insect migration: tracking resources through space and time (eds Drake VA, Gatehouse AG), pp. 3–29. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Stefanescu C, et al. 2013. Multi-generational long-distance migration in insects: studying the painted lady butterfly in the Western Palaearctic. Ecography 36, 474–486. ( 10.1111/j.1600-0587.2012.07738.x) [DOI] [Google Scholar]
- 7.Talavera G, Vila R. 2016. Discovery of mass migration and breeding of the butterfly Vanessa cardui in the Sub-Sahara: the Europe—Africa migration revisited. Biol. J. Linn. Soc. ( 10.1111/bij.12873) [DOI] [Google Scholar]
- 8.Wassenaar LI, Hobson KA. 2003. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes Environ. Health Stud. 39, 211–217. ( 10.1080/1025601031000096781) [DOI] [PubMed] [Google Scholar]
- 9.García-Pérez B, Hobson KA. 2014. A multi-isotope (δ2H, δ13C, δ15N) approach to establishing migratory connectivity of Barn Swallow (Hirundo rustica). Ecosphere 5, art21. ( 10.1890/ES13-00116.1) [DOI] [Google Scholar]
- 10.Hobson KA, Soto DX, Paulson DR, Wassenaar LI, Matthews JH. 2012. A dragonfly (δ2H) isoscape for North America: a new tool for determining natal origins of migratory aquatic emergent insects. Methods Ecol. Evol. 3, 766–772. ( 10.1111/j.2041-210X.2012.00202.x) [DOI] [Google Scholar]
- 11.Terzer S, Wassenaar LI, Araguás-Araguás LJ, Aggrawal PK. 2013. Global isoscapes for δ18O and δ2H in precipitation: improved prediction using regionalized climatic regression models. Hydrol. Earth Syst. Sci. Discuss. 10, 7351–7393. ( 10.5194/hess-17-1-2013) [DOI] [Google Scholar]
- 12.Brattström O, Bensch S, Wassenaar LI, Hobson KA, Åkesson S. 2010. Understanding the migration ecology of European red admirals Vanessa atalanta using stable hydrogen isotopes. Ecography 33, 720–729. ( 10.1111/j.1600-0587.2009.05748.x) [DOI] [Google Scholar]
- 13.R CoreTeam. 2015. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 14.Chapman BB, Hulthén K, Wellenreuther M, Hansson L-A, Nilsson J-Å, Brönmark C. 2014. Patterns of animal migration. In Animal movement across scales (eds Hansson L-A, Åkesson S), pp. 11–35. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Williams CB. 1958. Insect migration. London, UK: Collins. [Google Scholar]
- 16.Chapman JW, Nesbit RL, Burgin LE, Reynolds DR, Smith AD, Middleton DR, Hill JK. 2010. Flight orientation behaviors promote optimal migration trajectories in high-flying insects. Science 327, 682–685. ( 10.1126/science.1182990) [DOI] [PubMed] [Google Scholar]
- 17.Zwarts L, Bijlsma RG, van der Kamp J, Wymenga E. 2009. Living on the edge: wetlands and birds in a changing Sahel. Zeist, The Netherlands: KNNV Uitgeverij. [Google Scholar]
- 18.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press. [Google Scholar]
- 19.Symmons PM, Cressman K. 2001. Desert locust guidelines. 1. Biology and behaviour. Rome, Italy: FAO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this article are included as part of the electronic supplementary material.