Abstract
We report the discovery that the locomotor trajectories of Drosophila larvae follow the power-law relationship between speed and curvature previously found in the movements of human and non-human primates. Using high-resolution behavioural tracking in controlled but naturalistic sensory environments, we tested the law in maggots tracing different trajectory types, from reaching-like movements to scribbles. For most but not all flies, we found that the law holds robustly, with an exponent close to three-quarters rather than to the usual two-thirds found in almost all human situations, suggesting dynamic effects adding on purely kinematic constraints. There are different hypotheses for the origin of the law in primates, one invoking cortical computations, another viscoelastic muscle properties coupled with central pattern generators. Our findings are consistent with the latter view and demonstrate that the law is possible in animals with nervous systems orders of magnitude simpler than in primates. Scaling laws might exist because natural selection favours processes that remain behaviourally efficient across a wide range of neural and body architectures in distantly related species.
Keywords: power law, motor control, Drosophila, locomotion
1. Introduction
When we scribble our name on a piece of paper, the instantaneous angular speed is related to the local path curvature according to a power law [1]. The law is one of the best-studied characteristics of human voluntary movements, holding for hand drawing, pursuit eye movements, speech and walking [1–4]. Movements complying with the law are maximally smooth [2,4,5]. The law is not given a priori: even when the path is imposed, as in hand drawing, movement speed could, in principle, vary in infinite ways, as shown by systematic deviations from the power law for some movements [2,6]. Therefore, the law must result from physiological constraints, although its origin remains debated. According to one view, the law originates by decoding complex cortical processes; indeed, population vectors in motor cortex obey the power law during drawing [7]. According to another view, the law stems from simple harmonic motions [6]—such as those output by spinal central pattern generators (CPGs)—coupled with the viscoelastic properties of muscles [8].
To the best of our knowledge, the power law has only been studied in human and non-human primates [1–8]. Here, we report that Drosophila melanogaster larvae, whose movements are controlled by a much simpler neural system [9], display a speed–curvature power law while crawling. This demonstrates that the law can emerge from the interplay between relatively simple neural commands and biomechanics. Our findings support the view that, despite huge divergence in anatomy, functional complexity and ecological contingencies, basic principles of motor control resulting in efficient behaviour are shared across distantly related species [4,10].
2. Material and methods
Experimental procedures and tracking of larvae behaviour were the same as in [11]. Third-instar Drosophila melanogaster larvae in the foraging stage were washed in 15%-sucrose solution and transferred to a flat-lid arena coated with a 3%-agarose slab. Animals were tracked at 7 frames s−1, 90 μm pixel−1 for 5 min. Tracking was interrupted if the animal touched the plate boundaries. Custom-made tracking scripts [12] extracted the location of the centroid, head and tail from postural sequences. We used three groups of larvae exposed to different experimental conditions: overshoot, approach and dispersal [11,13]. Odour gradients were created by suspending a 10 μl droplet of ethyl butyrate from the arena top, out of animals’ reach.
For the main analyses, the x, y position samples of the centroid were low-pass filtered (second-order, zero-phase-lag Butterworth filter). A 0.07 Hz cut-off frequency was chosen after verifying that the power-spectrum density of the raw data was approximately flat up to ≈0.01 Hz, and then dropped rapidly. At 0.07 Hz, the power was down by ≈30 dB. We interpolated the filtered data with cubic splines, computed the time derivatives of the interpolating spline, instantaneous curvature C(t) and angular speed A(t) from standard differential geometry [1,3,14]. Least-squares orthogonal-regression of log10A(t) versus log10C(t) was performed to estimate the exponent (β) and the variance accounted for (r2) by the power law A(t) = kC(t)β. Statistically significant differences of β between experimental conditions were assessed using non-parametric tests (Kruskal–Wallis ANOVA by ranks followed by multiple comparisons), because the data were not normally distributed (Kolmogorov–Smirnov test).
3. Results
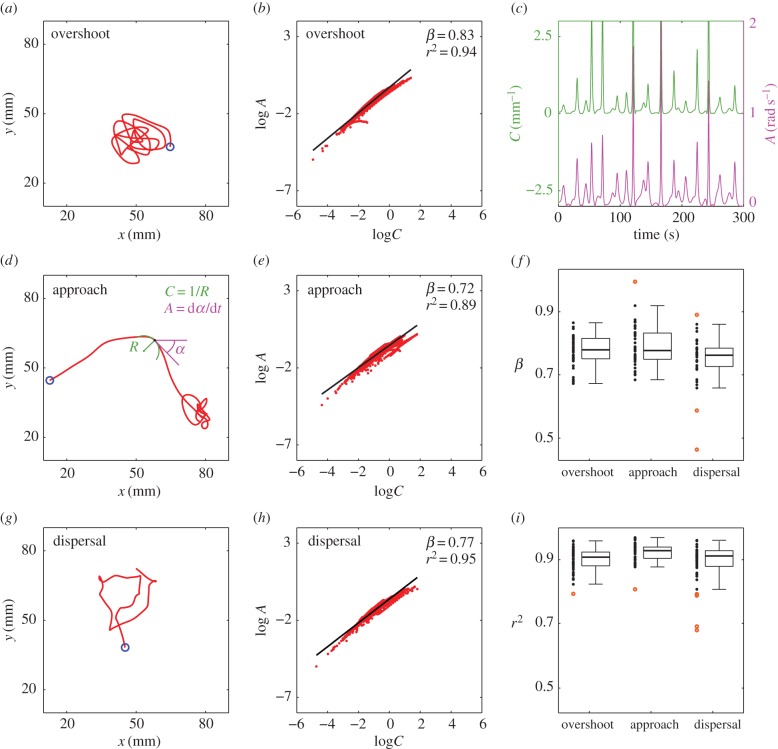
To induce animals to naturally ‘draw’ different types of trajectories, we tested different sensory environments [11]. In the overshoot condition during chemotaxis close to an odour source, the larval centroid traced complex trajectories resembling human scribbles (figure 1a). Trajectories were not associated with a constant progression speed or any simple kinematic pattern. Both the angular speed and path curvature were widely modulated, yet they covaried throughout (figure 1c). A log–log plot of angular speed versus curvature revealed a power law as a straight line whose slope corresponds to the power exponent (figure 1b). Power-law scaling extended over three or more orders of magnitude along both axes, consistent with typical requirements for robust power laws [15]. Similar results were obtained for all individual larvae in this condition.
Figure 1.
Relation between angular speed and path curvature in fly larvae tracing different trajectories. (a) Trajectory of the centroid position of one representative larva in the overshoot condition (blue circle indicates starting position). (b) Scatterplot of instantaneous angular speed A and local path curvature C on log10–log10 scale. All data points (red dots, n = 2100) sampled at equal time intervals along the same trajectory as in (a) were included. The data were fitted by the power function A(t) = kC(t)β (black line) with β-exponent and variance accounted for (r2) as indicated (top right). (c) Temporal evolution of the path curvature (green) and angular speed (magenta) for the same data as in (a–b). (d) Centroid trajectory of a larva in the approach condition. Key movement variables are identified at an arbitrary point along the trajectory: C is the curvature of the osculating circle of radius R, α is the phase angle of the tangent and the angular speed A is the time derivative of α. (e) Log–log plot of angular speed versus curvature for the same trajectory as in (d). (f) Summary boxplot statistics for β-exponent of individual animals in the three different groups: overshoot (n = 42), approach (n = 40) and dispersal (n = 41). Outliers are orange dots. (g) Centroid trajectory of a larva in the dispersal condition. (h) Log–log plot of angular speed versus curvature for the same trajectory as in g. (i) Summary boxplot statistics for r2 in the three groups. (Online version in colour.)
Next, we tested larvae subjected to other sensory environments, resulting in different exploratory strategies and movement trajectories. In the approach condition, individuals reached an odour source at the opposite side of the arena via progressively more curved paths (figure 1d). In the dispersal condition, larvae searching for food in the absence of olfactory cues moved in arbitrary directions tracing highly variable paths (figure 1g). Overall, the power law did not depend on the type of exploratory movements: overshooting, approaching and dispersing larvae complied with the power law (figure 1b,e,h,i). The median value of the power exponent was 0.78 (interquartile range = 0.06, n = 42), 0.78 (interquartile range = 0.08, n = 40) and 0.76 (interquartile range = 0.06, n = 41) for the overshoot, approach and dispersal conditions, respectively (figure 1f). The distribution of the power exponents did not differ significantly between the three groups (Kruskal–Wallis H2,123 = 5.29, p = 0.071; multiple comparisons p > 0.05).
Similar results were observed for trajectories traced by the animal's hindmost part (tail): across all animals and conditions, the median r2 for the power law was 0.89 (interquartile range = 0.06, n = 123), and the median value of the power exponent was 0.74 (interquartile range = 0.09).
Moreover, the results were not affected substantially by using different frequency cut-offs in filtering the position data [16]. In the electronic supplementary material, figure S1 reports the results for the overshoot condition, but very similar results were obtained in the other conditions. The power law accounted well for the results, irrespective of filtering (median r2 > 0.85 over the tested range of frequency cut-offs, including no-filtering). The value of β-exponent varied with frequency cut-off, but only to a limited extent (median = 0.77, interquartile range = 0.06). A few individuals did not comply with the law (especially in the dispersal condition; see outliers as orange dots in figure 1f,i), confirming that it is not an obligatory outcome of our analyses.
4. Discussion
We have reported that a fundamental law of human control is at work in the humble maggot. The power law for voluntary movements in human and non-human primates may well have different origins [7] from those in crawling larvae. Yet, it is remarkable that the law is compatible with comparatively simple nervous systems, and that it holds for movements differing in speed by several orders of magnitude, such as those generated by humans and fly larvae. The non-trivial nature of the law is demonstrated by both theoretical considerations [5] and the empirical finding of violations in humans [2,6] and in some larvae here.
The power exponent for human hand drawing is generally close to 0.66 (so-called two-thirds power law [1]), but it becomes 0.73 when drawing in water [17], the latter value being close to the present values in larvae. Therefore, not only do we find in the larvae the geometric–kinematic constraint dictated by the power law, but also hints of dynamic constraints in the power exponent as recently found in humans [2,5,8,17], where its specific value depends on the viscosity of the medium (air or water for hand drawing, agar support and thin liquid coat for larval locomotion) and the trajectory shape.
In Drosophila larvae, multiple CPGs in the abdominal and thoracic segments of the nervous system generate peristaltic waves of muscle contractions along the body axis that enable crawling [9]. Curvature and crawling speed are regulated by two distinct processes. The degree of symmetry and synchrony of neural activity on each side of the nervous system controls the instantaneous direction of movement and therefore path curvature, straighter trajectories resulting from more symmetrical contractions in amplitude and timing [18], whereas frequency determines movement speed. It is then possible that the speed–curvature power law emerges from these patterns of neural activity transformed in oscillatory body motion, although suprasegmental nervous structures as well as sensory feedback also contribute to the net motor output [9]. It is also conceivable that the speed–curvature power law is solely due to viscoelastic muscle properties (as suggested by previous modelling studies [8]), although the presence of violations would argue against this interpretation. Future studies might use appropriate mutants to genetically disrupt the power law and provide generative mechanistic models [15] to elucidate the relative role of neural structures, body mechanics and sensory feedback control in the generation of such movement trajectories.
Typical strategies of environment exploration for available resources involve the execution of quasi-random walks with relatively straight stretches of locomotion alternating with abrupt changes of direction [9,11–14,18]. Scaling laws hold both at the whole-trajectory level and at the scale of local and instantaneous movements. At a macroscopic level, the probabilities of locomotor stretch sizes and idling-durations obey power-law distributions [19]. At a mesoscopic level, speed and curvature are related by the power law described here. Why do these scaling laws exist and why are they important in flies, and other organisms? One hypothesis is that they both entail optimal behaviour: Lévy-like foraging in sparse environments [19] and instantaneous movement smoothness for speed–curvature power law [2,4,5]. Scaling laws tend to be ubiquitous in nature, possibly because natural selection favours processes that remain behaviourally efficient across wide ranges of size and structure in different contexts [4,10] and across phyla.
Supplementary Material
Acknowledgements
We thank Asif Ghazanfar, André Brown, Santiago Canals and Ibrahim Tastekin for valuable feedback.
Data accessibility
Data available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.37sb7.
Code accessibility
Code available from the SourceForge repository: https://sourceforge.net/projects/sos-track/.
Authors' contributions
All authors contributed to study design, data analysis and interpretation, and manuscript writing, and agree to be held accountable for the content therein and approved the final version of the manuscript.
Competing interests
Authors declare no competing interests.
Funding
This work was supported by the Italian Space Agency (Arianna grant no. 2014-008-R.0 to M.Z., COREA grant 2013-084-R.0 to F.L.) and by the Spanish Ministry of Economy and Competitivity (Severo Ochoa Center of Excellence programme SEV2013-0317 start-up funds to A.G.-M.).
References
- 1.Lacquaniti F, Terzuolo C, Viviani P. 1983. The law relating the kinematic and figural aspects of drawing movements. Acta Psychol. 54, 115–130. ( 10.1016/0001-6918(83)90027-6) [DOI] [PubMed] [Google Scholar]
- 2.Wann J, Nimmo-Smith I, Wing AM. 1988. Relation between velocity and curvature in movement: equivalence and divergence between a power law and a minimum-jerk model. J. Exp. Psychol. Hum. Percept. Perform. 14, 622–637. ( 10.1037/0096-1523.14.4.622) [DOI] [PubMed] [Google Scholar]
- 3.Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. 2002. Two-thirds power law in human locomotion: role of ground contact forces. Neuroreport 13, 1171–1174. ( 10.1097/00001756-200207020-00020) [DOI] [PubMed] [Google Scholar]
- 4.Flash T, Hochner B. 2005. Motor primitives in vertebrates and invertebrates. Curr. Opin. Neurobiol. 15, 660–666. ( 10.1016/j.conb.2005.10.011) [DOI] [PubMed] [Google Scholar]
- 5.Huh D, Sejnowski TJ. 2015. Spectrum of power laws for curved hand movements. Proc. Natl Acad. Sci. USA 112, E3950–E3958. ( 10.1073/pnas.1510208112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaal S, Sternad D. 2001. Origins and violations of the 2/3 power law in rhythmic three-dimensional arm movements. Exp. Brain Res. 136, 60–72. ( 10.1007/s002210000505) [DOI] [PubMed] [Google Scholar]
- 7.Schwartz AB. 1994. Direct cortical representation of drawing. Science 265, 540–542. ( 10.1126/science.8036499) [DOI] [PubMed] [Google Scholar]
- 8.Gribble PL, Ostry DJ. 1996. Origins of the power law relation between movement velocity and curvature: modeling the effects of muscle mechanics and limb dynamics. J. Neurophysiol. 76, 2853–2860. [DOI] [PubMed] [Google Scholar]
- 9.Berni J, Pulver SR, Griffith LC, Bate M. 2012. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr. Biol. 22, 1861–1870. ( 10.1016/j.cub.2012.07.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grillner S. 2003. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 47, 573–586. ( 10.1038/nrn1137) [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Marin A, Stephens GJ, Louis M. 2011. Active sampling and decision making in Drosophila chemotaxis. Nat. Commun. 2, 441 ( 10.1038/ncomms1455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Marin A, Partoune N, Stephens GJ, Louis M. 2012. Automated tracking of animal posture and movement during exploration and sensory orientation behaviors. PLoS ONE 7, e41642 ( 10.1371/journal.pone.0041642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zago M, Lacquaniti F, Gomez-Marin A. 2016. Data from: the speed–curvature power law in Drosophila larval locomotion. Dryad Digital Repository. ( 10.5061/dryad.37sb7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Marin A, Louis M. 2014. Multilevel control of run orientation in Drosophila larval chemotaxis. Front. Behav. Neurosci. 8, 38 ( 10.3389/fnbeh.2014.00038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stumpf MP, Porter MA. 2012. Critical truths about power laws. Science 335, 665–666. ( 10.1126/science.1216142) [DOI] [PubMed] [Google Scholar]
- 16.Maoz U, Portugaly E, Flash T, Weiss Y. 2006. Noise and the two-thirds power law. Adv. Neural Inf. Proc. Syst. 18, 851–858. [Google Scholar]
- 17.Catavitello G, Ivanenko YP, Lacquaniti F, Viviani P. 2016. Drawing ellipses in water: evidence for dynamic constraints in the relation between velocity and path curvature. Exp. Brain Res. 234, 1649–1657. ( 10.1007/s00221-016-4569-9) [DOI] [PubMed] [Google Scholar]
- 18.Berni J. 2015. Genetic dissection of a regionally differentiated network for exploratory behavior in Drosophila larvae. Curr. Biol. 25, 1319–1326. ( 10.1016/j.cub.2015.03.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds AM, Jones HB, Hill JK, Pearson AJ, Wilson K, Wolf S, Lim KS, Reynolds DR, Chapman JW. 2015. Evidence for a pervasive ‘idling-mode’ activity template in flying and pedestrian insects. R. Soc. Open Sci. 2, 150085 ( 10.1098/rsos.150085) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.37sb7.