Abstract
The immunocompetence handicap hypothesis posits that androgens in males can be a ‘double-edged sword’, actively promoting reproductive success, while also negatively impacting health. Because there can be both substantial androgen concentrations in females and significant androgenic variation among them, particularly in species portraying female social dominance over males or intense female–female competition, androgens might also play a role in mediating female health and fitness. We examined this hypothesis in the meerkat (Suricata suricatta), a cooperatively breeding, social carnivoran characterized by aggressively mediated female social dominance and extreme rank-related reproductive skew. Dominant females also have greater androgen concentrations and harbour greater parasite loads than their subordinate counterparts, but the relationship between concurrent androgen concentrations and parasite burdens is unknown. We found that a female's faecal androgen concentrations reliably predicted her concurrent state of endoparasitism irrespective of her social status: parasite species richness and infection by Spirurida nematodes, Oxynema suricattae, Pseudandrya suricattae and coccidia were greater with greater androgen concentrations. Based on gastrointestinal parasite burdens, females appear to experience the same trade-off in the costs and benefits of raised androgens as do the males of many species. This trade-off presumably represents a health cost of sexual selection operating in females.
Keywords: ecoimmunology, mammal, testosterone, masculinization, helminth
1. Introduction
An individual's position within a social hierarchy can significantly impact its reproduction, health and survival [1,2]: high-ranking males often enjoy reproductive benefits, but may experience greater parasitism than their subordinate counterparts [2]. This male trade-off between reproduction and health has been described in various, often polygynous [3,4], mammalian and avian [5,6] species and has been attributed to the action of androgens, predominantly testosterone (T) [7,8]. T functions to promote male-reproductive success via its role in growth and the development of secondary sex characteristics and in the modulation of aggressive behaviour [9]; yet T, either measured directly from blood or inferred from faecal androgen metabolites (FAM), also has been implicated in immunosuppression and parasitism [7]. Although a similar trade-off can be replicated in females by experimentally administering exogenous androgen [10,11], the consequences of natural variation in endogenous androgen for female health rarely have been investigated. Here, we examine if concurrent androgen concentrations in female meerkats (Suricata suricatta)—a species in which all females naturally experience raised androstenedione and T [12]—relate to parasite loads in a manner similar to that experienced by many males [4–8].
Meerkats are cooperatively breeding mongooses that live in stable groups or clans, comprising a dominant breeding pair and various subordinate helpers of both sexes [13]. Although subordinate females show some degree of hormonal ‘masculinization,’ the dominant female has significantly greater concentrations of total androgen than does any other group member [12], which may enable her to restrict the development and reproduction of potential rivals [14,15]. Dominance status dramatically increases reproductive success, but can also incur immunosuppressive costs, as evidenced by increased parasitism [16]. Whether or not this cost is associated with raised androgen concentrations in females is unknown. We predict that androgens will be positively related to parasitism in female meerkats.
2. Material and methods
(a). Study site and subjects
We conducted this study on a previously described, well-habituated, wild population of meerkats living in the Kuruman River Reserve in South Africa's Kalahari Desert [12–18]. Our focal subjects, from 12 clans (or groups), were 37 sexually mature female meerkats (11 dominant, 25 subordinate and 1 that achieved dominant status during the study and is represented in both social classes). Details about meerkat identification and monitoring can be found elsewhere [16].
(b). Sample collection
From January 2013 to March 2014, we opportunistically collected 55 faecal samples from our focal females. To evenly distribute parasite eggs and oocysts (hereafter eggs) throughout each sample, we gently homogenized each sample prior to dividing it for parasitology and endocrine analyses. Details on sample collection, processing and storage are provided elsewhere (for hormone assays [17]; for parasitology [16]).
(c). Parasitology
We encountered eggs from six endoparasite taxa—four nematodes (e.g. Strongylates, Toxocara suricattae, Spiurids, Oxynema suricattae), one cestode (e.g. Pseudandrya suricattae) and one apicomplexan (e.g. coccidia)—and quantified the infection status (i.e. presence/absence) and abundance of each taxon per sample (as in 16). Our estimations of abundance represent the number of conspecific parasite eggs per wet mount, scored as follows: 0 (no eggs), 1 (1 egg), 2 (2–7 eggs), 3 (7–20 eggs) and 4 (more than 20 eggs). We determined parasite species richness (PSR), or the number of parasite taxa, per sample.
(d). Enzyme immunoassays
Following extraction, we analysed FAM via enzyme immunoassay, using previously validated methods [12,17]. We analysed samples in duplicate (re-analysing those for which coefficients of variation exceeded 10%).
(e). Statistics
We used generalized linear models (GLMs) and generalized linear mixed models (GLMMs) to test for associations between social status, FAM and parasitism (infection status, abundance and PSR) using the lme4 (v. 1.1–6) and glmmADMB (v. 0.7.7) packages in R (v. 3.2.2). We used binomial, negative binomial and Poisson distributions for infection status, abundance and PSR, respectively. Our full models included, as explanatory variables, social status (dominant, subordinate), FAM concentration (log nanograms per gram) and their interaction. We specified covariables according to prior results [16]: cumulative rainfall (millimetres), subject weight (grams), pregnancy state (pregnant, not pregnant) and clan size (average number of animals over the previous 30 days). We dropped time of sample collection because it was not associated with parasitism or FAM (ps > 0.05). We controlled for repeated sampling by including individual identity as a random term. We determined the optimal random structure of our models, selected models (by removing non-significant terms sequentially based on AIC) and confirmed the validity of our final models, as previously described [16].
3. Results
Female meerkats were infected with a mean of 2.89 ± 0.194 standard errors (s.e.) parasite taxa (mean dominant PSR ± s.e.: 3.94 ± 0.32; mean subordinate PSR ± s.e.: 2.42 ± 0.20). One subordinate female showed no evidence of parasitism, whereas two dominant females were infected by all six parasite taxa.
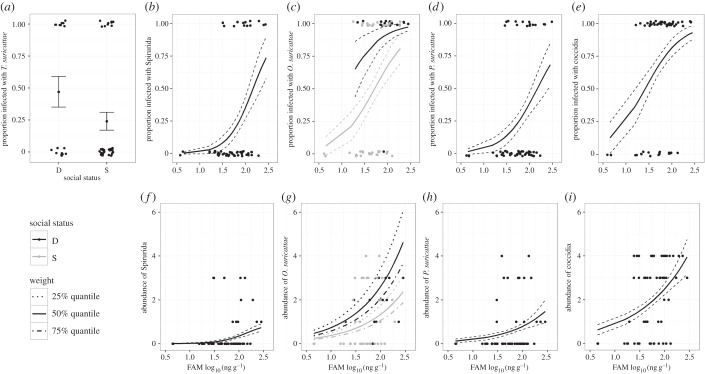
Consistent with a prior report [12], dominant females had significantly greater FAM concentrations than did subordinate females (electronic supplementary material). The females' log FAM concentrations (χ2(1) = 12.97, p < 0.001), but not social status (χ2(1) = 3.12, p = 0.078), were positively and significantly associated with PSR (figure 1). Likewise, their FAM concentrations were positively related to the prevalence and abundance of Spirurida nematodes, O. suricattae, P. suricattae and coccidia (figure 2; electronic supplementary material). Social status was linked only to infections of O. suricattae, which were more prevalent and abundant in dominant females than in subordinate females (figure 2; electronic supplementary material). The interaction between FAM and social status was not statistically significant (electronic supplementary material).
Figure 1.
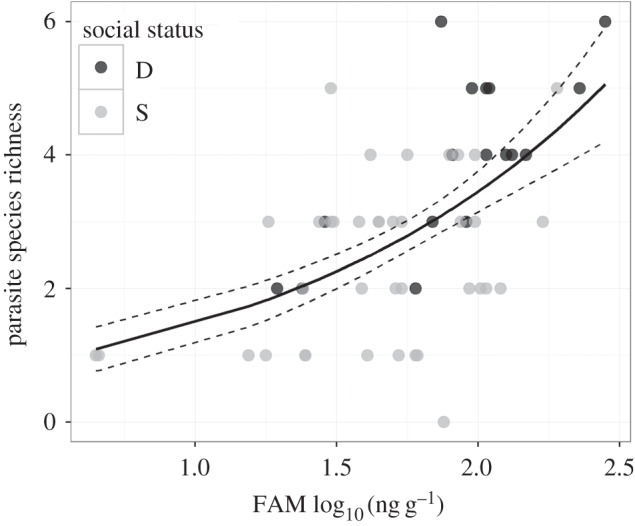
Relationship between parasite species richness (PSR) and faecal androgen metabolites (FAM) in female meerkats. Androgens were positively associated with PSR (GLM: χ2(1) = 12.97, p < 0.001). Black circles represent dominant females (D); grey circles represent subordinate females (S). The solid line represents the fit of the Poisson GLMM and dashed lines represent the standard errors of the linear fit.
Figure 2.
Predictors that significantly explained variance in parasitism in female meerkats (see electronic supplementary material, tables S1 and S2). Shown are the predicted fits for binomial GLMs (for infection status, a–e) and Poisson GLMs (for abundance, f–i). Error bars or hashed lines indicate s.e. estimates (a–i). For both values reflecting parasitism by Oxynema suricattae (c and g), separate predicted fits are shown for dominant (black) and subordinate (grey) females. For abundance of O. suricattae (g), predicted fits are plotted for the 25th quantile (540.75 g; dotted lines), 50th quantile (653.50 g; solid lines) and 75th quantile (750 g; hashed-dotted lines) of weight.
Strongyle infections were so common (present in 94% and 74% of faecal samples from dominant and subordinate females, respectively) that an analysis of infection status was precluded. Neither FAM nor social status was predictive of strongyle abundance. With the exception of weight, other potentially influential covariables [16] were unrelated to parasitism after including FAM in the models (figure 2).
4. Discussion
It is well established that androgens can have direct reproductive costs for females [19]: experimentally administering androgens can delay reproduction [20], reduce fecundity [21] and impair maternal behaviour [22]. Female meerkats have apparently overcome these reproductive limitations; yet, their naturally raised androgens make them susceptible to health costs. We present the first evidence in a wild mammal that the androgen-mediated trade-off between reproduction and health that often characterizes males can also apply to females. Female meerkats that had the greatest androgen concentrations were infected with the most species of parasites and harboured the greatest intensities of infection.
We had previously identified social status as the primary predictor of parasitism in meerkats [16] and had tentatively attributed this finding, in females, to their status-related differences in androgen concentrations. We now show that the relationship between androgens and parasitism transcends a female's social status: although, on average, dominant females had greater androgen concentrations than did subordinate females, there was significant overlap in their ranges and subordinates with the greatest androgen concentrations also evidenced increased parasitism. This pattern is consistent with the finding that all female meerkats are hormonally ‘masculinized’ [12] and that masculinization is merely exaggerated in dominant females. By virtue of their exceptionally raised androgens, dominant female meerkats are able to gain a competitive and reproductive advantage [15]; yet, these benefits come at a cost. For meerkats, the consequences of infection by gastrointestinal parasites are unknown, but for other species they include nutritional deficiencies [23] and impaired growth [24].
Across species, androgens can mediate parasitism in various ways. They can increase susceptibility directly via immunosuppression [25] or indirectly via shunting energy away from immune defence [26]. Additionally, by promoting aggression [17], androgens may increase exposure to those parasites directly transmitted by opponents [27] or indirectly acquired by consuming preferred resources [27]. Dominant female meerkats may suffer an additional risk: by virtue of their increased androgens, they occupy a central position within their clan's social network [22], potentially promoting exposure to parasites. Socially mediated exposure may explain why the prevalence of O. suricattae, a helminth that relies on direct transmission, was both positively related to androgen concentrations and greater in dominant females than in subordinate females. Thus, to the extent that androgens enhance sexually selected traits (e.g. aggressive behaviour) in females, increased parasitism may be a by-product of sexual selection.
Supplementary Material
Acknowledgements
We thank the Kalahari Research Trust and the Northern Cape for permission to conduct research and the neighbouring farmers for allowing access to their land. We thank Marta Manser for her support and input on the fieldwork at the KMP. We thank Nicholas Caruso for lending his unmatched expertise in data visualization and assisting with figures. We thank the volunteers and field managers, including Jessica Mitchell and Charli Davies, for assistance with sample collection.
Ethics
Protocols were approved by the University of Pretoria ethics committee (EC074-11) and the Duke University Institutional Animal Care and Use Committee (A143-12-05).
Data accessibility
Our dataset [28] is publicly available at Dryad: http://dx.doi.org/10.5061/dryad.m8q88.
Authors' contributions
K.N.S. and C.M.D. conceptualized the study. T.C.-B. provided access to the study population and life-history records. K.N.S. and L.K.G. collected samples and analysed faecal androgens. K.N.S. identified parasites and performed statistical analyses. K.N.S. and C.M.D. wrote the manuscript. L.K.G. and T.C.-B. provided comments. All authors agree to be held accountable for the content of this manuscript and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by the National Science Foundation (IOS 333-1264 to C.M.D.) and the Duke University Graduate School (Pre-Dissertation Travel Award, Fred and Barbara Sutherland Fellowship and Katherine Goodman Stern Fellowship to K.N.S.). Vehicle costs in the field were supported by Duke University. We relied on records of individual life histories and access to a field site maintained by the Kalahari Meerkat Project (KMP). During the span of this study, the KMP was supported by the University of Cambridge, University of Zurich, Duke University and a European Research Council grant (no. 294494) to T.C.-B.
References
- 1.Sapolsky RM. 2004. Social status and health in humans and other animals. Annu. Rev. Anthropol. 33, 393–418. ( 10.1146/annurev.anthro.33.070203.144000) [DOI] [Google Scholar]
- 2.Habig B, Archie EA. 2015. Social status, immune response and parasitism in males: a meta-analysis. Phil. Trans. R. Soc. B 370, 20140109 ( 10.1098/rstb.2014.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson K, Grenfell BT, Pilkington JG, Boyd HE, Gulland FM. 2004. Parasites and their impact. In Soay sheep: dynamics and selection in an island population (eds Clutton-Brock TH, Pemberton JM), pp. 113–165. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Ezenwa VO, Stefan Ekernas L, Creel S. 2012. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 26, 123–133. ( 10.1111/j.1365-2435.2011.01919.x) [DOI] [Google Scholar]
- 5.Zuk M, Johnsen TS, Maclarty T. 1995. Endocrine–immune interactions, ornaments and mate choice in red jungle fowl. Phil. Trans. R. Soc. B 260, 205–210. ( 10.1098/rspb.1995.0081) [DOI] [Google Scholar]
- 6.Muehlenbein MP, Watts DP. 2010. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. BioPsychoSoc. Med. 4, 1–12. ( 10.1186/1751-0759-4-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folstad I, Karter AJ. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622. ( 10.1086/285346) [DOI] [Google Scholar]
- 8.Muehlenbein MP, Bribiescas RG. 2005. Testosterone-mediated immune functions and male life histories. Am. J. Hum. Biol. 17, 527–558. ( 10.1002/ajhb.20419) [DOI] [PubMed] [Google Scholar]
- 9.Wingfield JC, Hegner RE, Dufty AM Jr, Ball GF. 1990. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846. ( 10.1086/285134) [DOI] [Google Scholar]
- 10.Kiyota M, Korenaga M, Nawa Y, Kotani M. 1984. Effect of androgen on the expression of the sex difference in susceptibility to infection with Strongyloides ratti in C57BL/6 mice. Aust. J. Exp. Biol. Med. Sci. 62, 607–618. ( 10.1038/icb.1984.58) [DOI] [PubMed] [Google Scholar]
- 11.Zysling DA, Greives TJ, Breuner CW, Casto JM, Demas G, Ketterson ED. 2006. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos. Horm. Behav. 50, 200–207. ( 10.1016/j.yhbeh.2006.03.004) [DOI] [PubMed] [Google Scholar]
- 12.Davies C, Smyth KN, Greene LK, Walsh D, Mitchell J, Clutton-Brock TH, Drea CM. 2016 Exceptional endocrine profiles characterize the meerkat: sex, status, and reproductive patterns. Sci. Rep . 6 , 35492. ( ) [DOI] [PMC free article] [PubMed]
- 13.Clutton-Brock TH, et al. 1998. Costs of cooperative behaviour in suricates. Phil. Trans. R. Soc. B 265, 185–190. ( 10.1098/rspb.1998.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T. 2006. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12 005–12 010. ( 10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodge SJ, Manica A, Flower TP, Clutton-Brock TH. 2008. Determinants of reproductive success in dominant female meerkats. J. Anim. Ecol. B 77, 92–102. ( 10.1111/j.1365-2656.2007.01318.x) [DOI] [PubMed] [Google Scholar]
- 16.Smyth KN, Drea C. 2016. Patterns of parasitism in the cooperatively breeding meerkat: a cost of dominance for females. Behav. Ecol. 27, 148–157. ( 10.1093/beheco/arv132) [DOI] [Google Scholar]
- 17.delBarco-Trillo J, Greene LK, Goncalves IB, Fenkes M, Wisse JH, Drewe JA, Manser MB, Clutton-Brock T, Drea CM. 2015. Beyond aggression: androgen-receptor blockade modulates social interaction in wild meerkats. Horm. Behav. 78, 95–106. ( 10.1016/j.yhbeh.2015.11.001) [DOI] [PubMed] [Google Scholar]
- 18.Drewe JA. 2010. Who infects whom? Social networks and tuberculosis transmission in wild meerkats. Phil. Trans. R. Soc. B 277, 633–642. ( 10.1098/rspb.2009.1775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. 2004. Androgen excess in women: experience with over 1000 consecutive patients. J. Clin. Endocrinol. Metab. 89, 453–462. ( 10.1210/jc.2003-031122) [DOI] [PubMed] [Google Scholar]
- 20.Clotfelter ED, et al. 2004. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm. Behav. 46, 171–178. ( 10.1016/j.yhbeh.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 21.Rutkowska J, Cichoń M, Puerta M, Gil D. 2005. Negative effects of elevated testosterone on female fecundity in zebra finches. Horm. Behav. 47, 585–591. ( 10.1016/j.yhbeh.2004.12.006) [DOI] [PubMed] [Google Scholar]
- 22.Pomerantz SM, Roy MM, Thornton JE, Goy RW. 1985. Expression of adult female patterns of sexual behavior by male, female, and pseudohermaphroditic female rhesus monkeys. Biol. Reprod. 33, 878–889. ( 10.1095/biolreprod33.4.878) [DOI] [PubMed] [Google Scholar]
- 23.Colditz IG. 2008. Six costs of immunity to gastrointestinal nematode infections. Parasite Immunol. 30, 63–70. ( 10.1111/j.1365-3024.2007.00964.x) [DOI] [PubMed] [Google Scholar]
- 24.Newman C, Macdonald DW, Anwar MA. 2001. Coccidiosis in the European badger, Meles meles in Wytham Woods: infection and consequences for growth and survival. Parasitology 123, 133–142. ( 10.1017/S0031182001008265) [DOI] [PubMed] [Google Scholar]
- 25.Olsen N, Kovacs W. 1996. Gonadal steroids and immunity. Endocr. Rev. 17, 369–384. ( 10.1210/edrv-17-4-369) [DOI] [PubMed] [Google Scholar]
- 26.Alonso-Alvarez C, Bertrand S, Sorci G. 2007. Energetic reserves, leptin and testosterone: a refinement of the immunocompetence handicap hypothesis. Biol. Lett. 3, 271–274. ( 10.1098/rsbl.2007.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawley DM, Etienne RS, Ezenwa VO, Jolles AE. 2011. Does animal behavior underlie covariation between hosts’ exposure to infectious agents and susceptibility to infection? Implications for disease dynamics. Integr. Comp. Biol. 51, 528–539. ( 10.1093/icb/icr062) [DOI] [PubMed] [Google Scholar]
- 28.Smyth K, Greene L, Clutton-Brock T, Drea C.2016. Data from: Androgens predict parasitism in female meerkats: a new perspective on a classic trade-off. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our dataset [28] is publicly available at Dryad: http://dx.doi.org/10.5061/dryad.m8q88.