Abstract
Phenotypic plasticity and diversified bet hedging are strategies for coping with variable environments. Plasticity is favoured when an organism can predict future conditions using environmental cues, while bet hedging is favoured when predictive cues are not available. Theoretical analyses suggest that many organisms should use a mixture of both strategies, because environments often present both scenarios. Here, we examine if the pea aphid wing polyphenism, a well-known case of plasticity, is potentially a mixture of plasticity and bet hedging. In this polyphenism, asexual females produce more winged offspring in crowded conditions, and wingless offspring in uncrowded conditions. We find that pea aphids use plasticity to respond to crowding and we find considerable genetic variation for this response. We further show that individual aphids produce both winged and wingless offspring, consistent with the variability expected in a bet hedging trait. We conclude that the pea aphid wing polyphenism system is probably a mixture of plasticity and bet hedging. Our study adds to a limited list of empirical studies examining mixed strategy usage, and suggests that mixed strategies may be common in dispersal traits.
Keywords: phenotypic plasticity, bet hedging, environmental heterogeneity, dispersal polyphenism, pea aphid, adaptive coin-flipping
1. Introduction
Phenotypic plasticity and diversified bet hedging are two strategies that have evolved in response to environmental heterogeneity [1]. Adaptive phenotypic plasticity produces different phenotypes from a single genotype in response to cues that predict future environmental conditions [2]. Diversified bet hedging also results in multiple phenotypes produced from a single genotype, but differs from plasticity because no predictive cues are used; the different phenotypes are always produced [3–5]. Bet hedging is favoured in fluctuating environments that lack predictive cues about the future [3–5].
Phenotypic plasticity and bet hedging are often discussed as alternative strategies for coping with environmental variability [6]. However, most environments consist of both predictable and unpredictable changes and theoretical models suggest that environmental cues with varying levels of predictive power should favour a joint strategy (also known as coin-flipping plasticity) [7–9]. In a joint strategy, a response to an environmental signal induces a phenotypic change, while the magnitude of this change differs between individuals [1]. While there is a growing body of theoretical work and simulation studies focused on scenarios where a mixed strategy is favoured [8–10], few empirical investigations have addressed this issue ([11,12]; see also references within [8]).
Here, we test for the usage of a joint strategy in the wing polyphenism of pea aphids (Acyrthosiphon pisum). During the summer, parthenogenetic females produce genetically identical winged and wingless daughters based upon environmental conditions experienced by the mother [13]. In optimal conditions, females produce wingless daughters with increased fecundity, while high population density and insufficient food cause the production of dispersal-capable, winged daughters (reviewed in [14]). While multiple studies have shown that pea aphids respond to these cues in a plastic manner, the magnitude of the response is variable and genotypes never produce 100% winged offspring [14,15]. These previous observations could be solely due to plasticity, or due to a combination of plasticity and bet hedging. If the response were purely plastic, we would expect individuals within a genotype to respond similarly. However, if the same conditions were to elicit different responses within a genotype, this would be strong evidence for bet-hedging. Our purpose here, therefore, was to take a closer look at the wing polyphenism, with special attention to the variance of response both among genotypes and among individual aphids within a genotype to consider the possibility that this well-known polyphenism is actually a mixture of plasticity and bet hedging.
2. Material and methods
Aphids were reared on Vicia faba plants covered with cages in climate-controlled rooms at approximately 19°C, 35% humidity, and 16 L : 8 D cycle. Aphids were maintained for three generations at low population density (approx. six females per plant) prior to the start of experiments. We allowed low-density adults to produce nymphs for 24 h; this was the pre-crowding treatment. Adults were then crowded for 24 h in groups of 10 in 35 mm Petri dishes containing moist filter paper to prevent desiccation. From the 40 crowded adults, 10 were moved to a single plant for each genotype; the remaining 30 were discarded. Females larviposited for four consecutive 24 h intervals and then were discarded. Offspring from each 24 h interval were moved to new plants to minimally disturb the adult females, which remained on the same plant for the 4 days. Rearing conditions should not affect offspring phenotypes because morph determination in the pea aphid is pre-natal [16]. The phenotype, winged or wingless, of each offspring was counted as adults (phenotypes cannot be visibly distinguished until third instar, and are easiest to phenotype as adults) for each genotype and time point (pre-crowding and four 24 h intervals post-crowding). This experiment was repeated three times.
We used three genotypes to investigate the offspring phenotypes of individual aphids, rather than groups, produced in response to crowding. Aphids were crowded as above and placed individually (as compared to the last experiment, where they were placed in groups of 10) on plants for 24 h. Offspring were phenotyped as adults. This experiment was performed in triplicate.
To determine if facultative bacterial endosymbionts affected wing induction (Regiella is thought to have an effect [17]), we tested each aphid genotype for the presence of Serratia, Hamiltonella, Regiella, Spiroplasma, Rickettsia, Rickettsiella, Wolbachia and X-type, with Buchnera as a positive control. Diagnostic PCRs were run for each symbiont using published primers [18] and repeated for confirmation (table 1).
Table 1.
Pea aphid genotypes tested, collection site and endosymbionts present.
| genotype | collection site | Regiella | Rickettsia | Spiroplasma |
|---|---|---|---|---|
| MA 1 | Berkley, MA | Y | N | N |
| MA 2 | Berkley, MA | N | Y | N |
| NY 1 | Rochester, NY | N | N | Y |
| NY 2 | Ithaca, NY | N | N | Y |
| NY 3 | Ithaca, NY | N | N | N |
| NY 4 | Rochester, NY | N | N | N |
| NY 5 | Rochester, NY | N | N | Y |
| NY 6 | Ithaca, NY | N | N | Y |
| NY 7 | Rochester, NY | Y | N | N |
| NY 8 | Rochester, NY | N | N | Y |
We used the lme4 package to make generalized linear models (GLM, quasibinomial, link logit function) and generalized linear mixed models (GLMM, binomial, link logit function) in R v. 3.2.5 [19,20]. Models were compared with an ANOVA using a Wald's χ2 statistic [21]. The effect sizes of independent variables were calculated as marginal R2 values [22].
3. Results
(a). Genotypic variation for the polyphenism exists in natural populations
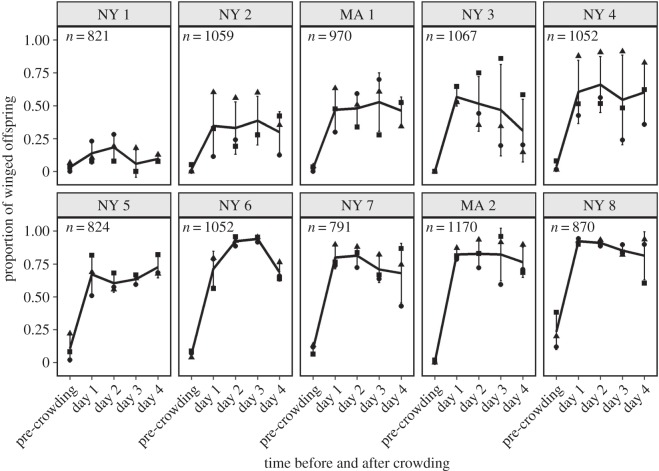
Genotype significantly impacted the proportion of winged offspring in the pre-crowding treatment (GLM, 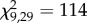 , p = 1.5 × 10−8), explaining 71% of the variance (figure 1). All genotypes produced more winged offspring after experiencing the crowding cue. The effect differed markedly among genotypes, with genotype and treatment (pre-crowding versus day 1 post-crowding) significantly affecting the response (GLM; genotype,
, p = 1.5 × 10−8), explaining 71% of the variance (figure 1). All genotypes produced more winged offspring after experiencing the crowding cue. The effect differed markedly among genotypes, with genotype and treatment (pre-crowding versus day 1 post-crowding) significantly affecting the response (GLM; genotype, 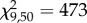 , p = 2.1 × 10−12; treatment,
, p = 2.1 × 10−12; treatment, 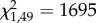 , p < 2.2 × 10−16; genotype × treatment,
, p < 2.2 × 10−16; genotype × treatment, 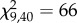 , p = 0.32). Genotype also had a large effect in the post-crowding treatment, explaining 69% of the variation. Finally, some genotypes had greater variance between replicates than others (ANOVA; genotype, F9,30 = 3.9, p
= 2.4 × 10−3, genotype accounts for 54% of the variance).
, p = 0.32). Genotype also had a large effect in the post-crowding treatment, explaining 69% of the variation. Finally, some genotypes had greater variance between replicates than others (ANOVA; genotype, F9,30 = 3.9, p
= 2.4 × 10−3, genotype accounts for 54% of the variance).
Figure 1.
Pea aphids respond to crowding via plasticity, by increasing the proportion of winged offspring. For each of the 10 genotypes (MA 1–2, NY 1–8), the winged offspring produced for the 24 h before and 4 days after a 24 h crowding treatment is illustrated. The genotypes are ordered from lowest to highest, by the proportion of winged offspring they produced on day 1. The lines indicate the mean and the points (circles, squares and triangles) differentiate the three experimental replicates (the offspring from the same sets of 10 females over time). Sample sizes indicate the number of offspring counted per genotype.
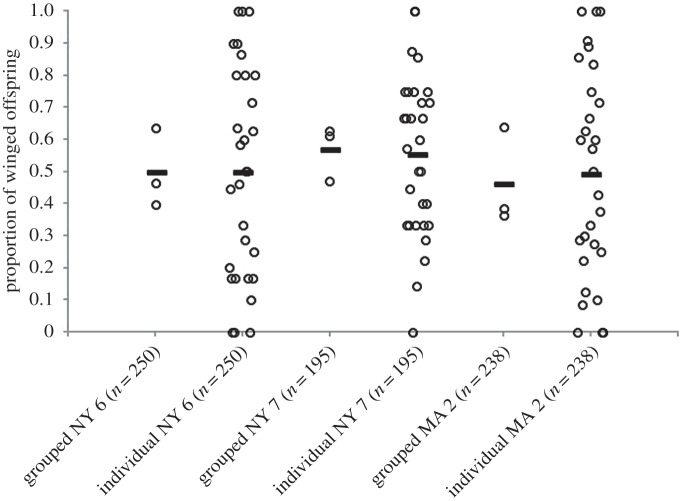
(b). Individuals produce a mixture of winged and wingless offspring
We examined the proportion of winged offspring produced by individual females within genotypes, with the prediction that they would produce both winged and wingless offspring if they bet hedge. We counted the phenotypes of the offspring produced by 30 individual females for three genotypes from the first 24 h after crowding. Quite strikingly, we observed that individuals produced a broad distribution of winged offspring proportions, ranging from 0 up to 1 (figure 2). We found that they were significantly over-dispersed from the expected binomial distribution (χ2 test of homogeneity; MA 2, 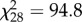 , p = 3.4 × 10−9; NY 6,
, p = 3.4 × 10−9; NY 6, 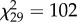 , p = 3.7 × 10−10; NY 7,
, p = 3.7 × 10−10; NY 7, 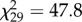 , p = 0.015).
, p = 0.015).
Figure 2.
Individual females almost always produce a mixture of winged and wingless offspring. Shown are the proportions of winged offspring produced by three genotypes in the first 24 h post-crowding. ‘grouped’ = offspring of sets of 10 adult females (n = 3), and ‘individual’ = those same offspring, broken down by individual females (n = 30). The bar shows the mean.
(c). No effect on the wing polyphenism from secondary endosymbionts
We tested the effect of the three facultative symbionts found in our genotypes, but none of them affected the level of wing-induction response elicited from the crowding treatment (GLMM; Regiella, 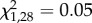 , p = 0.83; Spiroplasma,
, p = 0.83; Spiroplasma, 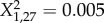 , p = 0.94; Rickettsia,
, p = 0.94; Rickettsia, 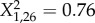 , p = 0.38).
, p = 0.38).
4. Discussion
(a). Pea aphids exhibit phenotypic plasticity in response to crowding
We confirmed earlier studies showing that pea aphids react to a high-density environment using phenotypic plasticity [13,23]. All 10 genotypes responded to the combination of crowding and starvation by increasing their production of winged offspring from the pre-crowding treatment (figure 1). This suggests that an experience of 24 h of crowding and starvation is predictive of future environmental deterioration in natural populations, because plasticity evolves when signals are predictive [10]. Moreover, we showed that there is genetic variation for this plasticity, consistent with previous studies [23,24]. It is notable that the slope of NY 1 (figure 1) differs from the other genotypes; however, we did not find a significant effect of slope (genotype × treatment).
(b). The wing polyphenism is probably a mixture of bet hedging and plasticity
Individual pea aphids stochastically produce different proportions of offspring phenotypes, resulting in high variability among females of a single genotype (figure 2). This stochasticity is consistent with bet hedging. Additionally, the significantly over-dispersed distribution of offspring phenotypes from individual females (figure 2) suggests that there is a biological process in this polyphenism that creates a near-flat distribution of offspring phenotype proportions.
Further rationale for calling this bet hedging can be stated in the context of Simons [6], who explicitly outlined six evidence categories for candidate bet-hedging traits. The first is the recognition of a candidate bet-hedging trait, which in this case is the production of winged and wingless offspring. The second is the identification of an unpredictable environmental factor. Crowding, starvation and interspecific interactions cause the induction of winged offspring (reviewed in [14]), but the winged offspring are not flight capable until they are adults. Thus, there is a time lag of approximately 10 days between the cue and the ability of the offspring to leave. In that time, predators can leave, plant quality can improve, or aphids can walk to better plants, so these cues are not 100% predictive of the future. The third category of evidence is that there is genotype-level candidate bet hedging. There is significantly more variability for winged offspring production in some genotypes than others (figure 1), which is evidence for a genetic basis of this trait in pea aphids. These first three categories have been sufficient to label a trait as bet hedging in other systems ([6] table S1, [12,25]). The remaining three categories (demonstrating variable fitness consequences, demonstrating that the candidate bet-hedging trait is advantageous under fluctuating selection, and showing that the degree of bet hedging matches the degree of fluctuating selection) await future studies.
In sum, we have demonstrated that pea aphids exhibit surprisingly high variability for the production of winged versus wingless offspring at many levels: in the absence and in the presence of high density cues, between genotypes, and among individual females. We conclude that pea aphids probably use a joint strategy of phenotypic plasticity and bet hedging to produce this variability. This work joins a limited number of empirical studies demonstrating mixed strategy usage. These studies have focused mostly on dormancy emergence traits, such as seed germination timing in desert annuals [26] and diapause break in killifish [12]. Our study suggests that mixed strategies may also be common in dispersal traits, such as previously shown in an amphicarpic annual plant [27] and now in the pea aphid wing polyphenism.
Acknowledgements
We thank Ryan Bickel, James Fry and Danielle Presgraves for valuable discussions.
Data accessibility
Data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.2bb5q [28].
Authors' contributions
M.E.G. and J.A.B. conceived of and designed the study, and drafted the manuscript. C.J.A., Y.X.Z. and B.R.O. carried out the study, and participated in drafting the manuscript. M.E.G. conducted data analyses. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Competing interests
The authors declare no competing interests.
Funding
This research was supported by award R01GM116867 from the NIGMS to J.A.B.
References
- 1.DeWitt TJ, Scheiner SM. 2004. Phenotypic plasticity: functional and conceptual approaches. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.West-Eberhard MJ. 2003. Developmental plasticity and evolution, xx, 794 p Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Seger J, Brockmann HJ. 1987. What is bet-hedging? In Oxford surveys in evolutionary biology (eds Harvey PH, Partridge L), pp. 182–211. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Cohen D. 1966. Optimizing reproduction in a randomly varying environment. J. Theor. Biol. 12, 119–129. ( 10.1016/0022-5193(66)90188-3) [DOI] [PubMed] [Google Scholar]
- 5.Cohen D. 1967. Optimizing reproduction in a randomly varying environment when a correlation may exist between conditions at time a choice has to be made and subsequent outcome. J. Theor. Biol. 16, 1–14. ( 10.1016/0022-5193(67)90050-1) [DOI] [PubMed] [Google Scholar]
- 6.Simons AM. 2011. Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc. R. Soc. B 278, 1601–1609. ( 10.1098/rspb.2011.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran NA. 1992. The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989. ( 10.1086/285369) [DOI] [Google Scholar]
- 8.Donaldson-Matasci MC, Bergstrom CT, Lachmann M. 2013. When unreliable cues are good enough. Am. Nat. 182, 313–327. ( 10.1086/671161) [DOI] [PubMed] [Google Scholar]
- 9.Wong TG, Ackerly DD. 2005. Optimal reproductive allocation in annuals and an informational constraint on plasticity. New Phytol. 166, 159–172. ( 10.1111/j.1469-8137.2005.01375.x) [DOI] [PubMed] [Google Scholar]
- 10.Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT. 2010. Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391–3400. ( 10.1098/rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert JJ, McPeek MA. 2013. Maternal age and spine development in a rotifer: ecological implications and evolution. Ecology 94, 2166–2172. ( 10.1890/13-0768.1) [DOI] [PubMed] [Google Scholar]
- 12.Furness AI, Lee K, Reznick DN. 2015. Adaptation in a variable environment: phenotypic plasticity and bet-hedging during egg diapause and hatching in an annual killifish. Evolution 69, 1461–1475. ( 10.1111/evo.12669) [DOI] [PubMed] [Google Scholar]
- 13.Lees AD. 1966. The control of polymorphism in aphids. In Adv. Insect Physiol (eds Beament JWL, Treherne JE, Wigglesworth VB), pp. 207–277. New York, NY: Academic Press. [Google Scholar]
- 14.Müller CB, Williams IS, Hardie J. 2001. The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol. Entomol. 26, 330–340. ( 10.1046/j.1365-2311.2001.00321.x) [DOI] [Google Scholar]
- 15.Walker TJ. 1986. Stochastic polyphenism: coping with uncertainty. Florida Entomol. 69, 46–62. ( 10.2307/3494744) [DOI] [Google Scholar]
- 16.Sutherland ORW. 1969. The role of crowding in the production of winged forms by two strains of the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 15, 1385–1410. ( 10.1016/0022-1910(69)90199-1) [DOI] [Google Scholar]
- 17.Leonardo TE, Mondor EB. 2006. Symbiont modifies host life-history traits that affect gene flow. Proc. R. Soc. B 273, 1079–1084. ( 10.1098/rspb.2005.3408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell JA, et al. 2013. Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol. Ecol. 22, 2045–2059. ( 10.1111/mec.12211) [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. 2015. R: A language and environment for statistical computing. Vienna, Austria: R Core Team. [Google Scholar]
- 20.Bates D, Machler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 21.Fox J, Weisberg S. 2011. An R Companion to Applied Regression, 2nd edn Thousand Oaks, CA: Sage. [Google Scholar]
- 22.Lefcheck JS. 2016. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 23.Lamb RJ, MacKay PA. 1979. Variability in migratory tendency within and among natural populations of the pea aphid, Acyrthosiphon pisum. Oecologia 39, 289–299. ( 10.2307/4215820) [DOI] [PubMed] [Google Scholar]
- 24.Braendle C, Friebe I, Caillaud MC, Stern DL. 2005. Genetic variation for an aphid wing polyphenism is genetically linked to a naturally occurring wing polymorphism. Proc. R. Soc. B 272, 657–664. ( 10.1098/rspb.2004.2995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford MJ, Roff DA. 1993. Bet hedging and the diapause strategies of the cricket Allonemobius fasciatus. Ecology 74, 1129–1135. ( 10.2307/1940482) [DOI] [Google Scholar]
- 26.Clauss MJ, Venable DL. 2000. Seed germination in desert annuals: an empirical test of adaptive bet hedging. Am. Nat. 155, 168–186. ( 10.1086/303314) [DOI] [PubMed] [Google Scholar]
- 27.Sadeh A, Guterman H, Gersani M, Ovadia O. 2007. Plastic bet-hedging in an amphicarpic annual: an integrated strategy under variable conditions. Evol. Ecol. 23, 373–388. ( 10.1007/s10682-007-9232-2) [DOI] [Google Scholar]
- 28.Grantham M, Antonio C, O'Neil B, Zhan YX, Brisson J.2016. Data from: a case for a joint strategy of diversified bet hedging and plasticity in the pea aphid wing polyphenism. Dryad Data Repository. (idoi:10.5061/dryad.2bb5q) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.2bb5q [28].